Abstract
Many viruses exploit cellular polarity to constrain the assembly and release of progeny virions to a desired surface. Influenza virus particles are released only from the apical surface of epithelial cells and this polarization is partly owing to specific targeting of the viral membrane proteins to the apical plasma membrane. The RNA genome of the virus is transcribed and replicated in the nucleus, necessitating nuclear export of the individual ribonucleoprotein (RNP) segments before they can be incorporated into budding virus particles. We show that the process of polarized virus assembly begins in the nucleus with the RNPs adopting a novel asymmetric distribution at the inner nuclear membrane prior to their export to the cytoplasm. The viral nucleoprotein, the major protein component of RNPs, displays the same polarized intranuclear distribution in the absence of other influenza virus components, suggesting the existence of a hitherto unrecognized polarity within the mammalian cell nucleus.
Keywords: leptomycin B, haemagglutinin, nucleoprotein, nuclear periphery
1. Introduction
Influenza A virus is a formidable pathogen that, despite an effective vaccination programme and antiviral drugs, retains the potential of causing global pandemics with mortality figures in millions (Webby & Webster 2003). Low pathogenicity influenza virus infections in mammals are limited to the respiratory epithelium, partly because of the distribution of host proteases necessary to activate the viral haemagglutinin (Baigent & McCauley 2003). However, another determinant of superficial infection is that virus budding from epithelial cells is polar, with progeny virions released only from the apical surface (Rodriguez-Boulan & Sabatini 1978). This polarity is partly determined by specific sorting of the viral membrane proteins to the apical plasma membrane (Barman et al. 2001). The virus genome is transcribed and replicated in the nucleus, where its eight segments of negative-sense RNA are encapsidated into ribonucleoproteins (RNPs) by the viral nucleoprotein (NP; Portela & Digard 2002). Newly assembled RNPs are subsequently exported to the cytoplasm to be packaged into virus particles. Nuclear export of RNPs results from their sequential binding by the viral M1 and NS2/NEP proteins, followed by recognition of a nuclear export signal in NEP by the cellular nuclear export receptor CRM1 (Cros & Palese 2003). Accordingly, inactivation of CRM1 in infected fibroblasts by the toxin leptomycin B (LMB) causes nuclear retention of RNPs and also causes them to redistribute to the nuclear periphery (Elton et al. 2001; Ma et al. 2001). Here, we show that, in epithelial cells, RNPs transiently accumulate at the nuclear periphery during normal infection. Furthermore, they do so with marked polarity; a finding (to the best of our knowledge) without precedent for a soluble component of the nucleoplasm.
2. Material and methods
Human embryonic kidney 293-T cells and colon carcinoma Caco-2 cells were cultured in Dulbecco-modified Eagle's medium, supplemented with 10% and 20% foetal calf serum, respectively. Baby hamster kidney (BHK) cells were cultured in Glasgow minimal essential medium supplemented with 10% newborn calf serum (NCS) and 0.3% tryptose phosphate broth. Cells were plated onto glass cover-slips or porous filters (Costar Transwells). LMB, influenza virus A/PR/8/34, anti-RNP and anti-β-actin sera and the NP-expression plasmid pCDNA-NP have been previously described (Elton et al. 2001; Carrasco et al. 2004). Antisera to nucleoporin 62 (Nup62), lamina-associated polypeptide-2 (LAP-2), karyopherin α/Rch1, Rcc1 and Ran were purchased from Transduction Laboratories. Antibodies to CRM1 (N19 and C20 used as a 1 : 1 mixture) were purchased from Santa Cruz Biotechnology. Anti-nucleolin was a gift from J. Hiscox.
Cells were infected at a multiplicity of 5. Plasmid transfections were performed as described (Elton et al. 2001). For immunofluorescence, cells were fixed for 20 min in phosphate‐buffered saline (PBS) containing 4% formaldehyde, blocked with PBS containing 1% NCS and probed with primary followed by fluorophore-conjugated antibodies diluted in PBS/NCS (Elton et al. 2001). Cells were visualized using a Leica TCS-NT confocal microscope. z-axis reconstructions were generated from serial optical planes of focus taken at approximately 0.5 μm intervals.
3. Results
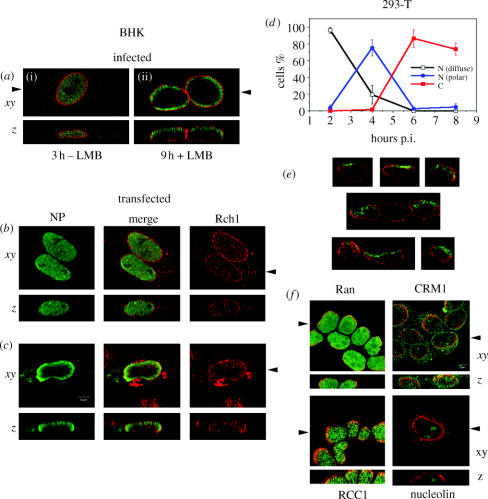
To examine the intranuclear distribution of RNPs in fibroblasts, infected BHK cells were stained for NP and Nup62, a component of the nuclear pore complex. At early times post infection (p.i.), speckles of NP distributed throughout the nucleoplasm with the exception of the nucleolus (figure 1a(i)). At late times p.i., after incubation in the presence of LMB, NP was retained within the nucleus, but as previously defined (Elton et al. 2001; Ma et al. 2001), it was now located predominantly at the nuclear periphery (figure 1a(ii)). Furthermore, when examined in 3D, RNP distribution was polar; rather than forming a complete shell inside the nuclear membrane, they formed a cap at the apex of the nucleus (figure 1a(ii), lower). The lack of NP staining towards the basal side of the nucleus contrasted with the non-polar staining observed for the same protein at 3 h p.i. (figure 1a(i), lower) and for Nup62 under all conditions.
Figure 1.
Polarized intranuclear localization of NP. (a–c) BHK cells were (a) infected and (i) fixed at 3 h p.i. or (ii) treated with 11 nM LMB and fixed at 9 h p.i. before immunofluorescent staining for NP (green) and Nup62 (red). (b,c) 5×104 cells were transfected with (b) 10 or (c) 100 ng of NP plasmid and stained 16 h later for NP (green) and Rch1 (red). (d) NP localization patterns were scored (nuclear (N) diffuse, N-polar or cytoplasmic (C)) through a time-course of infection in 293-T cells and plotted as the percentage of total cells per time-point. A minimum of 100 cells were counted per datum from two independent experiments and the mean±range plotted. (e) Gallery of images taken from 8 μm frozen sections cut through infected 293-T cells at 4.5 h p.i. stained for NP (green) and LAP-2 (red). (f) Infected 293-T cells were stained for NP (red) and the indicated cellular antigen (green) at 4.5 h p.i. Single optical sections through the midline of the nucleus (xy) and z-axis reconstructions (z) are shown. Arrowheads indicate the plane of matching z-sections. Scale bar, 5 μm.
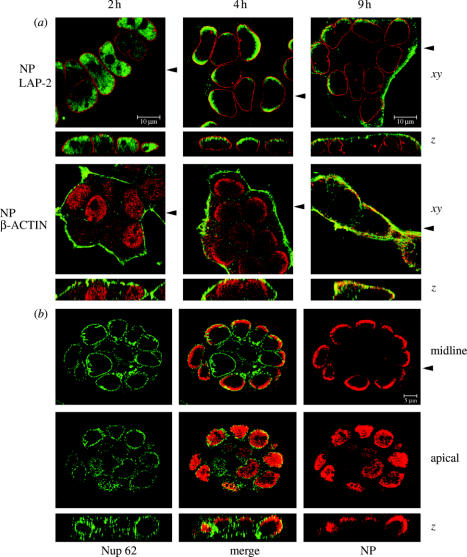
We examined whether this unusual finding held true in other cell types and without drug treatment. Early in infection in epithelioid 293-T cells, speckles of NP localized throughout the nucleus (figure 2a; 2 h). At late times, most NP was cytoplasmic, residing at the apical plasma membrane in close proximity to the cortical β-actin web (figure 2a; 9 h). However, preceding the onset of RNP nuclear export, NP remained within the nucleus but redistributed towards the inner nuclear membrane as defined by LAP-2 staining (figure 2a; 4 h). Importantly, this occurred in the absence of LMB treatment. z-axis reconstructions showed predominant accumulation of NP towards the apices of the nuclei, again in contrast to the homogeneous staining found earlier in infection (figure 2a; compare lower panels of 2 h and 4 h images). Furthermore, NP located at the nuclear periphery showed marked asymmetry in the x−y-plane, with a bias towards areas of the nuclear periphery facing away from regions of cell‐to‐cell contact (figure 2a; 4 h; lateral membranes visible by faint β-actin staining). This effect was particularly pronounced in small clumps of Caco-2 cells grown on permeable filters to promote formation of properly differentiated apical and basolateral surfaces. Optical sections taken through the midline at 4 h p.i. showed intranuclear NP arranged predominantly in short arcs against those areas of nuclear membrane closest to the exterior of the cell sheet (figure 2b). NP in cells surrounded by neighbours was almost solely at the uppermost surface of the nucleus, as shown by a single optical section towards the apical surface of the cells or z-axis reconstructions (figure 2b). Time-course experiments on infected cells showed that the polar–nuclear pattern appeared after 2.5 h p.i., was found in most cells around 4 h p.i. and disappeared after 6 h (figure 1d). Similar polarized intranuclear redistribution of RNPs midway through the virus life cycle was seen in a variety of other cell types including epithelial Madin-Darby canine kidney cells, human rhabdomyosarcoma RD cells and liver hepatocyte WIF-B cells, and with other strains of influenza A virus (data not shown). This polarity seemed unlikely to be a staining artefact because it was seen with various anti-NP sera under different cell fixation and permeabilization regimes (data not shown); it changed during the virus life cycle and double staining with many other antibodies showed that all regions of the nucleus were accessible. Furthermore, the asymmetrical NP staining was most marked in fully polarized cells grown on filters (figure 2b), and here antibody was applied to both apical and basolateral surfaces. Nevertheless, to further rule out an artefact, cells were infected, fixed at 4.5 h p.i., scraped off the dish, embedded in gelatin capsules and cut sections were stained for LAP-2 and NP. In many cases, NP was found in close proximity to discrete regions of the inner nuclear membrane (figure 1e). Although this approach loses orientation of apical and basolateral surfaces, where clumps of cells were sectioned, these asymmetric patches of NP were aligned similarly (figure 1e). Thus staining of cut sections produces results consistent with those seen for permeabilized whole cells. Overall, this strongly argues against this temporally distinct polarized intranuclear localization of NP resulting from a staining artefact. Thus NP shows three stages of intracellular localization in cells of epithelial origin: an early pattern of homogeneous nuclear speckles, late accumulation in the cytoplasm and an intermediate stage of redistribution towards regions of the nuclear periphery closest to apical membrane surfaces.
Figure 2.
Intranuclear localization of NP in epithelial cells. (a) Infected 293-T cells on cover-slips or (b) Caco-2 cells on filters were stained at (a) the indicated times p.i. or (b) at 4.5 h p.i. for NP (green) and LAP-2 (red) or NP (red) and Nup 62 or β-actin (green) as labelled. Scale bars: (a) 10 μm; (b) 5 μm.
We examined whether other influenza virus proteins involved in RNP trafficking showed a polarized distribution within the nucleus, but neither NEP nor M1 showed this behaviour (see figure 3 in the Electronic Appendix). We therefore examined the localization of NP expressed in the absence of other virus proteins. Transfected NP shuttles between nucleus and cytoplasm, with high‐level expression biasing it towards cytoplasmic accumulation and low‐level expression biasing it towards a nuclear distribution (Portela & Digard 2002). BHK cells were transfected with varying amounts of an NP-expressing plasmid. In cells that received 10 ng of plasmid, the majority of NP-expressing cells showed speckled nuclear fluorescence with no polarity in z-sections through the nuclei (figure 1b). In cells transfected with 100 ng of plasmid, the majority of cells displayed exclusively cytoplasmic fluorescence (data not shown). However, in the minority of cells that contained nuclear NP, it was distributed around the inner periphery of the nuclear membrane (figure 1c). Furthermore, z-sections showed NP distributed towards the upper surface (figure 1c). No polarity was observed in the distribution of the nuclear import receptor, Rch1 (figure 1b,c). Similar polarized-peripheral intranuclear distribution of NP but not of Rch1 was observed in transfected 293-T cells (data not shown). Thus, in the absence of other influenza virus proteins, NP adopts a polarized intranuclear distribution indistinguishable from that observed during virus infection. This strongly suggests that it interacts with a similarly arranged cellular structure. Normal nucleolar architecture seemed unaffected by infection (figure 1f). Because RNPs polarize at the nuclear periphery before they leave the nucleus, we examined the distribution of components of the cellular nuclear export apparatus. However, there was no observable asymmetry to the localization of CRM1, largely resident at the nuclear rim (figure 1f). The guanine-exchange factors Ran and RCC1 that regulate many nuclear transport factors showed diffuse nuclear staining patterns with only limited colocalization with peripheral NP (figure 1f). Furthermore, LMB-induced nuclear retention of cyclin A and transportin, cellular nucleo-cytoplasmic shuttling proteins, resulted in a non-polar nuclear diffuse staining pattern (see figure 3c in the Electronic Appendix). This does not support the hypothesis that the polarized accumulation of NP at the nuclear periphery results from its interaction with the nuclear export apparatus.
4. Discussion
To our knowledge, this is the first description of intranuclear polarization of a viral protein. The bias NP exhibits towards regions of the nucleus facing the apical plasma membrane prior to RNP nuclear export correlates with the apical distribution of the viral membrane proteins and indeed the site of virus budding (Barman et al. 2001). Thus polarized intranuclear trafficking of the RNPs may provide a functional advantage to the virus by directing the virus genome towards the correct surface of the cell for virion assembly before it leaves the nucleus. The hypothesis that asymmetry in nuclear pore complexes helps to ‘gate’ cellular transcription as a means of defining cellular polarity (Blobel 1985) has not met with universal support (Mahy et al. 2002). However, we propose that this instance provides the first example of ‘genome gating’ in which a virus polarizes nuclear export of its nucleic acid.
The striking nuclear asymmetry exhibited by NP is also almost without precedent for cellular components. Investigation of the nuclear architecture of the single-cell alga Chlamydomonas reinhardtii showed nuclear pore complexes concentrated at the posterior side of the nucleus (Colon-Ramos et al. 2003), whereas, in yeast, the Mlp family of nuclear pore components show similar asymmetry (Galy et al. 2004). However, it is not clear how this applies generally to metazoan cells because we found no evidence for asymmetric distribution of the pore complex in the vertebrate cell types examined here. Nevertheless, as NP exhibits intranuclear polarity in the absence of other viral proteins, it seems likely that it interacts with a similarly polarized nuclear peripheral component. Current views on organizational domains of the nucleus include a peripheral compartment, but there is little evidence to suggest it is polarized (Dundr & Misteli 2001). However, one candidate is chromatin as, at least in some cases, chromosomes occupy specific territories within the nucleus and also show a more general ‘Rabl’ type organization with the centromeres at the apex of the nucleus (Dundr & Misteli 2001). Indeed, one study has shown a polar-peripheral arrangement of DNAse hypersensitive chromatin in fibroblasts (Chan et al. 2000), which is very similar to the peripheral NP distribution we show here. Evidence suggests that influenza virus RNPs interact with chromatin or other insoluble components of the nucleus (Jackson et al. 1982), so we are testing the hypothesis that the interaction of RNPs with asymmetrically distributed chromatin domains is responsible for their polar localization within nuclei.
Acknowledgments
This work was supported by grants from the Royal Society, MRC (G0300009 and G9901213) and Wellcome Trust (059151 and 073126) to P.D. M.J.A. is supported by the Gulbenkian PhD programme in Biomedicine and the FCT-Portugal. We thank Gudrun Ihrke, Jeremy Skepper and Lyn Carter for invaluable help and discussion.
Supplementary Material
References
- Baigent S.J, McCauley J.W. Influenza type A in humans, mammals and birds: determinants of virus virulence, host-range and interspecies transmission. Bioessays. 2003;25:657–671. doi: 10.1002/bies.10303. [DOI] [PubMed] [Google Scholar]
- Barman S, Ali A, Hui E.K, Adhikary L, Nayak D.P. Transport of viral proteins to the apical membranes and interaction of matrix protein with glycoproteins in the assembly of influenza viruses. Virus Res. 2001;77:61–69. doi: 10.1016/s0168-1702(01)00266-0. [DOI] [PubMed] [Google Scholar]
- Blobel G. Gene gating: a hypothesis. Proc. Natl Acad. Sci. USA. 1985;82:8527–8529. doi: 10.1073/pnas.82.24.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Amorim M.J, Digard P. Lipid-raft dependent targetting of the influenza A virus nucleoprotein to the apical plasma membrane. Traffic. 2004;5:979–992. doi: 10.1111/j.1600-0854.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- Chan J.K.L, Park P.C, De Boni U. Association of DNAse sensitive chromatin domains with the nuclear periphery in 3T3 cells in vitro. Biochem. Cell Biol. 2000;78:67–78. [PubMed] [Google Scholar]
- Colon-Ramos D.A, Salisbury J.L, Sanders M.A, Shenoy S.M, Singer R.H, Garcia-Blanco M.A. Asymmetric distribution of nuclear pore complexes and the cytoplasmic localization of beta2-tubulin mRNA in Chlamydomonas reinhardtii. Dev. Cell. 2003;4:941–952. doi: 10.1016/s1534-5807(03)00163-1. [DOI] [PubMed] [Google Scholar]
- Cros J.F, Palese P. Trafficking of viral genomic RNA into and out of the nucleus: influenza, Thogoto and Borna disease viruses. Virus Res. 2003;95:3–12. doi: 10.1016/s0168-1702(03)00159-x. [DOI] [PubMed] [Google Scholar]
- Dundr M, Misteli T. Functional architecture in the cell nucleus. Biochem. J. 2001;356:297–310. doi: 10.1042/0264-6021:3560297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton D, Simpson-Holley M, Archer K, Medcalf L, Hallam R, McCauley J, Digard P. Interaction of the influenza virus nucleoprotein with the cellular CRM1-mediated nuclear export pathway. J. Virol. 2001;75:408–419. doi: 10.1128/JVI.75.1.408-419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy V, Gadal O, Fromont-Racine M, Romano A, Jacquier A, Nehrbass U. Nuclear retention of unspliced mRNAs in yeast is mediated by perinuclear Mlp1. Cell. 2004;116:63–73. doi: 10.1016/s0092-8674(03)01026-2. [DOI] [PubMed] [Google Scholar]
- Jackson D.A, Caton A.J, McCready S.J, Cook P.R. Influenza virus RNA is synthesised at fixed sites in the nucleus. Nature. 1982;296:366–368. doi: 10.1038/296366a0. [DOI] [PubMed] [Google Scholar]
- Ma K, Roy A.M, Whittaker G.R. Nuclear export of influenza virus ribonucleoproteins: identification of an export intermediate at the nuclear periphery. Virology. 2001;282:215–220. doi: 10.1006/viro.2001.0833. [DOI] [PubMed] [Google Scholar]
- Mahy N.L, Perry P.E, Gilchrist S, Baldock R.A, Bickmore W.A. Spatial organization of active and inactive genes and noncoding DNA within chromosome territories. J. Cell Biol. 2002;157:579–589. doi: 10.1083/jcb.200111071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela A, Digard P. The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication. J. Gen. Virol. 2002;83:723–734. doi: 10.1099/0022-1317-83-4-723. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E.R, Sabatini D.D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc. Natl Acad. Sci. USA. 1978;75:5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webby R.J, Webster R.G. Are we ready for pandemic influenza? Science. 2003;302:1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.