Abstract
Hosts often differ in their degree of parasitism and their expression of resistance. Yet very little is known about how the availability (and allocation) of resources to parasites at pre-infective stages influences their success in initiating parasitism, or in inducing and succumbing to resistance from hosts. We studied a damselfly–mite association to address how experimental variation in the age of first contact with hosts (timing) influenced subsequent parasite fitness. We demonstrate that timing influenced the ability of larval mites to make the transition to parasitism, but was not associated with measures of physiological resistance by hosts. Timing presumably relates to the availability of resources remaining for individuals to exploit their hosts. More research is needed on the importance of such factors, from variation in host resistance and parasite success and, ultimately, to the numbers and distributions of parasites on hosts.
Keywords: damselfly, mite, parasite fitness, resistance
1. Introduction
Various host-centred attributes including age, gender, nutrition and reproductive status can relate to immune or resistance expression (Sheldon & Verhulst 1996; Siva-Jothy et al. 1998; Kurtz et al. 2000; Leung et al. 2001; Rolff 2001). By comparison, the importance of parasite-centred attributes is often overlooked. For many parasites, infective stages are expected to eventually exhaust energy or other reserves in ‘searching’ or ‘waiting’ for hosts (Münchberg 1982). The variation in time spent searching for hosts could affect the likelihood of parasitizing hosts, or the likelihood of inducing or succumbing to host resistance.
We studied damselflies and their ectoparasitic mites to address how experimental variation in the timing of initial host contact by pre-infective mites (hereafter referred to as timing) influenced success in initiating parasitism. We also explored whether timing influences the magnitude of damselfly resistance, or the melanotic encapsulation of mites' feeding tubes, which results in their death (Yourth et al. 2001). Water mite parasitism appears costly to dragonflies, including the mites here studied (Forbes et al. 2004).
We used Lestes forcipatus (Rambur) damselflies and Arrenurus planus (Marshall) mites. Upon hatching, larval A. planus swim in search of a host; if a host is found, mites attach to it and become phoretic. Like other water mites, the larval mites abandon their larval host's exoskeleton at eclosion and crawl onto the newly forming adult (Smith 1988). Mites then attach to the ventral surface of the thorax, pierce the host's cuticle and produce a feeding tube (Smith 1988). Feeding tubes of A. planus form within 24 h of host emergence; melanotic encapsulation of the feeding tube is either mounted or not at that time. Mites not killed by resistance engorge and later drop off the host when hosts return to appropriate water bodies for mating and egg laying.
2. Materials and methods
(a) Manipulation of timing
Adult female A. planus were collected (see Forbes et al. (1999) for site location and description) and placed individually into 60 ml containers. Female A. planus lay multiple clutches of 20–40 eggs when fed ostracods ad libitum. Clutches were provided with fresh pond water and held at 22 °C until they hatched. Upon hatching, larval mites from each clutch were divided approximately evenly into two lots. To reduce the influence of maternal effects, mite larvae from each of these lots were combined with half of the mites from each of two other clutches that hatched the same day (making two batches of 40–80 mites). This procedure was repeated to produce enough batches of mites to challenge 120 hosts with 20 mites each. Mites from each batch were either presented with host larvae within 1 day of hatching, or left in jars without hosts for 4 days before being exposed to hosts. For Arrenurus papillator, a species closely related to A. planus, larval mites appear to exhaust their reserves after a week (Münchberg 1982). Four days were chosen as an upper limit of time to host contact. Mites waiting 7 days may fail to initiate parasitism on hosts, thereby precluding us from also examining melanotic encapsulation of feeding tubes of ‘delayed’ mites.
(b) Exposure
Final-instar L. forcipatus larvae were netted (during 18–26 June 2003) from the same site and brought to the laboratory in plastic 1 l containers with pond water. We removed all existing mites from larvae with a fine paintbrush and then placed each of 38 female and 25 male larval damselflies singly into 60 ml jars, each with 20 mites from a 1-day batch of mites. We also presented each of 31 females and 26 males to 20 mites from a 4-day batch. Hereafter, the treatment is referred to as mite group or timing.
After 24 h exposure at 22 °C, the numbers of mites attending to each larval host were counted using a dissecting microscope. Each host larva was subsequently placed into ‘mite free’ pond water and held individually at 22 °C. Damselfly larvae were then fed Daphnia until they emerged. We recorded the date of host emergence, mass (±0.01 g, Mettler AE100 Digital Scale), wing length (±0.01 mm, Mitutoyo digital calipers) and number of mites attached to each host, on the day of host emergence.
(c) Melanotic encapsualtion of feeding tubes
The proportion of feeding tubes melanized and the degree of the melanization response were assessed 24 h after a damselfly's emergence. To examine melanotic encapsulation, we prepared a damselfly by removing its head, wings, legs and abdomen. Each thorax was placed into a dram-vial (4.5 cm×1.5 cm) containing Andre's solution (1 : 1 : 1 chloral hydrate : acetic acid : water by mass) to ‘clear’ the cuticle and muscle tissue. This procedure left the feeding tubes, and responses to the feeding tubes, intact. The ventral portion of the thorax (with mites intact) was excised using a 26 gauge needle and placed on a microscope slide with a few drops of glycerol to prevent desiccation. For 165 out of 187 mites, we found feeding tubes; the proportion of tubes found did not differ between groups (logistic regression F1,42=2.54, p=0.12).
Feeding tubes were assessed for melanotic encapsulation and for the percentage coverage by melanin using phase contrast microscopy (250×). The percentage coverage of melanin was the proportion of the feeding tube with melanin (see Yourth et al. (2002) for a discussion of variably melanized feeding tubes). Scoring percentage coverage of melanin was highly repeatable between observers (Spearman's ρ=0.92, p<0.0001, n=20). T.R. scored samples ‘blind’ to their identity. The average percentage coverage of melanin across tubes was calculated for each damselfly.
Analyses were completed using R 1.9.1.(Ihaka & Gentleman 1996) and means are reported ±1 s.e. Unless otherwise stated, analyses were logistic regressions with quasibinomial errors because data were overdispersed (McCullagh & Nelder 1989). Both mass and wing lengths were log transformed for normality.
3. Results
The proportion of 20 mites that initially attended to host larvae was not influenced by timing, but was influenced by host sex (mite group: F1,119=0.36, p=0.55; sex: F1,119=6.47, p=0.01). Male larvae were attended to by proportionately fewer mites (1-day group: 0.14±0.020; 4-day group: 0.16±0.021) than were female larvae (1-day group: 0.20±0.021; 4-day group: 0.23±0.024).
Sixty-two damselflies emerged. The 39 females eclosed earlier (3.46±0.32 days) than the 23 males (4.96±0.55 days; ANOVA F1,61=6.06, p=0.02). There was no difference in days to emergence for larval hosts between mite groups (ANOVA F1,61=0.17, p=0.68). Also, no differences in mass or wing lengths were found either for emerging males, or for females, challenged with mites from different groups (table 1).
Table 1.
Comparisons of mean mass and mean wing lengths (±s.e.) separately for newly emerged male and female damselflies challenged with mites from the 1-day versus 4-day group. (The t-values for each comparison are presented with the associated degrees of freedom as subscripts, and p-values.)
| 1-day | 4-day | t-test | p | |
|---|---|---|---|---|
| males | ||||
| mass (g) | 0.0261±0.0012 | 0.0268±0.0007 | t23=0.54 | 0.60 |
| wing length (mm) | 13.20±0.12 | 12.86±0.20 | t23=1.46 | 0.17 |
| females | ||||
| mass (g) | 0.0329±0.0081 | 0.0293±0.0015 | t35=1.90 | 0.08 |
| wing length (mm) | 13.99±0.28 | 14.56±0.17 | t35=1.66 | 0.12 |
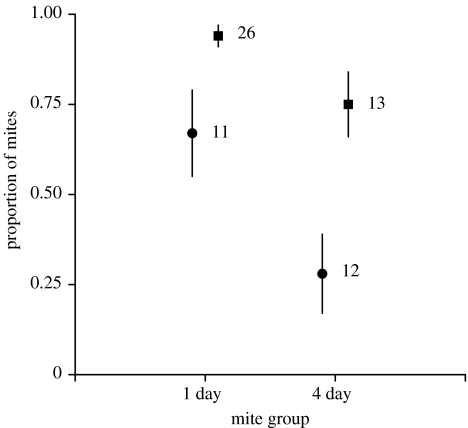
Of those mites that initially attended to the host, the proportion that successfully initiated parasitism was influenced by timing and host gender (figure 1). Mites from the 1-day group were more likely than 4-day mites to initiate parasitism (F1,59=10.12, p=0.002). Also, female hosts had a greater proportion of mites attaining this parasitic stage (F1,59=11.79, p=0.001).
Figure 1.
The proportion (±s.e.) of mites from the 1-day and 4-day groups that initiated parasitism. Proportions are predicted from a logistic regression with host sex and mite groups as dependent variables (numbers denote sample size; females, squares; males, circles). Three out of four host groups show similar success in emergence (44, 46 and 42% for males challenged with the mites from the 1-day and 4-day groups, and females challenged with mites from the 4-day group, respectively). However, females challenged with mites from the 1-day group had an unexplained higher emergence success (68%).
For examination of host response to feeding tubes, only 43 damselflies (33 hosts with mites from the 1-day group, and 10 hosts with mites from the 4-day group) emerged with mites. The proportion of feeding tubes showing melanotic encapsulation did not differ between sexes, nor was it dependent on mite group (sex: F1,41=0.16, p=0.69; mite group: F1,41=1.03, p=0.31). Males encapsulated 0.29±0.12 and 0.15±0.120 of the tubes from the 1-day group and the 4-day group of mites, respectively. Females encapsulated 0.35±0.088 and 0.18±0.12 of tubes from the 1-day and the 4-day group of mites, respectively. Average percentage coverage by melanin did not depend on either host sex or mite group (sex: F1,41=2.65, p=0.11; group: F1,41=0.25, p=0.62).
4. Discussion
That timing influenced a larval mite's ability to initiate parasitism could help to explain seasonal variation in mite infestation or other within-host-population variation in patterns of mite infestation (e.g. Forbes et al. 1999). By comparison, timing did not affect the host's encapsulation of feeding tubes or the degree of melanization. Yourth et al. (2001) found that mites with partially melanized tubes still died. Our measure of the proportion of tubes that were melanized should thus relate to future fitness expectation for larval mites. In other words, those subsets of mites that succeeded in initiating parasitism should have an equal expectation of future fitness, regardless of their treatment group. We expect that mites weakened by spending long periods of time searching for hosts obtain enough resources from non-responding hosts to complete their engorgement and development.
We did not observe any differences between sexes in either the proportion of tubes melanized or the degree of the melanization response. Other studies have found sex differences in allocation to immune parameters such as greater haemocyte concentration and greater phenoloxidase activity by females (e.g. Kurtz et al. 2000). However, variation in immune parameters does not always explain differences in mite parasitism (e.g. Robb et al. 2004). In our study, the proportion of mites initiating parasitism was greater for female than male hosts, and females had more mites attending them prior to emergence, but also emerged earlier than males. Larval mites appear more likely to attend larval damselflies closer to emergence and are more likely to initiate parasitism among those hosts (Leung et al. 1999). Another effective form of defence against mites is grooming by the hosts (Leung et al. 1999). We suspect that grooming is attenuated as quiescent hosts approach emergence, thereby explaining why females had more mites. Also, those mites have to spend less time waiting because female hosts emerged sooner, and this probably explains why more of the females' mites were successful in initiating parasitism (cf. Leung et al. 2001).
The importance of timing to success in initiating parasitism and expression of host resistance is understudied. Timing is one of several factors likely to affect resources available to pre-infective and later stages of parasites (cf. Ferrell et al. 2001). In our study, mites that had to wait longer (before they were exposed to hosts and while they were phoretic on hosts) were less able to make the transition to the parasitic phase. In nature, such ‘parasite effects’ may explain among-host variation in infestations, even if hosts have little variation in behavioural defence or physiological resistance. We did not find that resistance related to mite timing. Thus, the seasonal increases in resistance seen by L. forcipatus to A. planus (Yourth et al. 2002) cannot be explained by late-season damselfly hosts having mites that took a long time to find hosts. The relationship between parasite resources and resistance may be more important for parasites that counter host resistance following initial attachment or penetration. More work is needed to determine the importance of factors such as timing on variation in parasite success (whether mediated or not by host resistance) and, ultimately, the numbers and distributions of parasites on hosts.
Acknowledgments
Marc Lajeunesse and Silvia Laimgruber helped in the field and laboratory. We also thank two anonymous reviewers. Funding was provided by the Natural Sciences and Engineering Research Council of Canada to MRF. Bruce Smith and Rob Baker were very helpful in discussions.
References
- Ferrell D.L, Negovetich N.J, Wetzel E.J. Effect of temperature on the infectivity of metacercariae of Zygocotyle lunata (Digenea: Paramphistomidae) J. Parasitol. 2001;87:10–13. doi: 10.1645/0022-3395(2001)087[0010:EOTOTI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Forbes M.R, Muma K.E, Smith B.P. Parasitism of sympetrum dragonflies by Arrenurus planus mites: maintenance of resistance particular to one species. Int. J. Parasitol. 1999;29:991–999. doi: 10.1016/s0020-7519(99)00061-2. [DOI] [PubMed] [Google Scholar]
- Forbes M.R, Muma K.E, Smith B.P. Recapture of male and female dragonflies in relation to parasitism by mites, time of season, wing length and wing cell symmetry. Exp. Appl. Acarol. 2004;34:79–93. doi: 10.1023/b:appa.0000044441.60122.27. [DOI] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R. R: a language for data analysis and graphics. J. Comput. Graph. Stat. 1996;5:299–314. [Google Scholar]
- Kurtz J, Wiesner A, Götz P, Sauer K.P. Gender differences and individual variation in the immune system of the scorpionfly Panorpa vulgaris (Insecta: Mecoptera) Dev. Comp. Immunol. 2000;24:1–12. doi: 10.1016/s0145-305x(99)00057-9. [DOI] [PubMed] [Google Scholar]
- Leung B, Baker R.L, Forbes M.R. Grooming decisions by damselflies, age specific colonisation by water mites, and the probability of successful parasitism. Int. J. Parasitol. 1999;29:397–402. doi: 10.1016/s0020-7519(98)00223-9. [DOI] [PubMed] [Google Scholar]
- Leung B, Forbes M.R, Baker R.L. Nutritional stress and behavioural immunity of damselflies. Anim. Behav. 2001;6:1093–1099. [Google Scholar]
- McCullagh P, Nelder J.A. 2nd edn. Chapman & Hall; London: 1989. Generalized linear models. [Google Scholar]
- Münchberg V.P. On the parasitism on the wings of Sympetrum meridionale and fonscolombei Selys (Odonata) by Arrenurus (A.) papillator (Müll) (Hydrachnella, Acari), and on the specificity of and some hypotheses about the parasitism. Arch. Hydrobiol. 1982;152:353–368. [Google Scholar]
- Robb T, Forbes M.R, Jamieson I.G. Engorgement success of parasitic mites on adult sexes of the colour polymorphic mountain stone weta. New Zeal. J. Zool. 2004;31:249–254. [Google Scholar]
- Rolff J. Effects of age and gender on immunity of dragonflies (Odonata, Lestidae) from a wild population. Can. J. Zool. 2001;79:2176–2180. [Google Scholar]
- Sheldon B.C, Verhulst S. Ecological immunity: costly parasite defenses and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Siva-Jothy M.T, Tsubaki Y, Hooper R.E. Decreased immune response as a proximate cost of copulation and oviposition in a damselfly. Physiol. Entomol. 1998;23:274–277. [Google Scholar]
- Smith B.P. Host–parasite interaction and impact of larval water mites on insects. Annu. Rev. Entomol. 1988;33:487–507. [Google Scholar]
- Yourth C.P, Forbes M.R, Smith B.P. On understanding variation in immune expression of the damselflies Lestes spp. Can. J. Zool. 2001;78:815–821. [Google Scholar]
- Yourth C.P, Forbes M.R, Smith B.P. Immune expression in a damselfly is related to time of season, not to fluctuating asymmetry or host size. Ecol. Entomol. 2002;27:123–128. [Google Scholar]