Abstract
A common assumption in fish studies is that iteroparous fishes, once mature, normally reproduce in all consecutive seasons. An analysis of scales from Norwegian spring-spawning herring collected between 1935 and 1973 revealed a strong under-representation (47% of expected) of second-time spawners on the spawning grounds. This reduction is not satisfactorily explained by errors in scale-reading, suggesting that the second reproductive season is frequently skipped. Skipping a season may relate to trade-offs between growth, current and future reproduction, and survival, which are likely to be particularly strong for young adult herring.
Keywords: Clupea harengus, current and future reproduction, fishery management, life-history trade-off, migration cost, survival
1. Introduction
Each reproductive season, adults of iteroparous species face an important life-history decision: reproduce now or delay reproduction until next season? Investment in reproduction can reduce growth and survival, and thereby expected reproductive success later on. Large body size is often advantageous in terms of survival, mating success and the quality and number of offspring. This lifelong trade-off between growth, reproduction and survival strongly influences the life-history decision to reproduce in a given season. Theoretically, skipping reproduction is expected when the loss in fecundity this season is more than balanced by increased fecundity in the future, discounted by the survival probability up to that point (Roff 2002).
The extent to which reproduction is skipped is potentially an important factor affecting reproductive potential, and thereby sustainable yield, in commercially exploited fish populations. Nevertheless, the possibility of skipped reproduction has received only sporadic attention among fisheries biologists (Ivanov 1971; Oganesyan 1993; Burton et al. 1997; Rideout et al. 2000; Jørgensen et al. 2004).
This paper challenges the conventional idea of strictly annual reproduction for Norwegian spring-spawning herring, the largest stock of Atlantic herring (Clupea harengus). In this population, individuals mature at age 3–9 years and have a maximum lifespan of greater than 20 years. The adult herring undertake long annual migrations between productive summer‐feeding areas in the Norwegian Sea, overwintering areas off northwestern Norway, and spawning areas off western and southwestern Norway. The spawning migration incurs considerable energetic costs. The spawning grounds further to the south (favourable for offspring survival) are mainly reached by the larger, older herring, while the smaller, first-time spawning herring tend to spawn further north (Slotte & Fiksen 2000). Our results suggest, however, that a significant fraction of adult herring may skip the second spawning migration altogether.
2. Material and methods
Age, age at maturation (here, age at first spawning) and number of post-maturation years (i.e. years completed since first spawning) were obtained from scales of 84 116 adult Norwegian spring-spawning herring, sampled randomly between January and March of 1935–1973 in the spawning areas by the Institute of Marine Research (for details see Engelhard et al. 2003). Experienced scale readers distinguished between three types of growth layers (Runnström 1936; Engelhard et al. 2003): relatively wide ‘coastal’ and ‘oceanic’ rings, corresponding to the early and late immature stages, respectively; and narrow ‘spawning’ rings, corresponding to years after the first spawning event. For adult herring, the number of coastal and oceanic rings thus equals age at maturation, and the number of spawning rings equals the number of post-maturation years.
We examined the hypothesis that, after maturation, herring return annually to the spawning areas. For a given year-class, numbers of spawners sharing the same age at first spawning should then decrease in each consecutive spawning season solely as a function of mortality. Post-maturation survival was modelled based on the numbers of fish (n) sampled in consecutive years y and y+1 and with, respectively, p and p+1 post-maturation years. Log-transformation allows the use of the linear model:
| (2.1) |
where np,y and np+1,y+1 are the sampled numbers of fish of a given year-class and given age at maturation with p and p+1 post-maturation years, respectively, and cp is the pth element in a vector of survival coefficients, corresponding to the logarithm of change in numbers from p to p+1 post-maturation years. Ny and Ny+1 are the sampled numbers of fish of all year-classes and maturation ages included to reduce noise caused by the variable sampling effort.
Survival coefficients estimated with model (2.1) were similar for most transitions, except for those from 0→1 and 1→2 post-maturation years. This suggested constant annual survival and an under-representation of fish with 1 post-maturation year in samples. Model (2.1) was therefore modified as follows:
| (2.2) |
where c0 is a mean survival coefficient, cI is a coefficient of under-representation and Ip is an indicator variable with
Mean annual post-maturation survival is thus estimated as ec0, and the fraction of fish skipping the second spawning season as 1−ecI.
3. Results
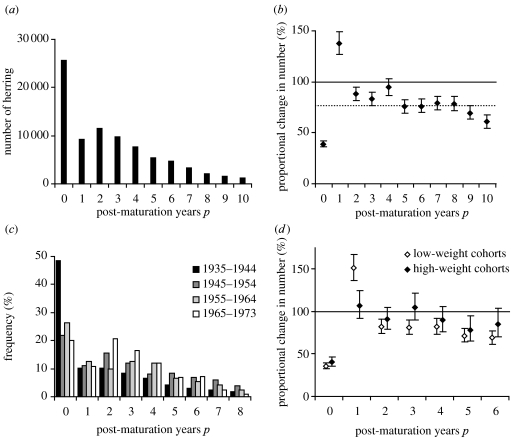
Figure 1a shows the numbers of Norwegian spring-spawning herring sampled in the spawning area for different numbers of post-maturation years. As expected, the samples show the highest numbers of fish with 0 post-maturation years (first-time spawners) and exponentially declining numbers of fish with increasing numbers of post-maturation years, indicating constant annual survival. Remarkably, however, the samples contain much fewer fish with 1 post-maturation year (second-time spawners) than expected. This is illustrated in figure 1b, showing proportional change in numbers of spawners between consecutive post-maturation years. In fact, numbers increase from 1→2 post-maturation years, indicating that processes other than survival must also be invoked to explain the observations. Applying model (2.2)—assuming constant survival but allowing for skipping of the second spawning season—it was found that 47% of potential second-time spawners were missing in the samples (cI=−0.63, s.e.=0.06). This fraction was significantly different from zero (F=126, p<0.0001). Mean annual post-maturation survival was estimated at 76% (c0=−0.28, s.e.=0.02).
Figure 1.
(a) Total numbers of herring sampled in the spawning area during 1935–1973 at different numbers of post-maturation years. First-time spawners have zero post-maturation years. (b) Proportional change (with s.e.) in numbers of herring, present in the spawning area, from p to p+1 post-maturation years (estimated with model (2.1)). Dotted line indicates mean annual survival, estimated with model (2.2). (c) Frequency distributions of herring with different numbers of post-maturation years by decade. (d) Proportional change (with s.e.) in numbers of herring, present in the spawning area, from p to p+1 post-maturation years (estimated with model (2.1)), shown separately for year-classes with lower- or higher-than-average mean weight as first-time spawners (overall mean, 213 g).
Skipped reproduction occurred throughout the time-series: frequency distributions by decade (figure 1c) show a consistent (albeit noisier) pattern of under-representation of fish with 1 post-maturation year in relation to the expectation of exponentially decaying numbers if survival alone were to explain the distribution. Higher noise may be attributed to the highly unequal sampling effort between years, large differences in year-class strength and possible variations in the extent of skipped reproduction. Applying model (2.2) by decade suggested significant (p<0.0005) under-representation of second-time spawners for the decades 1935–1944, 1945–1954 and 1965–1973 (estimated fraction skipping, respectively, 53%, 32% and 52%), but not for 1955–1964 (p=0.191; estimated fraction skipping 29%).
Figure 1d splits the results shown in figure 1b between year-classes that, as first-time spawners, showed either lower- or higher-than-average mean weights. The discrepancy in the transitions from 0→1 and 1→2 post-maturation years, compared with later transitions, appeared more marked for the low-weight group. This suggests that reduced weight of first-time spawners may be related to increased frequency of skipped second spawning seasons, even though the difference in under-representation was not statistically significant (low-weight group: cI=−0.72, s.e.=0.07, estimated fraction skipping 51%; high-weight group: cI=−0.50, s.e.=0.10, estimated fraction skipping 39%; F=3.047, p=0.081).
4. Discussion
This study suggests that almost one in two adult Norwegian spring-spawning herring may skip the second reproductive season. Skipping of later spawning seasons may also occur, but in our data this would easily go undetected: if skipping becomes unsynchronized after the second potential spawning season, absence of fish owing to skipping cannot be separated from absence owing to mortality.
How could extensive skipping of spawning have gone unnoticed? Skipping becomes visible in our data if (and only if) analysed by maturation cohorts: because the maturation of a single year-class of herring is spread over several years, frequent skipping of the second spawning season will not result in an easily detected reduction in the number of spawners in any single year. Furthermore, data collection on Norwegian spring-spawning herring has traditionally been concentrated in or near spawning areas where fish skipping reproduction could only be observed indirectly, through their absence.
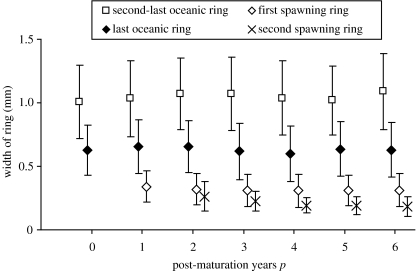
An alternative explanation is that the finding is simply artefactual. Although a team of experts made scale-readings, errors are inevitable. An apparent under-representation of second-time spawners would ensue if herring with 1 post-maturation year frequently have their first spawning ring misinterpreted as the last oceanic ring. This should be visible as first-time spawners having larger last oceanic rings than second-time spawners. This is not the case, nor do ring widths suggest any other obvious irregularities (figure 2). Thus, if misinterpretation had been frequent, it would have had to occur in a very specific manner leaving average ring widths independent of post-maturation years. We consider this unlikely; yet, this possibility cannot be excluded without independent validation of ring patterns (e.g. trace element analysis).
Figure 2.
Comparison of second-last and last oceanic, and first and second spawning rings (mean, s.d.) between herring with identical ages at maturation (5 years, the most common age at maturation), but different numbers of post-maturation years (i.e. caught at different ages). For each ring type, ring widths are consistent, suggesting consistency of the interpretation of ring patterns, and thereby, of the estimated numbers of post-maturation years.
Where are the fish that skip spawning? In recent years, surveys have been conducted in the coastal overwintering areas of the mature part of the stock. Analysis of these data shows an anomaly in post-maturation years similar to that in the spawning grounds (G. H. Engelhard and M. Heino, unpublished data). Do the fish that skip spawning join the schools of late immature fish, which overwinter oceanically in the Norwegian Sea (Dragesund et al. 1980)? The ultimate demonstration of skipped spawning in herring would require finding the non-reproducing, mature herring.
Why would herring not return to spawn in the year after first spawning? Obviously, reproduction implies costs, affecting future reproduction and survival. In Norwegian spring-spawning herring, predation risk is high in spawning areas because of a range of coastal predators targeting herring (Fernö et al. 1998). Moreover, since herring do not feed while migrating, only fish with sufficient energy stores are able to migrate and spawn successfully (Slotte & Fiksen 2000). Furthermore, the bioenergetics of swimming favour large fish (Ware 1978). Owing to these costs and trade-offs, participation in spawning migrations may only pay off in terms of fitness if individuals are sufficiently large and in sufficient condition to both migrate and spawn. We suggest that older, repeat-spawning herring, advantaged by large size, may normally be able to spawn annually. By contrast, first-time spawners which, owing to small size, rely more heavily on condition, may often need an extra year to regain the energy stores required for reproduction. This is in accordance with the tendency of stronger under-representation of second-time spawners for year-classes that had shown lower-than-average mean weights as first-time spawners (figure 1d), and agrees with models on cod (Gadus morhua) predicting skipped reproduction to be particularly frequent the year after first spawning (Jørgensen et al. 2004).
Skipped reproduction has been observed in a range of reptiles, birds and mammals (e.g. Reiter & Le Boeuf 1991; Danchin & Cam 2002; Broderick et al. 2003). While skipped reproduction has also been reported for fishes (Ivanov 1971; Dutil 1986; Oganesyan 1993; Burton et al. 1997; Rideout et al. 2000; Fredrich et al. 2003), it is usually treated more like an anomaly—a mere response to poor feeding conditions. The present study is, to our knowledge, the first one to empirically demonstrate that skipped reproduction may be an integral part of the life history of a commercially important species. Moreover, the possibility of extensive skipped reproduction having gone unnoticed in such a well-studied stock suggests that it may occur more frequently than commonly believed—an important consideration in fishery management, given that fishing typically shifts the adult composition of stocks towards younger ages where skipping reproduction may be most prevalent.
Acknowledgments
The European Research Training Network ModLife, funded through the Human Potential Programme of the European Commission (Contract HPRN-CT-2000-00051), and the Department for Environment, Food & Rural Affairs of the United Kingdom (Contract MFO322) supported this study. Michael Armstrong, Ewen Bell, Ulf Dieckmann, Olav Rune Godø, Jens Christian Holst, Christian Jørgensen, Lucia Privitera and Reidar Toresen provided valuable discussions.
References
- Broderick A.C, Glen F, Godley B.J, Hays G.C. Variation in reproductive output of marine turtles. J. Exp. Mar. Biol. Ecol. 2003;288:95–109. [Google Scholar]
- Burton M.P.M, Penney R.M, Biddiscombe S. Time course of gametogenesis in Northwest Atlantic cod (Gadus morhua) Can. J. Fish. Aquat. Sci. 1997;54(Suppl. 1):122–131. [Google Scholar]
- Danchin E, Cam E. Can non-breeding be a cost of breeding dispersal? Behav. Ecol. Sociobiol. 2002;51:153–163. [Google Scholar]
- Dragesund O, Hamre J, Ulltang Ø. Biology and population dynamics of the Norwegian spring-spawning herring. Rapp. P.-V. Réun. Cons. Int. Explor. Mer. 1980;177:43–71. [Google Scholar]
- Dutil J.-D. Energetic constraints and spawning interval in the anadromous Arctic charr (Salvelinus alpinus) Copeia. 1986;1986:945–955. [Google Scholar]
- Engelhard G.H, Dieckmann U, Godø O.R. Age at maturation predicted from routine scale measurements in Norwegian spring-spawning herring (Clupea harengus) using discriminant and neural network analyses. ICES J. Mar. Sci. 2003;60:304–313. [Google Scholar]
- Fernö A, Pitcher T.J, Melle W, Nøttestad L, Mackinson S, Hollingworth C, Misund O.A. The challenge of the herring in the Norwegian Sea: making optimal spatial decisions. Sarsia. 1998;83:149–167. [Google Scholar]
- Fredrich F, Ohmann S, Curio B, Kirschbaum F. Spawning migrations of the chub in the River Spree, Germany. J. Fish Biol. 2003;63:710–723. [Google Scholar]
- Ivanov S.N. An analysis of the fecundity and intermittent spawning of the Lake Balkhash wild carp [Cyprinus carpio (L.)] J. Ichthyol. 1971;11:666–672. [Google Scholar]
- Jørgensen, C., Ernande, B., Fiksen, Ø. & Dieckmann, U. 2004 Skipped spawning is common for the Northeast Arctic cod in a life-history energy allocation model. ICES CM 2004/K:28.
- Oganesyan, S. A. 1993 Periodicity of the Barents Sea cod reproduction. ICES CM 1993/G:64.
- Reiter J, Le Boeuf B.J. Life history consequences of variation in age at primiparity in northern elephant seals. Behav. Ecol. Sociobiol. 1991;28:153–160. [Google Scholar]
- Rideout R.M, Burton M.P.M, Rose G.A. Observations on mass atresia and skipped spawning in northern Atlantic cod, from Smith Sound, Newfoundland. J. Fish Biol. 2000;57:1429–1440. [Google Scholar]
- Roff D.A. Sinauer Associates; Sunderland, MA: 2002. Life history evolution. [Google Scholar]
- Runnström S. A study on the life history and migrations of the Norwegian spring-herring based on the analysis of the winter rings and summer zones of the scale. FiskDir. Skr. Ser. HavUnders. 1936;5:1–103. [Google Scholar]
- Slotte A, Fiksen Ø. State-dependent spawning migration in Norwegian spring-spawning herring. J. Fish Biol. 2000;56:138–162. [Google Scholar]
- Ware D.M. Bioenergetics of pelagic fish: theoretical change in swimming speed and ration with body size. J. Fish. Res. Board Can. 1978;35:220–228. [Google Scholar]