Abstract
The exact nature of many interspecific interactions remains unclear, with some evidence suggesting mutualism and other evidence pointing to parasitism for the same pair of interacting species. Here, we show spatial variation in the outcome of the cleaning relationship between Caribbean cleaning gobies (Elacatinus evelynae) and longfin damselfish (Stegastes diencaeus) over the distribution range of these species, and link this variation to the availability of ectoparasites. Cleaning interactions at sites with more ectoparasites were characterized by greater reductions in ectoparasite loads on damselfish clients and lower rates of removal of scales and mucus (i.e. cheating) by cleaning gobies, whereas the opposite was observed at sites where ectoparasite abundance was lower. For damselfish clients, cleaning was therefore clearly mutualistic in some locations, but sometimes neutral or even parasitic in others. Seasonal variability in ectoparasite abundance may ensure that locally low parasite availability, which promotes cleanerfish cheating, may be a transient condition at any given site. Conflicting conclusions about the nature of cleaning symbioses may, therefore, be explained by variation in ectoparasite abundance.
Keywords: Caribbean, ectoparasites, Elacatinus species, geographical variation, interspecific interactions
1. Introduction
Mutualisms are characterized by net benefits to both interacting participants. However, the costs and benefits of mutualistic interactions can change with varying environmental conditions, including variation in physical environment (e.g. Burdon et al. 1989) and in the presence of other species (i.e. competitors, co-mutualists, predators; e.g. Benkman et al. 2001). Mutualistic interactions may, therefore, occasionally become neutral or antagonistic, either temporarily or over parts of the range of the interacting species (Thompson & Cunningham 2002).
Fish cleaning behaviour offers an ideal model system to evaluate the nature of apparently mutualistic interactions and how environmental pressures may alter their outcome. A long-standing debate surrounds the exact nature of cleaning interactions, both in marine and terrestrial systems. The extent to which clients benefit from being cleaned is at the centre of this controversy. In the marine environment, reductions in ectoparasite load on fish clients by cleaner fish have been demonstrated in some (Grutter 1999; Cheney & Côté 2001), but not all studies (Gorlick et al. 1987; Grutter 1996). In addition, cleaner fish can cheat by removing client scales, mucus and tissue from their clients (Grutter 1997; Whiteman & Côté 2002), the amount of which may vary between sites (Grutter 1997; Bansemer et al. 2002). This removal of non-ectoparasitic material may have a negative impact on a client, thus pushing individual interactions closer to commensalism or even parasitism (Johnstone & Bshary 2002). As the magnitude of the benefit of being cleaned for clients is probably related to client ectoparasite loads (Bansemer et al. 2002), and ectoparasite intensity varies geographically (Grutter 1994; Cheney & Côté, 2003a), variation in the outcome of cleaning interactions should exist and be linked to ectoparasite availability.
In this study, we examine variability in the outcome of the symbiosis between Caribbean sharknose cleaning gobies (Elacatinus evelynae) and longfin damselfish (Stegastes diencaeus) across the distributional range of the species. We provide direct measurements of the costs, in terms of non-parasitic material removed by cleaners, and benefits, in terms of ectoparasite reduction, to longfin damselfish clients and then relate variation in these costs and benefits to concurrently measured ectoparasite availability.
2. Material and methods
(a) Study site and species
Our study was conducted on six Caribbean islands: Barbados, Curaçao, Tobago, Puerto Rico, St John (US Virgin Islands) and Jamaica, which vary considerably in ectoparasite availability (Cheney & Côté 2003a). Data were collected in July 2001 for Jamaica, and between February and July 2002 for the remaining islands. Both of our study species are found throughout the Caribbean. Longfin damselfish are medium-sized (total length approximately 80–100 mm) damselfish found on shallow-water fringing reefs (2–15 m) and defend mutually exclusive feeding territories. Sharknose cleaning gobies are predominantly found in pairs or groups on coral or sponge. They glean mainly ectoparasitic gnathiid isopod larvae from the body surface of a variety of reef fish clients. Cleaning gobies are the main cleaners of longfin damselfish, which are cleaned only rarely by other cleaner fish or shrimp (Cheney & Côté 2001). We have previously shown, through experimental removals, that cleaning gobies can significantly alter the ectoparasite loads of their damselfish clients (Cheney & Côté 2003b).
(b) Behavioural observations
Between 14 and 25 longfin damselfish were haphazardly selected and observed at each site. Approximately half of all damselfish at each site had a cleaning station within their territory, while the remainder did not. Four 15 min observation periods (between 8.00 and 17.00) were conducted on each damselfish, during which we recorded the amount of time each damselfish spent being inspected by cleaning gobies.
(c) Damselfish ectoparasite loads
The ectoparasite load of each observed damselfish was determined as in Cheney & Côté (2001). Individual damselfish were caught using barrier and hand nets, placed into a hermetically sealed plastic bag and euthanized with clove oil. Each fish was then placed in a 0.4% chloretone bath (BDH Chemicals, UK) for 1 h to remove all ectoparasites. The entire fish and its bag were rinsed thoroughly and all liquids were filtered. Ectoparasites were transferred from the filter paper into a Petri dish for identification using a binocular microscope. Twelve of the damselfish were scanned under a binocular microscope to ensure that all ectoparasites had been removed.
(d) Cleaning goby diet analysis
Between 12 and 17 adult cleaning gobies were collected from each site, using an overdose of clove oil. Fish were preserved whole in 75% alcohol immediately after collection. The entire gut was dissected under a binocular microscope and the percentage cover of each food item category (crustacean parasites, scales, mucus, crustacean non-parasites and unidentifiable digested items) was estimated. ‘Non-parasitic client-gleaned material’ includes scales and mucus, and provides evidence for dishonest cleaning (Bshary 2002; Bshary & Grutter 2002).
3. Results
(a) Variation in the benefit of being cleaned
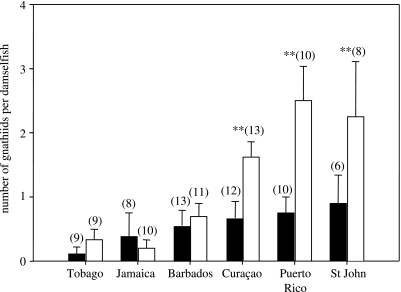
Individuals with cleaning stations in their territory spent significantly more time with cleaners than those without cleaners (all islands: Mann–Whitney U tests, U>6, p<0.006). Time spent with cleaners varied from (mean+s.d., s per 15 min) 8.1+2.4 and 0.6+0.4 for damselfish with and without cleaners in their territory respectively, in Tobago, to 16.4+5.7 and 3.8+1.3 for damselfish with and without cleaners in Puerto Rico. On average, damselfish without cleaning stations in their territories spent only 7–23% as much time with cleaners as their counterparts with a cleaning station. For this reason, the ectoparasite loads of damselfish without cleaners in their territories were used as site-specific ‘baselines’, representing parasite loads relatively unaffected by cleaners, from which reductions in parasite loads on damselfish that were regularly cleaned were measured.
Overall, ectoparasite loads varied significantly among islands (Kruskal–Wallis H5=26.4, p<0.001; figure 1). Differences in ectoparasites loads between fishes with and without cleaning stations were only significant at Curaçao, Puerto Rico and St John (Mann–Whitney U-tests: p<0.02; figure 1). Across islands, the benefit of being cleaned, calculated as the mean difference in ectoparasite numbers between damselfish with and without cleaning stations on their territories, was related to damselfish mean ectoparasite load (Pearson's correlation, r=0.84, n=6, p=0.03).
Figure 1.
Ectoparasite load (number of gnathiid larvae per fish) on longfin damselfish from different Caribbean islands. Black bars: damselfish with a cleaning station within their territory; white bars: damselfish without a cleaning station in their territory. Means are shown +1 s.e. Sample sizes are given in parentheses.
(b) Variation in the cost of being cleaned
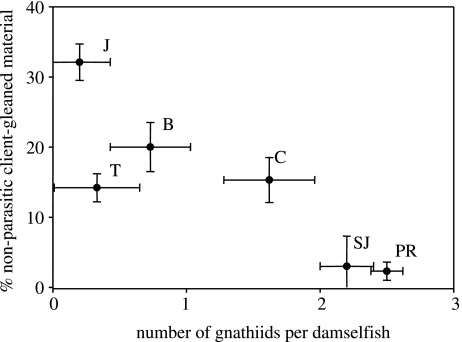
The mean percentage cover of different food items varied among islands (table 1). Two-thirds of cleaning gobies had ingested non-parasitic material (i.e. scales and mucus). There was significant variation among islands in the proportion of non-parasitic material ingested per cleaning goby (Kruskal–Wallis χ2(5)=15.3, p=0.01). The percentage cover of non-parasitic client-gleaned material ingested was negatively related to the mean ectoparasite load on longfin damselfish on each island (Pearson's correlation, r=−0.86, n=6, p=0.03; figure 2).
Table 1.
Mean (±s.e.) percentage cover of food items ingested by cleaning gobies Elacatinus evelynae at six Caribbean islands.
| island | n | parasites (gnathiids and caligids) | scales | mucus | other prey items (copepods and ostracods) | unidentifiable material |
|---|---|---|---|---|---|---|
| Barbados | 15 | 24.3±4.5 | 15.2±2.3 | 5.0±2.3 | 4.3±1.2 | 51.2±5.6 |
| Curacao | 17 | 14.3±3.9 | 12.5±3.7 | 2.8±1.2 | 5.6±2.3 | 64.8±3.2 |
| Jamaica | 13 | 5.2±3.4 | 28.2±1.2 | 3.9±1.8 | 14.3±3.9 | 48.4±2.1 |
| Puerto Rico | 13 | 62.3±5.4 | 2.0±1.3 | 0.3±0.1 | 0 | 35.4±5.2 |
| Tobago | 12 | 12.3±1.2 | 10.2±1.9 | 4.0±3.7 | 7.3±1.8 | 66.2±3.4 |
| St John | 13 | 34.7±6.3 | 2.5±2.1 | 0.5±0.3 | 0.4±1.2 | 61.9±6.5 |
Figure 2.
Percentage of non-parasitic client-gleaned material ingested per cleaning goby in relation to average ectoparasite load recorded on longfin damselfish from six Caribbean islands. Means are shown ±1 s.e. J=Jamaica, T=Tobago, B=Barbados, C=Curaçao, SJ=St John, PR=Puerto Rico.
4. Discussion
The absence of quantifiable benefits of being cleaned in some areas (Gorlick et al. 1987; Grutter 1996) and observations of cheating by cleaners (Bansemer et al. 2002; Bshary & Schaffer 2002) have challenged the long-held view of cleaning symbioses as paradigms of mutualism, suggesting instead that individual cleaning interactions may sometimes be more akin to parasitism on the part of cleaners (Losey 1987). We found that cleaning interactions at locations where clients have more ectoparasites result in greater reductions in ectoparasite loads and lower rates of removal of non-parasitic client-gleaned material by cleaner fish. The reverse was observed where ectoparasite abundance was low. Thus, from an individual client's perspective, cleaning interactions were often mutualistic at some sites, but neutral or even parasitic at others, with the outcome seemingly driven by ectoparasite availability. Bansemer et al. (2002) proposed a similar mechanism, based on behavioural correlates of cleaning intensity and cheating by cleaners rather than direct measurements of ectoparasite availability and removal rates, at two Great Barrier Reef sites.
The variation observed here in ectoparasite availability, and hence in the outcome of cleaning symbioses, could reflect permanent differences among locations. If so, cheating could erode cleaner–client relationships over time in parts of their range, unless gene flow among client populations occurs. Alternatively, our results could represent snapshots of spatially and temporally shifting outcomes, which are environmentally mediated. This interpretation seems very likely because ectoparasite load on reef fishes can vary within a site, sometimes by orders of magnitude, both seasonally and between years (Grutter 1994; Cheney & Côté 2003a). At Barbados, ectoparasite abundance varied significantly among three study years, with concomitant variation in the frequency of scale-eating by cleaners and benefit of being cleaned to longfin damselfish (Cheney 2003). Locally low parasite availability, which promotes cleaner fish cheating, may therefore be a transient condition at any given site.
The key to understanding the variable nature of cleaning symbioses thus lies in the mechanisms driving temporal and geographical variation in ectoparasite abundance. These mechanisms are not currently well understood but may include a variety of physical and ecological factors (Jacoby & Greenwood 1988; Grutter 1994; Sikkel et al. 2004-) which can affect rates of emergence of and predation on ectoparasites.
Our results suggest that the outcome of the same cleaner–client symbiosis can be variable over space and time and depends mainly on ectoparasite availability. Interspecific interactions, such as cleaning symbioses, and probably many other apparently mutualistic relationships, may, therefore, not be definable with a single label.
Acknowledgements
For logistical support we thank the staff at Bellairs Research Institute, Barbados; Discovery Bay Marine Laboratory, Jamaica; Isla Magueyes Marine Laboratory, Puerto Rico; CARMABI Institute, Curaçao; and Virgin Islands Environmental Resource Station, University of the Virgin Islands. We thank P. Sikkel, J. Mallela, M. Harvey, P. Frade and R. Jacinto for help in the field. K.L.C. was supported by a Biotechnology and Biological Sciences Research Council PhD studentship of the UK. We thank the University of East Anglia and the John and Pamela Salter Charitable Trust for financial support.
References
- Bansemer C, Grutter A.S, Poulin R. Geographic variation in the behaviour of the cleaner fish Labroides dimidiatus (Labridae) Ethology. 2002;108:353–366. [Google Scholar]
- Benkman C.W, Holimon W.C, Smith J.W. The influence of a competitor on the geographic mosaic of coevolution between crossbills and lodgepole pine. Evolution. 2001;55:282–294. doi: 10.1111/j.0014-3820.2001.tb01293.x. [DOI] [PubMed] [Google Scholar]
- Bshary R. Biting cleaner fish use altruism to deceive image-scoring client reef fish. Proc. R. Soc. B. 2002;269:2087–2093. doi: 10.1098/rspb.2002.2084. doi:10.1098/rspb.2002.2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bshary R, Grutter A.S. Asymmetric cheating opportunities and partner control in a cleaner fish mutualism. Anim. Behav. 2002;63:547–555. [Google Scholar]
- Bshray R, Schaffer D. Choosy reef fish select cleaner fish that provide high-quality service. Anim. Behav. 2002;63:557–564. [Google Scholar]
- Burdon J.J, Jarosz A.M, Kirby G.C. Pattern and patchiness in plant–pathogen interactions—causes and consequences. Annu. Rev. Ecol. Syst. 1989;20:119–136. [Google Scholar]
- Cheney, K. L. 2003 Interactions between Caribbean cleaning gobies (Elacatinus sp.) and territorial damselfish: costs, benefits and effects of scale. Ph.D. thesis, University of East Anglia.
- Cheney K.L, Côté I.M. Are Caribbean cleaning symbioses mutualistic? Costs and benefits of visiting cleaning stations to longfin damselfish. Anim. Behav. 2001;62:927–933. [Google Scholar]
- Cheney K.L, Côté I.M. Do ectoparasites determine cleaner fish abundance? Evidence on two spatial scales. Mar. Ecol. Prog. Ser. 2003a;263:189–196. [Google Scholar]
- Cheney K.L, Côté I.M. The ultimate effect of being cleaned: does ectoparasite removal increase reproductive success in a damselfish client? Behav. Ecol. 2003b;14:892–896. [Google Scholar]
- Gorlick D.L, Atkins P.D, Losey G.S. Effect of cleaning by Labroides dimidiatus (Labridae) on an ectoparasite population infecting Pomacentrus vaiuli (Pomacentriade) at Enewetak Atoll. Copeia. 1987:41–45. [Google Scholar]
- Grutter A.S. Spatial and temporal variations of the ectoparasites of 7 reef fish species from Lizard Island and Heron Island, Australia. Mar. Ecol. Prog. Ser. 1994;115:21–30. [Google Scholar]
- Grutter A.S. Experimental demonstration of no effect by the cleaner wrasse Labroides dimidiatus (Cuvier and Valenciennes) on the host fish Pomacentrus moluccensis (Bleeker) J. Exp. Mar. Biol. Ecol. 1996;196:285–298. [Google Scholar]
- Grutter A.S. Spatiotemporal variation and feeding selectivity in the diet of the cleaner fish Labroides dimidiatus. Copeia. 1997;1997:346–355. [Google Scholar]
- Grutter A.S. Cleaner fish really do clean. Nature. 1999;398:672–673. [Google Scholar]
- Jacoby C.A, Greenwood J.G. Spatial, temporal, and behavioural patterns in emergence of zooplankton in the lagoon of Heron Reef, Great Barrier Reef, Australia. Mar. Biol. 1988;97:309–328. [Google Scholar]
- Johnstone R.A, Bshary R. From parasitism to mutualism: partner control in asymmetric interactions. Ecol. Lett. 2002;5:634–639. [Google Scholar]
- Losey G.S. Cleaning symbiosis. Symbiosis. 1987;4:229–256. [Google Scholar]
- Sikkel P.C, Cheney K.L, Côté I.M. In situ evidence for ectoparasites as a proximate cause of cleaning interactions in reef fish. Anim. Behav. 2004;68:241–247. [Google Scholar]
- Thompson J.N, Cunningham B.M. Geographic structure and dynamics of coevolutionary selection. Nature. 2002;417:735–738. doi: 10.1038/nature00810. [DOI] [PubMed] [Google Scholar]
- Whiteman E.A, Côté I.M. Sex differences in cleaning behaviour and diet of a Caribbean cleaning goby. J. Mar. Biol. Assoc. UK. 2002;82:655–664. [Google Scholar]