Abstract
There are various ways to estimate ejaculate expenditure. Ejaculate size or sperm number (s) is an absolute number of units of ejaculate. Relative ejaculate expenditure (E) is the expenditure on the ejaculate as the proportion of the total expenditure on all aspects of the mating, including finding and acquiring a female, and so on. Relative testis size or gonadosomatic index (σ) is testes mass divided by body mass; it is assumed to reflect the product of mating rate (M) and ejaculate mass (s). In a new model, where mating rate, sperm competition and sperm allocation interact, and where the female's inter-clutch interval is assumed to be independent of s or M, we show that σ is directly proportional to the mean E for a species; across species σ and E increase monotonically with sperm competition. However, the relation between s and sperm competition across species depends on the range of sperm competition (low risk or high intensity): s increases with sperm competition at low risk levels, but decreases with sperm competition at high intensity levels. This situation arises because s∝E/M; both E and M increase with sperm competition, but E increases differently with sperm competition in its two ranges.
Keywords: sperm competition, relative testis size, ejaculate size, evolutionarily stable strategy
1. Introduction
Models of sperm expenditure (reviewed in Parker 1998) predict that ejaculate expenditure will increase with sperm competition level, viewed across species. In contrast, models examining sperm expenditure within a species sometimes predict reduced sperm expenditure as sperm competition increases (Parker et al. 1996). Here, we show that the hitherto robust prediction that sperm expenditure always increases across species as sperm competition increases depends on the measure of sperm expenditure. This prediction is correct for sperm expenditure as a proportion of the total expenditure for a given mating, but is correct only at low sperm competition risks if sperm expenditure is measured as sperm number per ejaculate. This analysis extends previous models by including the dependency between sperm competition and mating rate.
Relative testis size (here σ; often termed ‘gonado-somatic index’, e.g. Taborsky 1998) has often been correlated with sperm competition level across species (reviewed in Parker et al. 1997) and is now assumed to be a reliable index of sperm competition (e.g. Gage & Freckleton 2004, but see Tomkins & Simmons (2002)). However, σ is also likely to reflect the rate of sperm demand (product of ejaculate size and mating rate) on the male (Short 1977, 1979). Increased testicular size increases sperm production rate (Gomendio et al. 1998), and can increase fertilization success under competition (Preston et al. 2003). Therefore, both sperm competition level and mating rate affect σ. Our analysis concurs with empirical results, and suggests that σ increases with measures of sperm competition level across species, because of a positive relation between sperm competition and mating rate.
2. Analysis
(a) Relation between expenditure and mating rate
Sperm competition game models (e.g. Ball & Parker 2000) assume that each male has a (large) fixed budget, R units of energy, available for reproduction, a cost, C, is paid to obtain a mating, and the cost of each unit of ejaculate is D. Let T equal the average reproductive time-interval between clutches for the female. This is the (active) time taken by a female to produce a new batch of eggs during the reproductive season. During T, the male spends time searching, copulating and replenishing his mating energy. We write n for the number of matings a particular male achieves during T. Then a male strategy is (n,〈s〉) where 〈s〉 is the average units of ejaculate (=‘average sperm number’ for present purposes); (n*,〈s*〉) is the evolutionarily stable strategy (ESS; Maynard Smith 1982) value.
We have expressed sperm expenditure in two ways: (i) as the absolute value 〈s*〉 or D〈s*〉, or as the ratio of s in one condition relative to s in another condition; and (ii) as ‘relative sperm expenditure’, i.e. the ejaculate cost as a proportion of the entire expenditure on a given mating
| (2.1) |
(Parker 1990).
A male's mating rate is
| (2.2) |
Each male is subject to a constraint between his energy budget, R (assumed to be independent of s and n), available and his number of matings
| (2.3) |
(Parker et al. 1996). The simplest biological interpretation is that a male switches to food foraging (which is exclusive of mate-searching) at a critical energy deficit, then R energy units are accumulated, then reproductive activity resumes, and so on. The average cycle time T for the female is also assumed to be independent of s and n. So R≡R(T), i.e. R is a function of T. R is zero when T is zero, so, in a linear expansion
| (2.4) |
where k depends on the species. Taking the linear term is only a good approximation according to the above argument.
The assumptions leading to equation (2.4) are critical for the following analysis, which will require modification if T is strongly dependent on male strategy (s, n).
(b) Sperm allocation, mating rate and testes size
Combining equations (2.1)–(2.3), the ESS sperm allocation is
| (2.5) |
and from equation (2.4)
| (2.6) |
that is, average ejaculate mass is proportional to relative sperm expenditure divided by mating rate.
Relative testis size, σ, should reflect the rate of sperm demand (Short 1977, 1979). Assuming that σ increases linearly with the product of ejaculate mass and mating rate,
| (2.7) |
i.e. σ increases in direct proportion to E (see speculation in Parker 1998). However, unlike E, testis size cannot change at each mating in relation to current cues. Our previous calculations for E are therefore analogous to σ only where they relate to average sperm allocations for a species.
Note that the results of equations (2.6) and (2.7) follow directly from the definitions of sperm expenditure, equation (2.1); cost of a mating, equation (2.2); mating rate, equation (2.3); and the expansion, equation (2.4). They are therefore unaffected by such considerations as sperm limitation (Mesterton-Gibbons 1999) unless there are biological reasons for suspecting a strong dependency of T on s or n. For example, if females are sperm limited after just one mating, they may mate several times causing a significant extension of T. In general, we suspect that any such dependencies will usually be weak or insignificant. (This also means that k is independent of n or s.)
(c) Relation between adult males and adult females
For the population, the total number of matings of each sex must be equal (Ball & Parker 1998). Thus, if is the average number of matings a female has during the time T, then
| (2.8) |
where β is the adult sex ratio (adult males/adult females). We can thus write the constraint equation as:
| (2.9) |
Consider the composition of T. A male must remain in energy balance during T. Let equal the expected time taken for a male to gain each mating (a proportion of is spent mate-searching, and the remainder replenishing energy lost in searching), and let equal the time for a male to mate and replenish each of the s* sperm units expended (thus is the average time cost of the ejaculate). If food foraging (to gain energy) is mutually exclusive of mating or searching for females,
| (2.10) |
From equations (2.9) and (2.10)
giving an explicit value for k. The relative time expenditure equation, parallel to equation (2.1), is
(d) Modelling the relation between E*, s*, σ and sperm competition level
We used two approaches to model the range of sperm competition. The ‘risk’ model simulates low-level sperm competition in species where females may mate either once (with probability (1−q)), or at most twice (with probability q) in each reproductive cycle; thus , the average number of matings per female per cycle, is (1+q). The ‘intensity’ model simulates intense sperm competition where a mean of N ejaculates compete for each batch of eggs.
We assume that full fertility can be achieved with an arbitrary tiny amount of sperm, ∂, where ∂→0. Cases where significant infertility arises because ESS sperm levels limit fertilization (sperm limitation) require separate analysis (Mesterton-Gibbons 1999; Ball & Parker 2000).
For the risk model, we assume that under sperm competition, one of the two males is disfavoured. Let r (0<r<1) be the loading factor against the disfavoured male in the fertilization ‘raffle’. If, say, the second male to mate (male 2) is disfavoured, each sperm from male 2 counts only r against each sperm of male 1, and the probability of a given egg being fertilized by male 2 is rs2/(s1+rs2). Average ESS relative ejaculate expenditure is then
| (2.11a) |
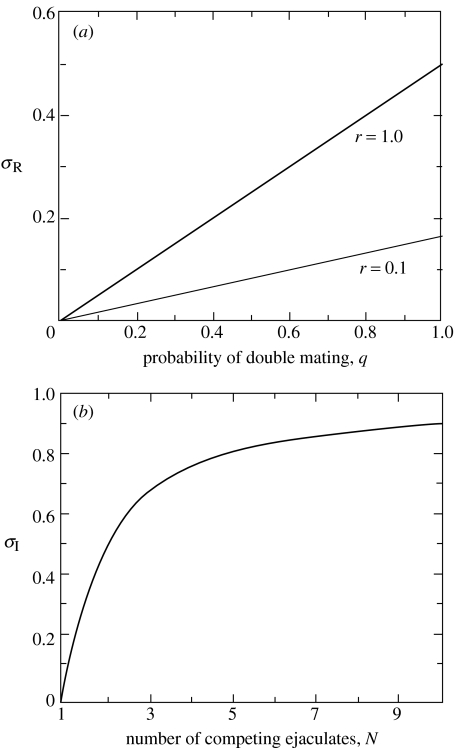
(Parker et al. 1997). Thus , and relative testis size, σR, increase linearly with sperm competition risk, q, at a rate of (or proportional to) 2r/(1+r)2 (figure 1a). If the raffle is ‘fair’ (r=1), .
Figure 1.
(a) Relative testis size (k=1.0) and ejaculate expenditure in the risk model, at r=1.0, r=0.1. (b) Relative testis size (k=1.0) and ejaculate expenditure in the intensity model.
Assuming that all sperm have equal chances, relative sperm expenditure in the intensity model is
| (2.11b) |
(Parker 1982), which increases monotonically with decreasing gradient (=1/N2) with intensity, N, to its asymptote of 1.0 at very high N. Relative testis size, σI, is proportional to (figure 1b).
Thus relative testis size and relative sperm expenditure increase monotonically with sperm competition across species (Parker 1998), whatever the range of sperm competition (risk or intensity).
Ejaculate costs for the risk model are
| (2.12a) |
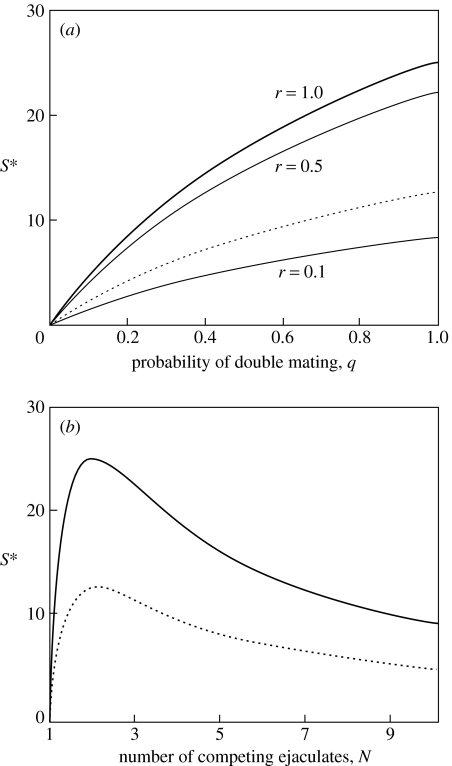
Thus across species with similar parameters, sperm numbers increase monotonically with decreasing gradient with risk of sperm competition, q (figure 2a).
Figure 2.
(a) Sperm numbers (ejaculate units) in the risk model, at r=1.0, r=0.5, r=0.1. (b) Sperm numbers (ejaculate units) in the intensity model. T=100 (continuous curve); T=50, r=1.0 (dotted curve); k=β=D=1.0.
However, for the intensity model
| (2.12b) |
Although equation (2.12b) obeys the same EM−1 rule as the risk model, it is not monotonic in N, having gradient=kβT(2−N)N3, which is positive for N<2, and negative for N>2. Thus across species with similar kβT/D, sperm numbers increase between N=1 and 2 (the range of the risk model) then decline for all intensities greater than N=2 (figure 2b shows how a range of such species should vary as sperm competition intensity increases).
3. Discussion
Using equation (2.4), we have shown that average relative sperm expenditure, E, and relative testis size, σ, are equivalent, and increase with sperm competition across both risk and intensity ranges (figure 1). However, average sperm numbers, 〈s*〉, increase across species with sperm competition risk, but decrease with sperm competition intensity (figure 2). Thus sperm allocation does not always increase across species with sperm competition, as was previously thought.
This effect arises because 〈s*〉∝E/N (equation (2.7)); both E and M increase with sperm competition, but E increases differently with sperm competition in its two ranges. In the risk range, E is linear; in the intensity range, E is monotonic decreasing, allowing 〈s*〉 to decrease with N.
Do these results invalidate the support given to sperm allocation theory from empirical tests? We suspect that generally they do not. Attempts to measure relative sperm expenditure, E, are understandably rare. However, relative testis size, σ, is much measured and increases with sperm competition (whatever its range), in concurrence with our results. Measurements of sperm numbers have often been in the risk range, where a positive relation with sperm competition risk, both across and within species, is predicted (and found; reviewed in Parker et al. 1997). In the intensity range, Pilastro et al. (2002) measured changes in sperm numbers, s, with the number of males, Ni, present at the time of spawning in two goby species, to test the prediction E should decline with Ni within each species as
| (3.1) |
where N is the mean number of competing ejaculates for the species (Parker et al. 1996).
Recalculating,
| (3.2) |
so that the same qualitative relation obtains between D〈s*〉 and Ni as between E and Ni. The Pilastro et al. (2002) results therefore remain good support for sperm allocation theory.
No evidence yet appears to be available for a given group to support the simultaneous predictions that average sperm expenditure, D〈s*〉, decreases with sperm competition intensity across species, while relative testis size, σ, increases. Across tettigoniid species (some of which mate many times per lifetime), ejaculate weight is known to decrease with female re-mating rate (Wedell 1993) and hence with mean N, though whether σ increases is not yet known.
Acknowledgments
We thank Dr K. Vahed and Dr L. W. Simmons for discussions.
References
- Ball M.A, Parker G.A. Sperm competition games: energy dependence and competitor numbers in the continuous external fertilization model. IMA J. Math. Appl. Med. Biol. 1998;15:87–96. [Google Scholar]
- Ball M.A, Parker G.A. Sperm competition games: a comparison of loaded raffle models and their biological implications. J. Theor. Biol. 2000;206:487–506. doi: 10.1006/jtbi.2000.2142. [DOI] [PubMed] [Google Scholar]
- Gage M.J.G, Freckleton R.P. Relative testis size and sperm morphometry across mammals: no evidence for an association between sperm competition and sperm length. Proc. R. Soc. B. 2004;270:625–632. doi: 10.1098/rspb.2002.2258. (doi:10.1098/rspb.2002.2258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomendio M, Harcourt A.H, Roldan E.R.S. Sperm competition in mammals. In: Birkhead T.R, Møller A.P, editors. Sperm competition and sexual selection. Academic Press; London: 1998. pp. 667–756. [Google Scholar]
- Maynard Smith J. Cambridge University Press; Cambridge: 1982. Evolution and the theory of games. [Google Scholar]
- Mesterton-Gibbons M. On sperm competition games: incomplete fertilization risk and the equity paradox. Proc. R. Soc. B. 1999;266:269–274. (doi:10.1098/rspb.1999.0632) [Google Scholar]
- Parker G.A. Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes. J. Theor. Biol. 1982;96:281–294. doi: 10.1016/0022-5193(82)90225-9. [DOI] [PubMed] [Google Scholar]
- Parker G.A. Sperm competition games: raffles and roles. Proc. R. Soc. B. 1990;242:120–126. [Google Scholar]
- Parker G.A. Sperm competition and the evolution of ejaculates: towards a theory base. In: Birkhead T.R, Møller A.P, editors. Sperm competition and sexual selection. Academic Press; London: 1998. pp. 3–54. [Google Scholar]
- Parker G.A, Ball M.A, Stockley P, Gage M.J.G. Sperm competition games: individual assessment of sperm competition intensity by group spawners. Proc. R. Soc. B. 1996;263:1291–1297. [Google Scholar]
- Parker G.A, Ball M.A, Stockley P, Gage M.J.G. Sperm competition games: a prospective analysis of risk assessment. Proc. R. Soc. B. 1997;264:1793–1802. doi: 10.1098/rspb.1997.0249. (doi:10.1098/rspb.1997.0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilastro A, Scaggiante M, Rasotto M.B. Individual adjustment of sperm expenditure accords with sperm competition theory. Proc. Natl Acad. Sci. USA. 2002;99:9913–9915. doi: 10.1073/pnas.152133499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston B.T, Stevenson I.R, Pemberton J.M, Coltman D.W, Wilson K. Overt and covert competition in a promiscuous mammal: the importance of weaponry and testes size to male reproductive success. Proc. R. Soc. B. 2003;270:633–640. doi: 10.1098/rspb.2002.2268. (doi:10.1098/rspb.2002.2268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short R.V. Proceedings of the Canberra Symposium on Reproduction and Evolution. Australian Academy of Sciences; Canberra: 1977. Sexual selection and the descent of man; pp. 3–19. [Google Scholar]
- Short R.V. Sexual selection and its component parts, somatic and genital selection, as illustrated by man and the great apes. Adv. Stud. Behav. 1979;9:131–158. [Google Scholar]
- Taborsky M. Sperm competition in fish ‘bourgeois’ males and parasitic spawning. Trends Evol. Ecol. 1998;16:222–227. doi: 10.1016/s0169-5347(97)01318-9. [DOI] [PubMed] [Google Scholar]
- Tomkins J.L, Simmons L.W. Measuring relative investment: a case study of testes investment in species with alternative male reproductive tactics. Anim. Behav. 2002;63:1009–1013. [Google Scholar]
- Wedell N. Spermatophore size in bushcrickets: comparative evidence for nuptial gifts as a sperm protection device. Evolution. 1993;47:1203–1212. doi: 10.1111/j.1558-5646.1993.tb02147.x. [DOI] [PubMed] [Google Scholar]