Abstract
This study investigated whether monkeys recognize when a human experimenter imitates their actions towards an object. Two experimenters faced 10 pigtailed macaques, who were given access to an interesting object. One experimenter imitated the monkeys' object-directed actions, the other performed temporally contingent but structurally different object-directed actions. Results show a significant visual preference for the imitator during manual object manipulations, but not mouthing actions. We argue that the ability to match actions could be based on both visual–visual and kinaesthetic–visual matching skills, and that mirror neurons, which have both visual and motor properties, could serve as a neural basis for recognizing imitation. However, imitation recognition as assessed by visual preference does not necessarily imply the capacity to attribute imitative intentionality to the imitator. The monkeys might implicitly recognize when they are being imitated without deeper insight into the mental processes of others.
Keywords: imitation recognition, matching system, mirror neurons, pigtailed macaques, preferential looking
1. Introduction
Motor imitation occurs when an individual replicates an action that it has observed being performed by another individual. Imitation seems to require a matching system that allows convertion of observed actions by others into actions executed by oneself. In other words, visual input needs to be transformed into corresponding motor output.
Imitation seems to be a rare phenomenon, and so far, only humans and great apes have provided convincing evidence for it (Whiten et al. 1996). Monkeys are often regarded as incapable of imitating in the sense that they do not repeat a model's action that is novel to their own behavioural repertoire (Visalberghi & Fragaszy 2002; but compare with Voelkl & Huber 2000). Thus, monkeys might be lacking a ‘like-me mechanism’ (Meltzoff & Gopnik 1993) that is involved in both creating a perceptual match between self and others' actions and in reproducing observed actions.
The capacity to recognize when one is being imitated may require similar mechanisms (Nadel 2002). Here, actions performed by oneself need to be matched with actions performed by others, which means that one's own visual–motor output needs to be matched with visual input originating from the imitator. Studies in humans show that 14-months-old infants can recognize when they are being imitated (Meltzoff 1990, 1996), and a recent study with one chimpanzee is suggestive of imitation recognition in great apes (Nielsen et al. 2004).
Recognizing imitation appears to be a cognitively simpler task than producing imitation. In order to achieve imitation, an individual must process the target action and try to physically reconstruct it. This means that imitative abilities rely on memory capacities as well as planning skills and inhibitory control. Recognizing imitation, on the other hand, requires processing anothers' actions only after a self-produced act, and finding a match between the two. Hence elements such as planning and selecting appropriate motor acts are not required.
In comparisons with apes, monkeys show poor planning skills and inhibitory control (e.g. in virtual maze tasks; Fragaszy et al. 2003). Such cognitive limitations may be sufficient to hamper the production of imitation. However, it remains possible that monkeys possess a ‘like me’ mechanism that allows matching of self and others' actions, in which case, monkeys would still be capable of detecting imitation. To test this hypothesis, we adapted Meltzoff's (1990, 1996) paradigm from studies with human infants to test if monkeys can recognize when they are being imitated.
2. Methods
(a) Subjects
Subjects were 10 pigtailed macaques (Macaca nemestrina), six male and four female, all captive-born and aged between 4 and 18 years (mean 8.6 years). Although all monkeys were housed individually (cage measures: 100×160×100 cm), their home cages were part of an interconnected cage system, which allowed social interactions between two or more individuals. The monkeys were not food deprived, and received their normal diet several hours before the start of each experimental session. Water was available ad libitum. All procedures complied with ASAB guidelines and European law on the humane care and use of laboratory animals.
(b) Procedure
All monkeys were tested individually in their home cage. A table (measures: 104×80×51 cm) was placed in front of the cage and two familiar experimenters were seated behind it, facing the monkey but avoiding direct eye contact in order to minimize any influence on the monkeys' preferential gazing. A wooden cube (edge=5.5 cm) with a small hole drilled into each side was given to each experimenter. A digital video camera placed between the two experimenters recorded all sessions with only the monkey in view, thus allowing blind scoring.
At the start of each test trial, a 5 min baseline of visual preference was conducted. During this baseline, the monkey could observe both experimenters manipulating their respective cubes with hands and mouth, mimicking common actions of the monkeys towards the object such as biting, twisting, poking at the holes, and so on. Experimenters were not matched for actions and did not act in synchrony. After the baseline period, an identical cube was placed on the table within reach of the monkey, and the test period started as soon as the monkey contacted the cube. During the 5 min test period, one experimenter imitated the monkey's cube-directed actions in as structurally accurate and temporally contingent a manner as possible, while the second experimenter performed monkey—typical actions that were temporally contingent but structurally different. For example, if a monkey mouthed the cube, the imitator would also mouth while the non-imitator might poke at the cube's holes. If a monkey let go of the cube to engage in other activities (such as locomotion, social interactions with other monkeys, etc.), both experimenters placed their cubes onto the table and remained still until the monkey touched the cube again. Identity and position of the imitator was counterbalanced between subjects. At least 24 h after the first trial, a second trial was conducted with each monkey using an identical set up, except that the role of the imitator was reversed between experimenters.
3. Analysis
All tapes were digitally analysed (25 frames per second) by a rater blind to the experimental condition, and the number of frames spent looking at each experimenter was recorded. Twenty-five percent of sessions were coded a second time to assess intra-observer reliability; agreement between both codings was high (Pearson's correlation: r=0.98, p<0.001). Raw scores from both trials were added for each monkey and divided by 2, so that the following statistical analyses were conducted on the average time from both trials, each monkey had spent looking at imitator and non-imitator.
4. Results
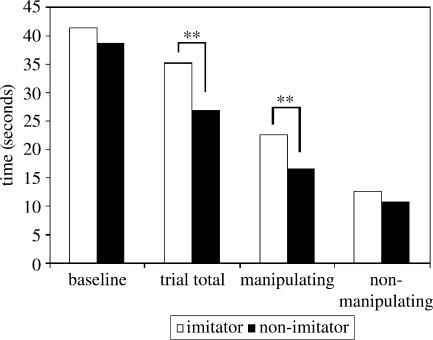
As figure 1 shows, the monkeys had no significant visual preference for either experimenter during the baseline period, which was confirmed by related sample t-tests (t9=0.946, p>0.05). In the test period, the monkeys' preference shifted and they looked significantly longer at the imitator (t9=2.651, p=0.026).
Figure 1.
Average time in seconds per monkey spent looking at imitator and non-imitator. ** indicates statistical significance with p<0.05.
Since the experimenters only imitated the monkeys' cube-directed actions and not other activities, visual preferences were analysed separately for each group of activities. Results show that the preference for the imitator was expressed only during manipulation of the cube (t9=2.344 p=0.044), not during non-manipulation (t9=1.988, p>0.05). There was no effect of identity or spatial position of the imitator, and no difference in looking times between trial 1 and 2 (all p>0.05).
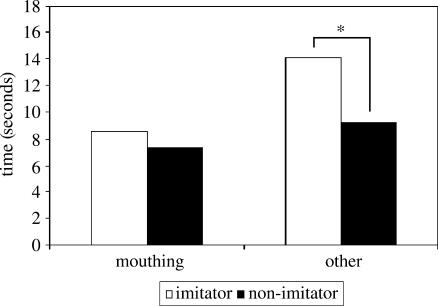
It could be argued that the monkeys' visual preference might stem from a greater interest in the mouthing action of an experimenter rather than an interest in matching of actions as such. To test this hypothesis, looks at the experimenters when mouthing and performing other actions were analysed separately. Figure 2 shows that when the monkeys were performing other cube-directed manipulations, visual preference for the imitator reached borderline significance (t9=2.255, p=0.051) but no visual preference was found during mouthing (t9=0.877, p>0.05).
Figure 2.
Average time in seconds per monkey spent looking at imitator and non-imitator during mouthing and other manipulations. * indicates borderline significance with p=0.051.
We also calculated a preferential looking index which gives a measure of the relative proportion of monkeys looking more frequently at the imitator or non-imitator (see Agnetta & Rochat 2004). This index was calculated as follows: (imitator−non-imitator)/(imitator+non-imitator). The resulting value represents a preference for the non-imitator if negative, and a preference for the imitator if positive (ranging from −1 to +1). We applied one-sample t-tests to these index values comparing them against zero as chance performance, and found no significant preference during the baseline period (t9=1.105, p>0.05). However, there was a significant preference during the trial period (t9=3.672, p=0.005), during manipulation of the object (t9=2.532, p=0.032), and in particular, during non-mouthing actions (t9=2.624, p=0.028), but not mouthing actions (t9=0.922, p>0.05).
5. Discussion
The results show that pigtailed macaques preferentially look at an experimenter imitating the monkeys' object-directed actions compared with an experimenter manipulating an identical object but not imitating their actions. Since both experimenters acted (as much as possible) in synchrony with the monkeys, the monkeys based this preference not on temporal contingency, but took into account the structural components of the experimenters' actions.
It could be argued that this match between the experimenters' and the monkeys' own actions was achieved through kinaesthetic–visual matching, i.e. the ability to conceive of the visual form of one's own felt body postures and movements (Mitchell 2002). Another possible mechanism is visual–visual matching, which is the ability to match the visual input of own and other's actions. These mechanisms are not mutually exclusive, and the monkeys might have used both to recognize imitation. However, their own mouthing behaviours might be difficult or even impossible to visually monitor, and in fact, the monkeys did not show a visual preference during mouthing. This could be interpreted as an absence of kinaesthetic–visual matching skills, and reliance on visual–visual matching skills alone. On the other hand, the monkeys frequently used their canines to mouth the object, for which they turned the head to one side. This might have resulted in less attention being paid to the experimenters and therefore fewer opportunities to establish a match between actions.
Clear evidence of kinaesthetic–visual matching is found at a neuronal level in the macaque brain. Mirror neurons, found in the ventral premotor area F5 (Rizzolatti et al. 1996) and the inferior parietal cortex of the macaque (Gallese et al. 2002), discharge both when a monkey performs an action, and when it sees a similar action being performed by an experimenter or another monkey. Area F5 in the macaque brain contains mirror neurons for both hand and mouth actions (Ferrari et al. 2003). The discharge of mirror neurons when the self is performing an action does not depend on the visual input from the action, as they also fire when the monkey executes a hand action without the possibility of seeing its own hand (Rizzolatti et al. 1996). Mirror neurons therefore have both visual and kinaesthetic properties.
It has been proposed that mirror neurons serve as the basis of action recognition (Rizzolatti et al. 2001), as they match the observed actions onto the internal motor repertoire. Mirror neurons, therefore, appear to be a suitable neural basis not only for kinaesthetic–visual matching, but also for a ‘like me’ mechanism that would allow imitation as well as recognition of imitation. In agreement with this hypothesis, a recent PET study in humans found common activated focuses for imitation and recognizing imitation corresponding to brain areas that coincide with the locus of the mirror system (Decety et al. 2002).
One unresolved issue not addressed in the present study concerns the cognitive level at which the match between actions is achieved. Nadel (2002) proposed several levels of understanding at which an individual could produce and recognize imitation. At its most basic, the production of matching motor movements could be related to simple ‘resonance’ mechanisms (Rizzolatti et al. 2002), which facilitate motor actions but do not result from any intention to reproduce a seen action. Similarly, the most basic level of recognizing imitation could merely consist of a capacity to recognize structural and temporal contingencies without any attribution of imitative intentionality to the imitator. Higher levels of understanding involve a deeper insight into the mental processes that might guide others' actions. For producing imitation, this means that an individual forms a concept of the model's goals or intentions. A higher level of recognizing imitation requires the imitatee to understand the imitator as an intentional agent, who holds the intention or goal to imitate. These explicit understandings of producing and recognizing imitation both rely on attributing mental states to others, and therefore require a theory of mind (TOM).
At present, it is unclear whether monkeys possess an implicit or explicit understanding of being imitated. Nadel (2002) proposed that while implicit recognition of being imitated might lead to increased visual attention, behavioural strategies to test the imitator (e.g. sudden changes of movement while looking at the imitator) could be indicative of explicit recognition. In the present study, a visual preference for the imitator was found, but testing behaviours were not noted. This is suggestive of implicit recognition of being imitated. An absence of explicit recognition is in accordance with current research findings in animal cognition, since so far, no strong behavioural evidence of TOM has been found in monkeys (Tomasello & Call 1997).
References
- Agnetta B, Rochat P. Imitative games by 9-, 14-, and 18-month-old infants. Infancy. 2004;6:1–36. [Google Scholar]
- Decety J, Chaminade T, Grezes J, Meltzoff A.N. A PET exploration of the neural mechanisms involved in reciprocal imitation. Neuroimage. 2002;15:265–272. doi: 10.1006/nimg.2001.0938. [DOI] [PubMed] [Google Scholar]
- Ferrari P.F, Gallese V, Rizzolatti G, Fogassi L. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. Eur. J. Neurosci. 2003;17:1703–1714. doi: 10.1046/j.1460-9568.2003.02601.x. [DOI] [PubMed] [Google Scholar]
- Fragaszy D.M, Johnson-Pynn J, Hirsh E, Brakke K. Strategic navigation of two-dimensional alley mazes: comparing capuchin monkeys and chimpanzees. Anim. Cogn. 2003;6:149–160. doi: 10.1007/s10071-002-0137-8. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti R. Action representation in the inferior parietal lobe. In: Prinz W, Hommel B, editors. Common mechanisms in perception and action. Oxford University Press; Oxford: 2002. pp. 334–355. [Google Scholar]
- Meltzoff A.N. Foundations for developing a concept of self: the role of imitation in relating self to other and the value of social mirroring, social modelling, and self practice in infancy. In: Cicchetti D, Beeghly M, editors. The self in transition: infancy to childhood. University of Chicago Press; Chicago: 1990. pp. 139–164. [Google Scholar]
- Meltzoff A.N. The human infant as imitative generalist: a 20-year progress report on infant imitation with implications for comparative psychology. In: Heyes C.M, Galef B.G, editors. Social learning in animals: the roots of culture. Academic Press; London: 1996. pp. 347–370. [Google Scholar]
- Meltzoff A.N, Gopnik A. The role of imitation in understanding persons and a theory of mind. In: Baron-Cohen S, Flusberg H, Cohen D, editors. Understanding other minds. Oxford University Press; Oxford: 1993. pp. 335–366. [Google Scholar]
- Mitchell R.W. Imitation as a perceptual process. In: Dautenhahn K, Nehaniv C.L, editors. Imitation in animals and artefacts. MIT Press; Cambridge, MA: 2002. pp. 441–469. [Google Scholar]
- Nadel J. Imitation and imitation recognition: functional use in preverbal infants and nonverbal children with autism. In: Meltzoff A.N, Prinz W, editors. The imitative mind: development, evolution, and brain bases. Cambridge University Press; Cambridge: 2002. pp. 42–62. [Google Scholar]
- Nielsen M, Collier-Baker E, Davis J.M, Suddendorf T. Imitation recognition in a captive chimpanzee (Pan troglodytes) Anim. Cogn. 2004;8:31–36. doi: 10.1007/s10071-004-0232-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cogn. Brain Res. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Motor and cognitive functions of the ventral premotor cortex. Curr. Opin. Neurobiol. 2002;12:149–154. doi: 10.1016/s0959-4388(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Call J. Oxford University Press; New York: 1997. Primate cognition. [Google Scholar]
- Visalberghi E, Fragaszy D.M. “Do monkeys ape?” Ten years after. In: Dautenhahn K, Nehaniv C, editors. Imitation in animals and artefacts. MIT Press; Cambridge, MA: 2002. pp. 471–499. [Google Scholar]
- Voelkl B, Huber L. True imitation in marmosets. Anim. Behav. 2000;60:195–202. doi: 10.1006/anbe.2000.1457. [DOI] [PubMed] [Google Scholar]
- Whiten A, Custance D.M, Gomez J.C, Teixidor P, Bard K.A. Imitative learning of artificial fruit processing in children (Homo sapiens) and chimpanzees (Pan troglodytes) J. Comp. Psychol. 1996;110:3–14. doi: 10.1037/0735-7036.110.1.3. [DOI] [PubMed] [Google Scholar]