Abstract
Recent studies have shown that some clupeid fishes, including shad and menhaden, can detect ultrasound (sound with frequencies higher than 20 kHz) and actively avoid it. However, other clupeids, including sardines and anchovies, do not detect ultrasound. The hearing abilities of herring are of particular interest because of their commercial importance, our reliance on acoustics to monitor their populations and behavioural evidence of responses to high-frequency sound by some clupeid species. We measured the hearing sensitivity of Pacific herring (Clupea pallasii) using the auditory brainstem response and found that they were unable to detect ultrasonic signals at received levels up to 185 dB re 1 μPa. Herring had hearing thresholds at lower frequencies (100–5000 Hz) that were typical of other non-ultrasound‐detecting clupeids. This lower‐frequency hearing sensitivity could explain the results of several earlier studies showing responses to broadband sounds.
Keywords: auditory brainstem response, Pacific herring, Clupea, ultrasound
Abbreviations: ABR, auditory brainstem response
1. Introduction
Management of herring stocks (Clupea spp.), the fourth largest worldwide fishery by tonnage (FAO 2001), relies on critical measurements of biomass using active acoustic sonar surveys that typically use ultrasonic frequencies (frequencies higher than 20 kHz). Recent studies have shown that some clupeid fishes, including shad and menhaden, can detect ultrasound (sound with frequencies higher than 20 kHz) and actively avoid it, thereby affecting the ability to perform stock assessment using these acoustic methods (Dunning et al. 1992; Nestler et al. 1992; Ross et al. 1995, 1996; Mann et al. 1997, 1998, 2001). Other clupeids, including sardines and anchovies, do not detect ultrasound (Mann et al. 2001). Major efforts have been undertaken to investigate and limit herring responses to lower‐frequency vessel noise (Fernandes et al. 2000), but concerns remain for the sonar itself. If herring avoid the survey sonar, as has been demonstrated for blueback herring and alewives (Dunning et al. 1992; Nestler et al. 1992), then it could greatly limit the usefulness of this technique for determining their abundance, distribution and behaviour.
Several reports have suggested that herring may be able to detect ultrasound, whereas others have suggested they do not. Fishing nets, for example, equipped with high-frequency pingers (typically greater than 10 kHz) had reduced Atlantic herring catches in one study (Kraus et al. 1997), but not in two others (Trippel et al. 1999; Culik et al. 2001). In another study, Pacific herring were shown to change their behaviour in response to simulated echolocation clicks (Wilson & Dill 2002). The acoustic signals in these studies were often broadband and contained energy from less than 4 kHz to ultrasonic frequencies. Thus, in studies where there was a behavioural response, it was not clear whether the herring were responding to the lower‐frequency components or to the ultrasound. The goal of this study was to determine the audiogram of Pacific herring using pure tone stimuli, and thereby assess their ability to detect ultrasonic pingers and echosounders.
2. Material and methods
Live Pacific herring (Clupea pallasii) were purchased from a commercial bait supplier on Vancouver Island, British Columbia (BC), Canada in June 2003 and were transported to the Division of Fisheries and Oceanography station in Nanaimo, BC. Testing was completed within 3 days of transportation. The auditory brainstem response (ABR; n=7; stimulus level (SL)=116–135 mm) was measured in response to pulsed tones at the following frequencies: 200, 300, 400, 600, 800, 1000, 2000, 4000, 5000, 6000, 8000, 10 000, 20 000, 40 000, 60 000 and 80 000 Hz. Water temperature was 13 °C.
The test tank was 72×6×26 cm3 (length×width×depth). Sounds were 20 ms duration. Hanning-windowed tone pulses were presented 11 times per second, and were generated with a Tucker-Davis technologies ABR workstation with BioSig software. Two transducers were used. An aqua-synthesis underwater speaker located 5 cm from the far wall opposite the fishes (57 cm to the fishes) was used for stimuli from 200 to 20 000 Hz. An ITC-1042 transducer located 40 cm from the far wall (this transducer was closer to provide maximum sound levels; i.e. 22 cm from the fishes) was used to generate signals at 40, 60 and 80 kHz. Sounds were presented from low sound levels to higher sound levels. Signals were calibrated with a Reson hydrophone and showed no frequencies outside the test frequency.
Fishes were temporarily anaesthetized in MS-222 and restrained in a nitex mesh sling and held 19 cm below the water surface. Fishes were allowed to recover from anaesthesia prior to testing. No MS-222 was supplied during ABR acquisition. To record ABR signals, a ground electrode was placed in the test tank, a recording needle electrode (Rochester Electro-Med) was placed subdermally just above the skull, and a reference needle electrode was placed in the dorsal musculature. Impedances were typically less than 1 kΩ. All electrodes were insulated except for the tip. ABR signals were amplified 10 000× and bandpass filtered from 10 to 15 000 Hz. ABR signals were acquired for 50 ms and measured by averaging the amplified evoked potentials from up to 2000 signal presentations. Thresholds were calculated as the lowest sound levels that visually yielded a consistent evoked potential above background levels. Controls were run with dead fishes to verify that all evoked potentials were not electrical artefacts.
All procedures were approved by the Institutional Animal Care and Use Committees of the University of South Florida and the University of British Columbia.
3. Results
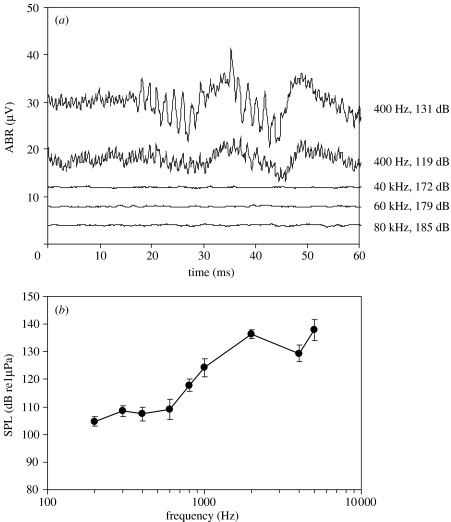
Herring showed ABR responses to signals of up to 5000 Hz, but never to ultrasound (maximum sound pressure levels tested owing to transducer limitations were 40 kHz, 172 dB re 1 μPa; 60 kHz, 179 dB re 1 μPa, 80 kHz, 185 dB re 1 μPa; figure 1a). Herring also showed no overt behavioural response to ultrasound presentation while in the test apparatus.
Figure 1.
(a) ABR signals from an individual herring in response to presentation of a 20 ms 400 Hz tone at 131 dB re 1 μPa and 119 dB re 1 μPa with 200 averages (top two traces). The start of sound presentation begins at time 1 ms (owing to the group delay of the amplifier). The traces are offset on the y-axis so that they can be compared (note that there is no direct current (DC) offset in the recordings). The evoked response contains a signal at 800 Hz (twice the stimulus frequency) beginning at 16 ms, as well as peaks at 35 and 50 ms. The lower three traces show no response to presentation of high levels of ultrasonic signals in the same fishes after 2000 averages. (b) Audiogram of Pacific herring (n=7; mean± s.e.) from 200 to 5000 Hz.
Evoked potentials to low-frequency sounds (200–1000 Hz) showed two signals. One was a series of troughs and peaks from 25 to 50 ms, and the other was a frequency doubling of the test signal (figure 1a). Evoked potentials to higher‐frequency stimuli (2000, 4000 and 5000 Hz) showed the troughs and peaks, but did not have the frequency doubling signal. Hearing sensitivity was best from 200 to 500 Hz, with decreasing sensitivity up to 5000 Hz (figure 1b). No evoked potentials were obtained at 10 or 20 kHz in response to sound presented at 151 dB (the maximum level that the low-frequency transducer could generate).
4. Discussion
The results of the evoked potential measurements showed that low-frequency thresholds of herring were typical of other clupeids and had a similar shape but less sensitivity than the neural recordings performed with Atlantic herring (Enger 1967). Like other members of the same subfamily, Clupeinae, the Pacific herring did not show any responses (evoked potential or behavioural) to ultrasound presentation. By contrast, American shad and gulf menhaden show evoked responses to the same stimuli at similar sound intensities, and swim vigorously in response to ultrasound presentation (Mann et al. 2001; Plachta & Popper 2003; Plachta et al. 2004).
The sensitivity of herring to sounds at frequencies of up to 5 kHz could explain the results of previous studies using stimuli containing a range of frequencies. This is an interesting result because it suggests that herring may use their upper-frequency hearing range to detect predators. A similar response to mid-frequency sounds has been hypothesized for some sciaenid fishes that change vocalization patterns in response to dolphin playbacks (Luczkovich et al. 2000).
All Clupeiforme fishes have two pairs of air bubbles in their inner ears that aid in sound detection, and are thought to widen their hearing bandwidth in comparison to other fishes without these specializations (Wohlfhart 1936; Blaxter et al. 1981). In existing studies, only members of the subfamily Alosinae, which include the shads and menhaden, have been found to detect ultrasound (Mann et al. 2001). Herring (Clupea spp.) are classified as members of the subfamily Clupeinae. Two other species of Clupeinae, the Spanish sardine and scaled sardine, have not been found to detect ultrasound (Mann et al. 2001). Our study suggests that like other Clupeinae, herring have sensitivities up to 5 kHz, but not into the ultrasonic range. Significantly, there is a subtle difference in the ears of Clupeinae and Alosinae that may provide a mechanical explanation for why only the Alosinae are able to detect ultrasound (Higgs et al. 2004).
Shipbased scientific echosounders used in scientific herring surveys typically broadcast pulses at 38 kHz or higher frequencies, with source levels of up to about 220 dB re 1 μPa. While a fish close to the sonar would receive sound levels above those we tested, it would drop to about 186 dB within 50 m, and 180 dB within 100 m. Pre-spawning herring typically school at between 100 and 150 m in depth (Maravelias et al. 2000), and therefore sound levels at the fishes would be at or below the levels we tested and so inaudible to the fishes even if they could detect these frequencies at higher levels. It is important to note that American shad and menhaden would be able to detect these ultrasonic signals, even at these depths. This finding shows that the sonar used in herring surveys that restrict acoustic signals to ultrasonic frequencies (greater than 20 kHz) should not affect their behaviour, and thus are an appropriate means to survey this commercially important species. At the same time, as all clupeids are able to detect sounds up to 5 kHz, it is important that future designs for sonar systems and other equipment used in fish surveys minimize energy bleeding into frequencies below 5 kHz.
Finally, recent reports show that herring produce high-frequency broadband sounds from 1.7 kHz to at least 22 kHz by releasing bubbles (Wahlberg & Westerberg 2003; Wilson et al. 2004). Although the function of bubble release is not known, the audiogram suggests that herring should be able to detect these sounds, while most other marine fishes without hearing specializations would not.
Acknowledgements
This work was supported by grant DC03936 from the National Institute of Deafness and Other Communicative Disorders of NIH. We thank Doug Hay for his assistance and use of facilities at the Pacific Biological Station, Department of Fisheries and Oceans, Canada.
References
- Blaxter J.H.S, Denton E.J, Gray J.A.B. Acoustico-lateralis systems in clupeid fishes. In: Tavolga W.N, Popper A.N, Fay R.R, editors. Hearing and sound communication in fishes. Springer; New York: 1981. pp. 39–59. [Google Scholar]
- Culik B.M, Koschinski S, Tregenza N, Ellis G.M. Reactions of harbour porpoises (Phocoena phocoena) and herring (Clupea harengus) to acoustic alarms. Mar. Ecol. Prog. Ser. 2001;211:255–260. [Google Scholar]
- Dunning D.J, Ross Q.E, Geoghegan P, Reichle J.J, Menezes J.K, Watson J.K. Alewives in a cage avoid high-frequency sound. North Am. J. Fish. Man. 1992;12:407–416. [Google Scholar]
- Enger P.S. Hearing in herring. Comp. Biochem. Physiol. 1967;22:527–538. doi: 10.1016/0010-406x(67)90615-9. [DOI] [PubMed] [Google Scholar]
- FAO Capture Production 2001.
- Fernandes P.G, Brierley A.S, Simmonds E.J, Millard N.W, McPhail S.D, Armstrong F, Stevenson P, Squires M. Fish do not avoid survey vessels. Nature. 2000;404:35. doi: 10.1038/35003648. [Addendum in Nature 2000 407, 152. [DOI] [PubMed] [Google Scholar]
- Higgs D.M, Plachta D.T.T, Rollo A.K, Singheiser M, Hastings M.C, Popper A.N. Development of ultrasound detection in American shad (Alosa sapidissima) J. Exp. Biol. 2004;207:155–163. doi: 10.1242/jeb.00735. [DOI] [PubMed] [Google Scholar]
- Kraus S.D, Read A.J, Solow A, Baldwin K, Spradlin T, Anderson E, Williamson J. Acoustic alarms reduce porpoise mortality. Nature. 1997;388:525. [Google Scholar]
- Luczkovich J.J, Daniel H.J, III, Hutchinson M, Jenkins T, Johnson S.E, Pullinger R.C, Sprague M.W. Sounds of sex and death in the sea: bottlenose dolphin whistles suppress mating choruses of silver perch. Bioacoustics. 2000;10:323–334. [Google Scholar]
- Mann D.A, Lu Z, Popper A.N. A clupeid fish can detect ultrasound. Nature. 1997;389:341. [Google Scholar]
- Mann D.A, Lu Z, Hastings M.C, Popper A.N. Detection of ultrasonic tones and simulated dolphin echolocation clicks by a teleost fish, the American shad (Alosa sapidissima) J. Acoust. Soc. Am. 1998;104:562–568. doi: 10.1121/1.423255. [DOI] [PubMed] [Google Scholar]
- Mann D.A, Higgs D.M, Tavolga W.N, Souza M.J, Popper A.N. Ultrasound detection by clupeiform fishes. J. Acoust. Soc. Am. 2001;109:3048–3054. doi: 10.1121/1.1368406. [DOI] [PubMed] [Google Scholar]
- Maravelias C.D, Reid D.G, Swartzman G. Seabed substrate, water depth and zooplankton as determinants of the prespawning spatial aggregation of North Atlantic herring. Mar. Ecol. Prog. Ser. 2000;195:249–259. [Google Scholar]
- Nestler J.M, Ploskey G.R, Pickens J, Menezes J, Schilt C. Responses of blueback herring to high-frequency sound and implications for reducing entrainment at hydropower dams. North Am. J. Fish. Man. 1992;12:667–683. [Google Scholar]
- Plachta D.T.T, Popper A.N. Evasive responses of American shad (Alosa sapidissima) to ultrasonic stimuli. Acoust. Res. Lett. Online (ARLO) 2003;4:25–30. doi:10.1121/1.1558376 [Google Scholar]
- Plachta D.T.T, Song J, Halvorsen M.B, Popper A.N. Neuronal encoding of ultrasonic sound by a fish. J. Neurophysiol. 2004;91:2590–2597. doi: 10.1152/jn.01200.2003. [DOI] [PubMed] [Google Scholar]
- Ross Q.E, Dunning D.J, Menezes J.K, Kenna M.J, Tiller G. Reducing impingement of alewives with high-frequency sound at a power plant on Lake Ontario. North Am. J. Fish. Man. 1995;15:378–388. [Google Scholar]
- Ross Q.E, Dunning D.J, Thorne R, Menezes J.K, Tiller G.W, Watson J.K. Response of alewives to high-frequency sound at a power plant intake on Lake Ontario. North Am. J. Fish. Man. 1996;16:548–559. [Google Scholar]
- Trippel E.A, Strong M.B, Terhune J.M, Conway J.D. Mitigation of harbour porpoise (Phocoena phocoena) by-catch in the gillnet fishery in the lower Bay of Fundy. Can. J. Fish. Aquat. Sci. 1999;56:113–123. [Google Scholar]
- Wahlberg M, Westerberg H. Sounds produced by herring (Clupea harengus) bubble release. Aquat. Living Resour. 2003;16:271–275. [Google Scholar]
- Wilson B, Dill L.M. Herring respond to simulated odontocete echolocation sounds. Can. J. Fish. Aquat. Sci. 2002;59:543–553. [Google Scholar]
- Wilson B, Batty R.S, Dill L.M. Pacific and Atlantic herring produce burst pulse sounds. Biol. Lett. 2004;271:S95–S97. doi: 10.1098/rsbl.2003.0107. doi:10.1098/rsbl.2003.0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfhart T.A. Das ohrlabyrinth der sardine (Clupea pilchardus Walb.) und seine beziehungen zur zchwimmblase und seitenlinie. Z. Morphol. Oekol. Tiere. 1936;31:371–410. [Google Scholar]