Abstract
The bright colours of feathers are among the most striking displays in nature and are frequently used as sexual signals. Feathers can be coloured by pigments or by ordered tissue, and these mechanisms have traditionally been treated as distinct modes of display. Here we show that some yellow plumage colour is created both by reflection of light from white structural tissue and absorption of light by carotenoids. Thus, structural components of feathers contribute substantially to yellow ‘carotenoid’ displays, but the effect of variation in structural components on variation in colour displays is, to our knowledge, unstudied. The presence of structural colour in some carotenoid-based colour displays will have to be considered in studies of colour signalling.
Keywords: plumage colour, nanostructure, sexual selection, honest signalling
1. Introduction
The coloration of feathers can be caused by carotenoids (usually producing yellow, orange and red), melanins (usually producing brown, black and grey), other pigments (such as found in some parrot feathers) or by nano-scale reflective tissues (usually producing UV-blue, white and iridescent coloration; Gill 1995). Coloration produced by the latter mechanism is typically referred to as ‘structural coloration’ (Gill 1995) and structural coloration and pigment-based coloration have traditionally been treated as distinctly different modes of colour display (e.g. Fitzpatrick 1998). However, many structural mechanisms require melanin pigments either to serve as layers in thin-film reflectors or to absorb incoherently backscattered light from reflective keratin and air matrices (Prum 1999). Moreover, many colour displays involve a combination of structures and pigments—most obviously in the green plumage of many species that results from blue-green structures and yellow carotenoids (Dyck 1971; Prum 1999). Here we consider the potential contribution of structural elements to colour production in yellow carotenoid-pigmented feathers.
Spectral measurements reveal that yellow feathers with carotenoid pigmentation frequently have reflectance curves with a peak in the ultraviolet and a plateau in the visible spectrum (Eaton & Lanyon 2003; MacDougall & Montgomerie 2004). This peak and plateau are probably not created by reflection of light from the carotenoid pigments themselves, as the high extinction coefficients (a measure of the light-absorbing properties of molecules) of carotenoids suggest that they strongly absorb, rather than reflect light (Bauerfeind 1981). Rather, carotenoids probably create a colour display by absorbing light from another reflective substance, and we propose that, in feathers, this reflective substance is the structural white tissue in which they are deposited (Mason 1923).
2. Materials and methods
We plucked yellow breast feathers from five study skins of alternate plumage American goldfinches (Carduelis tristis) in the Auburn University vertebrate museum, and collected recently moulted white breast feathers from the pens of five different domestic chickens (Gallus gallus) at the Auburn University Poultry Science Department.
To remove carotenoids and thus reveal the underlying colour of barbs, we placed five feathers from each bird in glass tubes, added 2 ml of acidified pyridine (Hudon & Brush 1992; three drops of HCl in 50 ml pyridine) and capped the tubes. We then incubated the solution for 3 h at 95 °C. As a control, we performed the same procedures on white chicken feathers. After cooling to room temperature, feathers were removed from the solution, allowed to air dry, and taped in stacks of five to gloss-free black construction paper before spectral measurements were obtained (see below).
To disrupt structural colour, we attached separate groups of de-pigmented and intact yellow feathers from each bird in stacks of five to black construction paper and white index cards, and saturated them with Cresol (Sigma, St Louis, MI) using the blunt end of a pair of forceps. Cresol has a refractive index (RI) identical to that of keratin (RI≅1.54). The scattering of light as it moves between materials of different RIs causes structural colour (Prum 1999). Thus this treatment prevents structural colour reflectance in barbs by replacing air (RI=1.00) with cresol and eliminating the variation in the RI that causes colour production. Many structurally coloured tissues thus become transparent upon treatment with cresol (Mason 1923).
We obtained spectral reflectance curves while the feathers were saturated (see below). The construction paper and cards became saturated with cresol in the area where the feathers were attached, and this background was visible and hence recorded spectrophotometrically when the feathers became transparent owing to cresol treatment. To account for these changes in the background coloration, we took spectral measurements before and after they were treated with cresol.
We obtained reflectance curves using an Ocean Optics S2000 spectrometer (range 250–880 nm; Dunedin, FL, USA) with a UV (deuterium bulb) and a visible (tungsten–halogen bulb) light source. Using a block sheath that excluded ambient light, we held a bifurcated micron fibre optic probe at a 90° angle 5 mm from the yellow feather surface, creating a measurement area of 2 mm in diameter. All data were generated relative to a white standard (WS-1, Ocean Optics). We used OOIbase software to record and average 20 spectra sequentially, and recorded and averaged measurements from five arbitrarily chosen points on each sample.
To determine the location of carotenoids and structural tissues within feather barbs, we examined them using light microscopy and transmission electron microscopy (TEM). To prepare barbs for light microscopy, we embedded whole feathers in tissue freezing medium (Electron Microscopy Sciences, Hatfield, PA), froze them at −25 °C and cut 20 μm thin sections of the coloured portions of barbs on a cryotome (FrigoCut, Riechert-Jung, Germany). We prepared coloured feather barbs for TEM using standard methods (see Shawkey et al. (2003) for details) and viewed them on a Phillips EM301 (Veeco FEI, Inc., Hillsboro, OR) transmission electron microscope.
3. Results and discussion
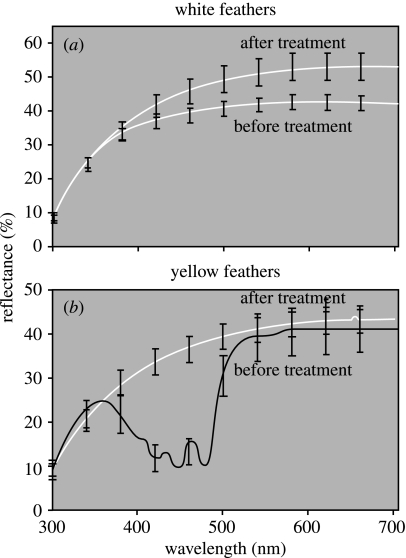
To test for the importance of the structural component of yellow feathers, we first treated yellow American goldfinch feathers and white chicken feathers (which served as controls) with acidified pyridine to remove carotenoids. White chicken feathers increased slightly in achromatic brightness following treatment, whereas the yellow feathers turned white (figure 1). This treatment demonstrates that yellow carotenoid feathers have a white background.
Figure 1.
Spectral reflectance curves (±1s.e.) of (a) white breast feathers from chickens and (b) yellow breast feathers from American Goldfinches before and after carotenoid extraction with acidified pyridine. The untreated feathers had reflectance curves typical of carotenoid-pigmented barbs whereas pyridine-treated feathers had typical reflectance curves for white feathers (b). n=5 in all cases.
We next treated feathers with cresol, a liquid with the same RI as keratin that removes the structural component of coloration. Whether de-pigmented or naturally white, white feathers became transparent after cresol treatment (figure 2a). Yellow feathers treated with cresol became nearly transparent with a faint yellow cast (figure 2b) when placed against a black background. When these cresol-treated yellow feathers were placed against a white background, however, they once again appeared bright yellow (figure 2c). On a black background, coloration comes only from the direct reflection of light from the carotenoid pigments and this produces minimal coloration. On a white background, carotenoids absorb reflected light from the white background and again appear bright yellow. We obtained similar results when we applied the same methods to yellow feathers of prothonotary warblers (Prothonotaria citrea), cedar waxwings (Bombycilla cedrorum), and great crested flycatchers (Myiarchus crintus) (M. D. Shawkey, unpublished data). Thus, these results appear to apply to yellow carotenoid-pigmented feathers generally.
Figure 2.
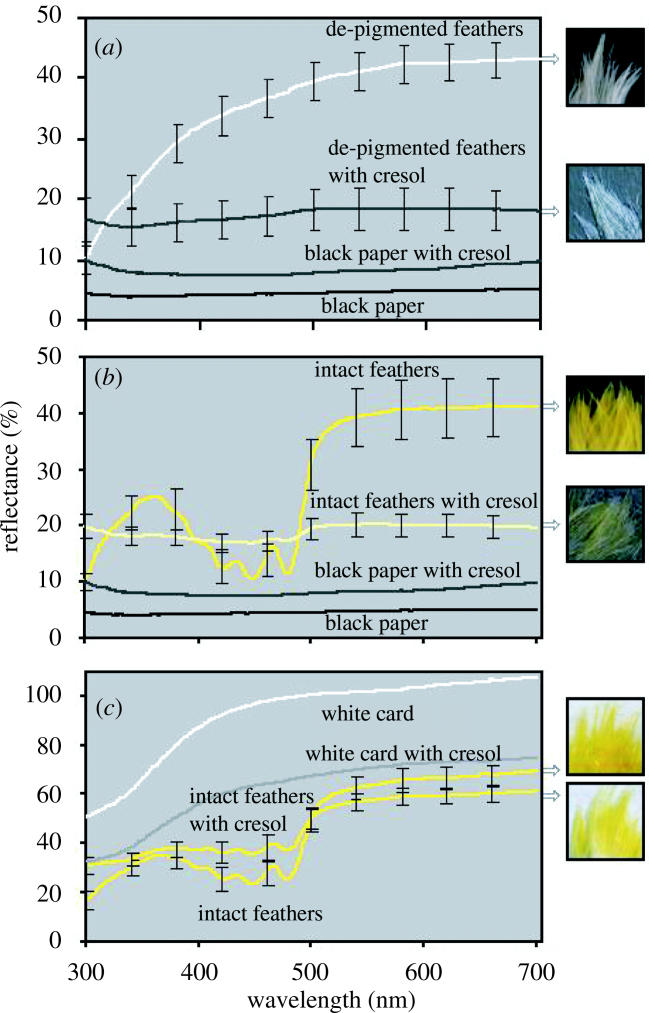
Spectral reflectance curves (±1s.e.) of cresol-treated breast feathers of American Goldfinches (a) against a black background after being chemically de-pigmented (b) against a black background intact, and (c) against a white background intact. Panel A shows that de-pigmented feathers became transparent after cresol treatment, as their reflectance matched that of the black background after being treated with cresol, except for a small increase in overall reflectance. Panel B shows that cresol-treated yellow feathers against a black background are nearly transparent with a faint yellow cast, as indicated by the small increase in brightness from 500 to 700 nm. c shows that cresol-treated yellow feathers against a white background are once again bright yellow, as carotenoids absorb light from the white background. n=5 for all feather reflectance curves. Because all feathers were measured on the same piece of background paper, n=1 for these curves.
The yellow feathers of male American Goldfinches have the carotenoid pigments canary xanthophylls a and b (Stradi et al. 1995; McGraw et al. 2001), which are purported to produce the yellow feather colour. Here we show, however, that the yellow feathers of goldfinches, and probably of other species, also have white structural coloration that plays a key role in producing their yellow and UV reflectance peaks. Our observations show that carotenoids function primarily to absorb light at wavelengths from approximately 400 nm to 500 nm from the structural white colour of the barbs.
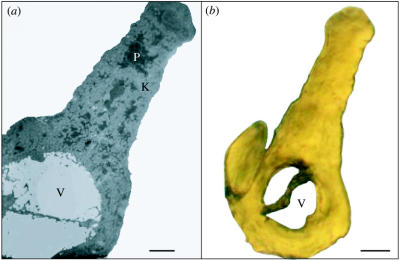
Our microscopic examinations revealed that carotenoid pigments are scattered haphazardly throughout the keratin substrate of feather barbs (figure 3a). This spatial arrangement may allow carotenoids to absorb light from the white keratin substrate while weakly reflecting yellow light, resulting in the pure yellow coloration seen in figure 3b. Thus, carotenoids probably serve as semi-transparent filters in yellow feathers. The mechanisms of white colour production in feather barbs are largely unknown (Prum 1999), however, and elucidation of these mechanisms by precise physical modelling is needed before we can state definitively how white tissue and carotenoids interact to produce colour. Our results clearly show that both white structural colour and pigments are needed for the production of yellow colour, and these results have several important implications for the study of plumage coloration.
Figure 3.
(a) TEM micrograph (1900×) of a yellow American goldfinch (Carduelis tristis) feather barb. P, carotenoid pigments; K, keratin substrate; and V, air-filled vacuole. Scale bar, 1 μm. (b) Light micrograph (1000×) of a yellow American goldfinch (C. tristis) feather barb. V, Vacuole. Scale bar, 2 μm.
First, while the characterization of colour displays as pigment-based or structural has heuristic value and should continue to be used, researchers should understand that many pigmentary mechanisms have a structural component. Here, we have shown that carotenoid displays rely critically on underlying white structural coloration.
Second, the blurring of the distinction between mechanisms of production may add to the complexity of feather signalling properties. It has been shown that changes in red and yellow coloration, particularly in hue and saturation (i.e. colour purity), are proportional to changes in types and concentrations of carotenoids in the feather (Hill 2002; Saks et al. 2003). In turn, birds in poor condition and with greater parasite loads tend to be duller and have lower carotenoid concentration than those in good condition (Hill 2002). More carotenoids can cover the white structure more completely than fewer carotenoids, resulting in a more saturated and pure colour. Thus, that saturation and purity of carotenoid coloration would dependably reflect pigment concentration, which in turn could signal foraging ability or parasite load (Hill 2002). Other elements of yellow coloration, however, such as UV chroma may be affected by variation in structural, as well as pigmentary components. Indeed, differences in UV reflectance between male and female yellow-breasted chats (Icteria virens) cannot be explained by differences in carotenoid concentration alone, and thus are presumably influenced by differences in structural coloration as well (Mays et al. 2004).
The brightness, or total reflectance, of carotenoid-containing tissues is probably also strongly influenced by structural components of barbs, as the reflection of light (as opposed to the subtraction of light) is primarily a function of the underlying white structural colour. Recent studies of variation in achromatic brightness of white plumage patches suggest a signalling function for white plumage (Doucet et al. 2005). The production mechanisms and extent of variation in white coloration, however, remain largely unknown (Mennill et al. 2003). Clearly, more information on these topics is needed. Perhaps the structural and pigmentary components of feather coloration signal different, or redundant, information that is assessed simultaneously by the receiver. In this way, single feather patches could contain multiple ornaments (Candolin 2003).
Finally, these results may partially explain the widespread occurrence of UV reflectance in avian plumage. Ultraviolet reflectance is close to ubiquitous among birds with pigmented feathers (Eaton & Lanyon 2003). This pattern may partly be caused by the near-ubiquity of structural white plumage colour in carotenoid-pigmented feathers. However, some pigments may reflect in the UV and not all structural white feathers reflect well in the UV, so further testing of this hypothesis is needed. Our results and the white colour of the proximal portions of many pigmented feathers suggest that white structural colour may be found commonly in conjunction with carotenoids and other pigments. Structural colour could be the foundation on which much ‘pigment-based’ plumage colour is based and its presence and variation may have to be taken into account in future studies of avian plumage colour signalling.
Acknowledgements
The idea for this manuscript was inspired by discussions with S. Andersson, R. Montgomerie, H. L. Mays Jr., and G. E. H.'s laboratory group. We thank M. Toivio-Kinnucan for preparing feather barbs for TEM and A. G. Moss for help with light microscopy. The manuscript was improved by comments from L. K. Estep, S. M. Doucet, K. J. Navara and members of G. E. H's laboratory group. This work was supported in part by research grants from the American Ornithologists’ Union and Birmingham Audubon Society to M. D. S. and NSF grants DEB 0077804 and IBN 0235778 to G. E. H.
References
- Bauerfeind J.C. Academic Press; New York, USA: 1981. Carotenoids as colorants and vitamin A precursors. [Google Scholar]
- Candolin U. The use of multiple cues in mate choice. Biol. Rev. 2003;78:575–595. doi: 10.1017/s1464793103006158. [DOI] [PubMed] [Google Scholar]
- Doucet S.M, Mennill D.J, Montgomerie R, Boag P.T, Ratcliffe L.M. Achromatic plumage reflectance predicts reproductive success in male black-capped chickadees. Behav. Ecol. 2005;16:218–222. [Google Scholar]
- Dyck J. Structure and spectral reflectance of green and blue feathers of the Lovebird (Aganorpis roseicollis) Biol. Skr. 1971;18:1–67. [Google Scholar]
- Eaton M.D, Lanyon S.M. The ubiquity of avian ultraviolet plumage reflectance. Proc. R. Soc. B. 2003;270:1721–1726. doi: 10.1098/rspb.2003.2431. doi:10.1098/rspb.2003.2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick S. Colour schemes for birds: structural coloration and signals of quality in feathers. Ann. Zool. Fenn. 1998;35:67–77. [Google Scholar]
- Gill F.B. W. H. Freeman; New York: 1995. Ornithology. [Google Scholar]
- Hill G.E. Oxford University Press; New York: 2002. A red bird in a brown bag: the function and evolution of ornamental plumage coloration in the House Finch. [Google Scholar]
- Hudon J, Brush A.H. Identification of carotenoid pigments in birds. Methods Enzymol. 1992;213:312–321. [Google Scholar]
- MacDougall A.K, Montgomerie R. Assortative mating by carotenoid-based plumage colour: a quality indicator in American goldfinches, Carduelis tristis. Naturwissenschaften. 2004;90:464–467. doi: 10.1007/s00114-003-0459-7. [DOI] [PubMed] [Google Scholar]
- McGraw K.J, Hill G.E, Stradi R, Parker R.S. The influence of carotenoid acquisition and utilization on the maintenance of species-typical plumage pigmentation in male American goldfinches (Carduelis tristis) and northern cardinals (Cardinalis cardinalis) Physiol. Biochem. Zool. 2001;74:843–852. doi: 10.1086/323797. [DOI] [PubMed] [Google Scholar]
- Mason C.W. Structural colors in feathers. J. Phys. Chem. 1923;27:201–251. [Google Scholar]
- Mays H.L, Jr, McGraw K.J, Ritchison G, Cooper S, Rush V, Parker R.S. Sexual dichromatism in the yellow-breasted chat (Icteria virens): spectrophotometric analysis and biochemical basis. J. Avian Biol. 2004;35:125–134. [Google Scholar]
- Mennill D.J, Doucet S.M, Montgomerie R, Ratcliffe L.M. Achromatic color variation in black-capped chickadees Poecile atricapilla: black and white signals of sex and rank. Behav. Ecol. Sociobiol. 2003;53:350–357. [Google Scholar]
- Prum R.O. The anatomy and physics of avian structural colours. In: Adams N, Slotow R, editors. Proceedings in 22nd International Ornithology Congress. University of Natal; Durban: 1999. pp. 1633–1653. [Google Scholar]
- Saks L, McGraw K.J, Horak P. How feather colour reflects its carotenoid content. Funct. Ecol. 2003;17:555–561. [Google Scholar]
- Shawkey M.D, Estes A.M, Siefferman L.M, Hill G.E. Nanostructure predicts intraspecific variation in ultraviolet-blue plumage colour. Proc. R. Soc. B. 2003;270:1455–1460. doi: 10.1098/rspb.2003.2390. doi:10.1098/rspb.2003.2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stradi R, Celentano G, Rossi E, Rovati G, Pastore M. Carotenoids in bird plumage- I. The carotenoid pattern in a series of Palearctic Carduelinae. Comp. Biochem. Physiol. 1995;110:131–143. [Google Scholar]