Abstract
The ability of malaria parasites to respond positively to the presence of feeding mosquito vectors would clearly be advantageous to transmission. In this study, Anopheles stephensi mosquitoes probed mice infected with the rodent malaria parasite, Plasmodium chabaudi. Growth of asexual stages was accelerated and gametocytes appeared 1–2 days earlier than in controls. This first study, to our knowledge, of the effects of mosquitoes on ‘in-host’ growth and development of Plasmodium has profound implications for malaria epidemiology, suggesting that individuals exposed to high mosquito numbers can contribute disproportionately high numbers of parasites to the transmission pool.
Keywords: mosquito, probing, saliva, plasmodium chabaudi, gametocytogenesis
1. Introduction
Blood-feeding arthropods produce in saliva numerous biologically active, pharmacological agents that improve their feeding success on vertebrate hosts (Ribeiro 1995). Anticoagulants target clotting pathways (Waidhet-Kouadio et al. 1998), vasodilators increase blood flow around the bite (Ribeiro et al. 1994) and immodulators reduce inflammation responses and host rejection (Ribeiro et al. 1984; Wikel et al. 1994; Zeidner et al. 1999).
Arthropod-borne protozoa and viruses can exploit salivary gland pharmacology to aid transmission (Titus & Ribeiro 1988; Edwards et al. 1998; Jones et al. 1992). Anopheles mosquitoes transmit human malaria parasites, resulting in some 200 million cases per annum. Transmission from the vertebrate host to the mosquito vector requires the formation of gametocytes, a process known as gametocytogenesis (Carter & Graves 1988). Parasites would be expected to optimize infectivity to the mosquito (Sinden 1983); this might be achieved by developmental mechanisms, increasing survival and numbers of the gametocytes, reducing gametocyte numbers if they are harmful in large numbers to the vector, or increasing the probability of gametocyte ingestion by mosquitoes. In this preliminary study, we describe the accelerated growth and modified gametocyte production rate of Plasmodium chabaudi resulting from probing by Anopheles stephensi mosquitoes on mice. This adaptive response points to hitherto unforeseen plasticity in the regulation of parasite development.
2. Material and methods
Twelve MF1 mice were infected with avirulent P. chabaudi 96DK by serial passage of 100 μl blood at 7% parasitaemia. Mice were lightly anaesthetized with Ropmun-Vetalar (Almeida & Billingsley 1998) daily for 14 days, and exposed for 20 min to 25 or 50 hungry female A. stephensi. Control mice were only anaesthetized. Excessive mosquito feeding alters malaria parasitaemia in mice owing to removal of erythrocytes (L. S. Snook and P. F. Billingsley, unpublished observations). Mice were therefore moved across the mosquito cage every few seconds, allowing mosquitoes to probe but not feed. Blood was removed daily from the tail and duplicate bloodsmears were Giemsa stained on blind-coded slides. The percentage of parasitaemia and proportion of parasites at mature gametocyte stage were determined by examination of 100 fields (100× objective) of each duplicate.
The exponential growth model of Buckling et al. (1999) was used to compare the observed and expected rates of gametocytogenesis. The maturation period of gametocytes was taken as 2 days and the proportion of parasites producing gametocytes on day t was described by the number of gametocytes at t+2 days. We assumed that gametocyte survival was unaffected, gametocyte half life was 12 h, and the asexual cycle took 24 h. Parasitaemias and the proportion of infected cells at the gametocyte stage were used to estimate gametocyte production rate. One mouse in each group demonstrated atypical infections (dotted lines in figure 1a–c) and failed to produce gametocytes, so only three mice per group were included in the analyses.
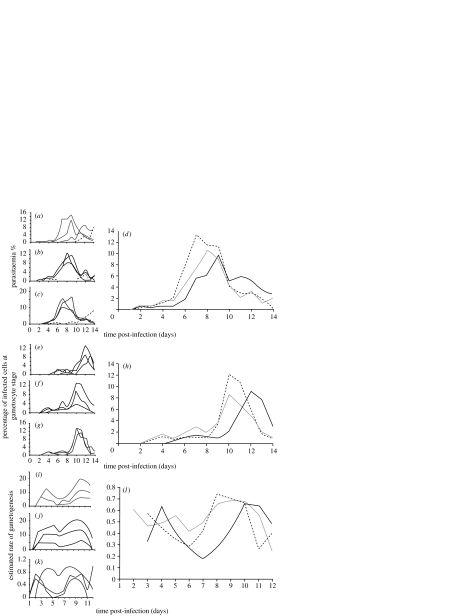
Figure 1.
Effect of probing by 25 or 50 female Anopheles stephensi on parasite (a–d) and gametocyte (e–h) populations of Plasmodium chabaudi 96DK within MF1 mice. Parasite (a–c) and gametocyte (e–g) populations in individual hosts are averaged to show the mean parasitaemia (d) and gametocyte rate (h). Individual (i–k) and mean (l) estimated rates of gametogenesis are calculated using the model of Buckling et al. (1999). In the small panels each line represents the characteristics of infection in an individual mouse. In the large panels, geometric mean values for control (black) mice, or mice probed by 25 (grey) or 50 (dashed line) were calculated from four (d) or three (h,l) mice.
The mean time to 50% and 80% maximum cumulative parasitaemia and gametocyte rate, respectively, were calculated from figure 2a,b using probit analysis. Life-history traits were estimated using the analysis of Eisen & Schall (2000) and compared using Student's t-test.
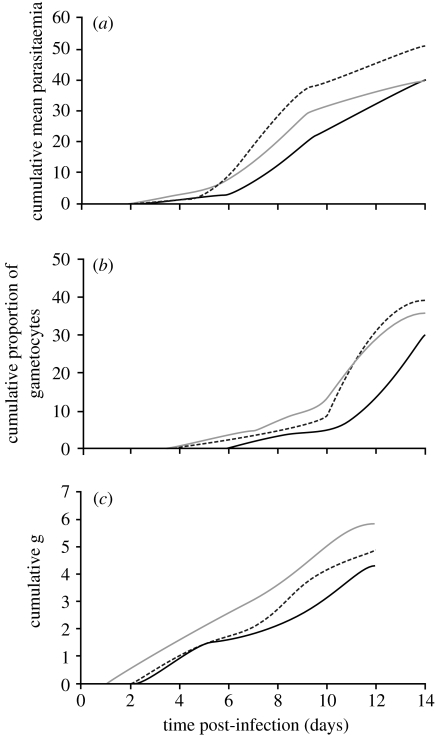
Figure 2.
Cumulative parasitaemia (a), proportion of infected cells at the gametocyte stage (b) and estimated rate of gametocytogenesis of Plasmodium chabaudi in control (solid) mice and mice probed daily by 25 (grey) or 50 (dashed line) Anopheles stephensi mosquitoes.
3. Results
The presence and number of probing mosquitoes positively influenced in-host P. chabaudi growth (figure 1). Three control mice showed typical infections, exhibiting peaks on days 8–11 p.i. Mean parasitaemia peaked at 7.3% on day 8 and the proportion of gametocytes at 9.1% of the infected red cells on day 12. The parasite population grew more rapidly to a higher and earlier peak in direct relation to the number of probing mosquitoes (figure 1a–d). Mean parasitaemia peaked at days 7 (8.0%) and 6 (9.9%) post-infection in mice exposed daily to 25 and 50 mosquitoes, respectively. Over the course of infection, mice probed daily by mosquitoes produced significantly more parasites (figure 2a; Wilcoxon signed: rank test-5 mosquitoes, Z=−2.726, p=0.006; 50 mosquitoes, Z=−2.84, p=0.005). The time until 50% and 80% of the maximum cumulative mean parasitaemia was advanced by 1.20–1.64 days and 0.99–1.57 days, respectively (table 1). The time to peak parasitaemia and the mean duration of parasite increase were increased but not significantly for mice probed by mosquitoes, while the rate of parasite increase was increased only in mice probed by 50 mosquitoes per day (table 2).
Table 1.
Time until 50 and 80% Plasmodium chabaudi cumulative parasitaemia and gametocyte rate in mice probed by Anopheles stephensi.
| time (days) | ||||
|---|---|---|---|---|
| control | 25 mosquitoes | 50 mosquitoes | ||
| cumulative parasitaemia | 50%a | 9.26 (8.30–0.33) | 7.62 (6.62–8.76) | 8.06 (7.03–9.25) |
| 80%a | 11.80 (10.58–13.17) | 10.23 (8.89–11.77) | 10.81 (9.43–12.41) | |
| cumulative gametocyte rate | 50%a | 11.84 (10.83–13.11) | 9.37 (8.19–10.70) | 9.84 (8.68–11.15) |
| 80%a | 14.96 (13.46–16.63) | 12.52 (10.95–14.31) | 12.81 (11.30–14.52) | |
Times to 50% and 80% maximum of the cumulative parasitaemia and proportion of infected cells at the gametocyte stage were calculated using probit analysis of the cumulative curves.
Table 2.
Analysis of parasite and gametocyte growth traits of Plasmodium chabaudi in mice probed by Anopheles stephensi. (Growth characteristics were analysed according to Eisen & Schall (2000).)
| control | 25 mosquitoes | 50 mosquitoes | |
|---|---|---|---|
| meana time to first parasite (days) | 1.33±0.33 | 0.67±0.33 | 1.33±0.33 |
| mean time to peak parasitaemia (days) | 10.00±1.00 | 8.33±0.33 | 7.67±0.67 |
| mean duration of parasite increase (days) | 8.67±1.20 | 6.67±0.33 | 6.00±1.00 |
| mean rate of parasite increase (percentage per day) | 1.35b±0.26 | 1.55±0.23 | 2.39b±0.34 |
| mean time to first gametocyte (days) | 6.00a,b±0.58 | 3.33a±0.33 | 3.33b±0.33 |
| mean time to peak gametocyte rate (days) | 12.33a,b±0.33 | 10.00a±0.00 | 10.33b±0.33 |
| mean duration of gametocyte increase (days) | 6.33±0.33 | 6.67±0.33 | 7.00±0.58 |
| mean rate of gametocyte increase (proportion per day) | 1.41±0.21 | 1.10±0.31 | 1.70±0.21 |
Means=arithmetic mean±s.d.
Numbers with the same letters in the same rows are significantly different from one another, Student's t-test, p<0.05.
Gametocytes were detected and peaked 2 days earlier at 3 days p.i. in mice probed by mosquitoes (figure 1e–h), compared with controls. Peak gametocyte rates occurred on day 10 in the experimental groups, 2 days earlier than in controls. A higher proportion of mature gametocytes in the parasite population (12.1%) was seen in mice probed by 50 mosquitoes at the peak on day 10, and the cumulative proportion of gametocytes produced increased in direct relation to the number of probing mosquitoes (control=32.7%; 25 mosquitoes=37.1%; 50 mosquitoes=39.9%; Z=−3.059, p=0.002 for both groups, Wilcoxon signed rank test; figure 2b). The estimated time until 50% and 80% of the maximum cumulative mean gametocyte rate was advanced by 2–2.47 days and 2.15–2.44 days, respectively (table 1). Mean times to first gametocyte and peak gametocyte rate were advanced significantly by approximately 3 days in both experimental groups (table 2).
The estimated rate of gametocytogenesis varied over the infection period. Two peaks were evident, associated with early infection around day 4 and later infection around days 8–10 (figure 1d,i). For mice probed by mosquitoes, the second peak was shifted forward by 1–2 days and the cumulative estimated rate of gametocytogenesis was increased in both experimental groups (figure 2c).
4. Discussion
Vector saliva can enhance parasite transmission from vector to vertebrate host. Serially passaged Leishmania are more likely to establish in mice in the presence of sandfly saliva (Titus & Ribeiro 1988) and transmission of subpatent viral infections between ticks on the same host results from salivary-mediated co-feeding transmission (Jones et al. 1992). In Plasmodium, while experiments suggest that sporozoites from the salivary glands are more infectious than those from the oocyst (Touray et al. 1992), differential infectivity may be owing more to sporozoite maturation than interaction with salivary factors (Al-Olayan et al. 2002). Vector feeding can also affect pathogens within the mouse host; New Jersey virus titres increased in mice fed upon by mosquitoes and LaCrosse virus infections were of longer duration, higher titre and more pathogenic when delivered by mosquito bite rather than injection (Osorio et al. 1996; Limesand et al. 2000). Here, we similarly demonstrate that in-host dynamics of a more complex organism, Plasmodium, can be modulated by mosquitoes.
It is interesting that both asexual growth and gametocytogenesis are modified by mosquito probing. Both of these will of course ultimately increase gametocyte numbers. The reasons for increased growth are unknown. In the same parasite, gametocyte formation was enhanced following drug-induced increases in reticulocytes (Gautret et al. 1996) and perhaps a similar haemopoiesis is stimulated by mosquito salivary factors. We have no evidence from this preliminary study whether reticulocyte function is affected in any way. We also cannot rule out that the limited amount of blood removal that occurred during these experiments could also be a determining factor in the altered parasite growth.
The shift in rate of gametocytogenesis represents an additional response to the presence of vector mosquitoes, and identifies a hitherto unknown plasticity in developmental modulation of the Plasmodium life cycle. Gametocytes are subject to finely tuned density-dependent processes, such that maximum transmissibility to the mosquito may not correlate with the maximum gametocytaemia (Fleck et al. 1994). The forward shift of gametocyte production may counteract down-regulation of transmission at high parasite densities later in the infection by increasing gametocyte numbers before non-specific inhibitory factors are stimulated. The logical next step in these studies will be to examine infectivity of gametocytes under different mosquito exposure conditions. A similar pattern of increased gametocyte densities in response to mosquito exposure and feeding has been observed for Plasmodium praecox-infecting sparrows (Missiroli 1939), but neither the numbers of mosquitoes used nor the prevention of blood removal were controlled.
Although the mechanisms underlying the parasite response are unknown, the results raise some interesting epidemiological considerations. Some individuals are exposed to greater numbers of mosquito bites (Charlwood et al. 1995) and such individuals will receive and transmit disproportionately high numbers of parasites (Woolhouse et al. 1997), thereby driving heterogeneity in parasite populations and maintaining parasite populations at low transmission intensities (Dye & Hasibender 1986). Detailed examination of gametocyte-to-mosquito ratios could provide information essential to our understanding of transmission dynamics (Killeen et al. 2000). While 25 or 50 per mouse represents a high number of mosquitoes relative to the body size of a human, mosquito biting rates can reach several hundred per person per night (Charlwood et al. 1995) and there is clearly potential for feeding by uninfected mosquitoes to influence parasite infections within the human host.
Acknowledgments
The authors thank J. P. Webster (University of Oxford), D. Champagne (University of Georgia), J. Valenzuela, J. M. C. Ribeiro (both National Institutes of Health) and R. Paul (Institut Pasteur), and A. Read (University of Edinburgh) for invaluable feedback, and D. Walliker (University of Edinburgh) for parasites.
References
- Almeida A.P.G, Billingsley P.F. Induced immunity against the mosquito Anopheles stephensi Liston (Diptera: Culicidae): effects on mosquito survival and fecundity. Int. J. Parasitol. 1998;28:1721–1731. doi: 10.1016/s0020-7519(98)00149-0. [DOI] [PubMed] [Google Scholar]
- Al-Olayan E.M, Beetsma A.L, Butcher G.A, Sinden R.E, Hurd H. Complete development of mosquito phases of the malaria parasite in vitro. Science. 2002;295:677–679. doi: 10.1126/science.1067159. [DOI] [PubMed] [Google Scholar]
- Buckling A, Crooks L, Read A. Plasmodium chabaudi: effect of antimalarial drugs on gametocytogensis. Exp. Parasitol. 1999;93:45–54. doi: 10.1006/expr.1999.4429. [DOI] [PubMed] [Google Scholar]
- Carter R, Graves P.M. Gametocytes. In: Wernsdorfer W.H, McGregor I, editors. Malaria: principles and practice of malariology. Churchill Livingstone; Edinburgh: 1988. pp. 253–305. [Google Scholar]
- Charlwood J.D, Kihonda J, Sama S, Billingsley P.F, Hadji H, Verhave J.P, Lyimo E, Luttikhuizen P.C, Smith T. The rise and fall of Anopheles arabiensis (Diptera: Culicidae) in a Tanzanian village. Bull. Entomol. Res. 1995;85:37–44. [Google Scholar]
- Dye C, Hasibender G. Population dynamics of mosquito-borne disease—effects of flies which bite some people more frequently than others. Trans. R. Soc. Trop. Med. Parasitol. 1986;80:69–77. doi: 10.1016/0035-9203(86)90199-9. [DOI] [PubMed] [Google Scholar]
- Edwards J.F, Higgs S, Beaty B. Mosquito feeding-induced enhancement of Cache Valley Virus Bunyaviridae infection in mice. J. Med. Entomol. 1998;35:261–265. doi: 10.1093/jmedent/35.3.261. [DOI] [PubMed] [Google Scholar]
- Eisen R.J, Schall J.J. Life history of a malaria parasite (Plasmodium mexicanum): independent traits and basis for variation. Proc. R. Soc. B. 2000;267:793–799. doi: 10.1098/rspb.2000.1073. doi:10.1098/rspb.2000.2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck S.L, Butcher G.A, Sinden R.E. Plasmodium berghei—serum-mediated inhibition of infectivity of infected mice to Anopheles stephensi mosquitos. Exp. Parasitol. 1994;78:20–27. doi: 10.1006/expr.1994.1002. [DOI] [PubMed] [Google Scholar]
- Gautret P, Miltgen F, Gantier J.-C, Chabaud A.G, Landau I. Enhanced gametocyte formation by Plasmodium chaubuadi in immature erythrocytes: pattern of production, sequestration, and infectivity to mosquitoes. J. Parasitol. 1996;82:900–906. [PubMed] [Google Scholar]
- Jones L.D, Matthewson M, Nuttall P.A. Saliva-activated transmission SAT of Thogoto virus—dynamics of SAT factor activity in the salivary glands of Rhipicephalus appendiculatus, Amblyomma variegatum and Boophilus microplus ticks. Exp. Appl. Acarol. 1992;13:241–248. doi: 10.1007/BF01195081. [DOI] [PubMed] [Google Scholar]
- Killeen G.F, McKenzie F.E, Foy B.D, Billingsley P.F, Beier J.C. Simplified model for predicting entomological inoculation rates from vector population characteristics and infectious reservoir size. Am. J. Trop. Med. Hyg. 2000;62:535–544. doi: 10.4269/ajtmh.2000.62.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limesand K.H, Higgs S, Pearson L.D, Beaty B.J. Potentiation of vesicular stomatitis New Jersey virus infection in mice by mosquito saliva. Parasite Immunol. 2000;22:461–467. doi: 10.1046/j.1365-3024.2000.00326.x. [DOI] [PubMed] [Google Scholar]
- Missiroli A. Modificazioni periodiche del numero de gametociti do Plasmodium praecox. Riv. Parassitol. 1939;3:279–285. [Google Scholar]
- Osorio J.E, Godsey M.S, Defoliart G.R, Yuill T.M. LaCrosse viremias in white tailed deer and chipmunks exposed by injection or mosquito bite. Am. J. Trop. Med. Hyg. 1996;54:338–342. doi: 10.4269/ajtmh.1996.54.338. [DOI] [PubMed] [Google Scholar]
- Ribeiro J.M.C. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect. Agents Dis. 1995;4:143–152. [PubMed] [Google Scholar]
- Ribeiro J.M.C, Sarkis J.J.F, Rossignol P.A, Spielman A. Salivary apyrase of Aedes aegypti: characterization and secretory fate. Comput. Biochem. Physiol. B. 1984;79:81–86. doi: 10.1016/0305-0491(84)90081-6. [DOI] [PubMed] [Google Scholar]
- Ribeiro J.M.C, Nussenzweig R.H, Tortorella G. Salivary vasodilators of Aedes triseriatus and Anopheles gambiae (Diptera: Culicidae) J. Med. Entomol. 1994;31:747–753. doi: 10.1093/jmedent/31.5.747. [DOI] [PubMed] [Google Scholar]
- Sinden R.E. Sexual development of malarial parasites. Adv. Parasitol. 1983;22:153–216. doi: 10.1016/s0065-308x(08)60462-5. [DOI] [PubMed] [Google Scholar]
- Titus R.G, Ribeiro J.M.C. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- Touray M.G, Warburg A, Laughinghouse A, Krettli A.U, Miller L.H. Developmentally regulated infectivity of malaria sporozoites for mosquito salivary glands and the vertebrate host. J. Exp. Med. 1992;175:1607–1612. doi: 10.1084/jem.175.6.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waidhet-Kouadio P, Yuda M, Katsushito A, Chinzei Y. Purification and characterisation of a thrombin inhibitor from the salivary glands of a malaria mosquito, Anopheles stephensi. Biochim. Biophys. Acta. 1998;1381:227–233. doi: 10.1016/s0304-4165(98)00026-9. [DOI] [PubMed] [Google Scholar]
- Wikel S.K, Ramachandra R.N, Bergman D.K. Tick-induced modulation of the host immune response. Int. J. Parasitol. 1994;24:59–66. doi: 10.1016/0020-7519(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Woolhouse M.E.J, et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl Acad. Sci. USA. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidner N, Higgs S, Happ C.M, Beaty B, Miller B.R. Mosquito feeding modulates Th1 and Th2 cytokines in flavirus susceptible mice: an effect mimicked by injection of sialokinins, but not demonstrated in flavivirus resistant mice. Parasite Immunol. 1999;21:35–44. doi: 10.1046/j.1365-3024.1999.00199.x. [DOI] [PubMed] [Google Scholar]