Abstract
Previous work has shown how, in the case of cytotoxic T-lymphocyte (CTL) responses to persistent viral infections, pathology may arise as a consequence of cell destruction directly by the virus or indirectly due to the CTL response, leading to maximum pathology at intermediate efficacy of the immune response. We expand these studies to consider pathology arising during acute infections with intracellular pathogens controlled by the CTL response. We show that, in contrast to persistent infections, pathology during acute infections is minimized with increasing efficacy of the immune response. The implications of these results for vaccination are discussed.
Keywords: pathology, intracellular pathogens, cytotoxic T-lymphocyte response, mathematical model
1. Introduction
There is a large and rapidly growing body of literature on the bacterial and viral genes required for the expression of virulence factors of pathogens (Finlay & Falkow 1997). Advances in a number of areas, including the sequencing of the genomes of pathogens using molecular and cellular methods, have resulted in remarkable progress in the identification and characterization of such virulence genes. These studies have shown how the vast array of virulence factors can be broadly classified into categories which include toxins, adhesins, invasins, molecules that prevent intracellular bacteria being degraded, molecules for evasion of the immune response, molecules for acquisition of host resources, molecules involved in gene regulation (of other virulence determinants) and molecules belonging to the machinery required for the secretion of virulence factors. This largely qualitative collection of taxonomic data is a prerequisite for understanding how pathogens harm their hosts. The next step is the development of a quantitative framework of the interaction between the replicating pathogen and the host's immune response. The necessity for this step can be seen from the observation that even when hosts die following infection, the total biomass of the pathogen within is usually small—the causes of host's death are likely to be found in the interaction between the pathogen population within the host and the host's response (Levin & Antia 2001).
Pathology may arise during an infection in a variety of ways. In the case of infections with intracellular pathogens (such as viruses and intracellular bacteria), pathology may arise because of the direct killing of infected cells by the pathogen or the destruction of infected cells by the cytotoxic T-Lymphocyte (CTL) response. Krakauer & Nowak (1999) proposed that pathology during viral infections can be measured as the reduction in the total number of uninfected and virus-infected cells as compared with an uninfected host. In the case of persistent infections, their studies suggested that pathology arising following infections with weakly cytolytic viruses should be maximal when the CTL response has intermediate efficacy (Krakauer & Nowak 1999; Wodarz & Krakauer 2000). In this paper, we extend these studies and consider the contribution of the CTL response to intracellular pathogens in generating pathology during acute infections.
2. Results and discussion
We focus our analysis on acute infections (defined as infections of a short duration where the pathogen is cleared before or shortly after the peak of the immune response). To model the dynamic of intracellular pathogens (such as intracellular bacteria and viruses) and the CTL response, we track the populations of the free pathogen, V, uninfected target, T, and pathogen-infected, I, cells, and the CTL response, Z:
| (2.1) |
| (2.2) |
| (2.3) |
| (2.4) |
where T0 is the number of uninfected cells prior to infection, d is the turnover rate of uninfected cells, β is per capita infection rate of uninfected cells by the free pathogen, d+α is the death rate of pathogen-infected cells; h is the killing rate of pathogen-infected cells by the CTL response, p is the production rate of the free pathogen by infected cells, c is the death rate of the free pathogen; s is the maximum proliferation rate of the immune cells and k is the pathogen-infected cell density at which CTLs proliferate at half-maximum rate.
Following Antia et al. (1994) and De Boer et al. (2001), we have employed a simple term for the dynamics of the immune response expanding in a pathogen-dependent fashion early during the response and being independent of the pathogen at later time points. Equation (2.4) describes the expansion phase of the CTL response during acute infections; it generates robust dynamics for the expansion phase, and changing this term to encompass a discrete proliferation phase (De Boer et al. 2001), or to a programmed proliferation model (Antia et al. 2003), does not generate qualitatively different dynamics or alter the conclusions arising from the analysis in this paper (see the Electronic Appendix). Finally, as we consider acute infections which result in the clearance of the pathogen prior to the peak of the response, we ignore the subsequent contraction and memory phases of the CTL response (Ahmed & Gray 1996). Note that since our model is deterministic, the pathogen never goes to extinction. We define clearance of the pathogen when the pathogen density falls below 1, and since the CTL response does not contract, pathogen clearance is always assured.
Extending the study by Krakauer & Nowak (1999), we define pathology P(t) at time t in terms of the reduction in the number of uninfected T(t) and infected I(t) host cells by the equation
| (2.5) |
where T0 is the initial number of uninfected cells prior to infection. Importantly, infection of host cells even by non-cytolytic pathogens (with α=0) may nevertheless affect regular functions performed by such cells (De La Torre & Oldstone 1992; Buchmeier & Zajac 1998). The parameter f thus accounts for the functionality of the pathogen-infected cells (f=1 when infected cells are fully functional as uninfected cells and f=0 when they are non-functional). Note that in previous studies of persistent infections, functionality was fixed at one (Krakauer & Nowak 1999).
Pathology during acute infections could be defined in a number of ways including: (i) the maximum value that pathology reaches during the infection, , where Δ is the duration of infection; and (ii) the average pathology over the infection period, . As we obtained comparable results for both these measures of pathology, we illustrate our results using the maximum pathology reached during the infection.
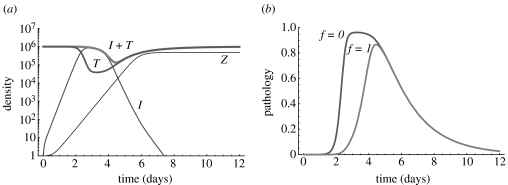
Figure 1a illustrates the dynamics of the host target and pathogen-infected cells and the CTL response. The model captures the basic dynamics of the pathogen as well as the expansion phase of the immune response. In figure 1b we plot the level of pathology observed during the course of infection calculated in accord with equation (2.5) for functional (f=1) and non-functional (f=0) infected cells. Note that, as may be expected, pathology is generally higher when infected cells are non-functional. In the Electronic Appendix we analyse how pathology depends on pathogen and host factors. In particular, we show that (i) pathology increases with the increasing pathogen infectivity β; (ii) pathology decreases with the increasing pathogen-induced death rate α; and (iii) pathology decreases with the increasing rate of target cell turnover d.
Figure 1.
Dynamics of the pathogen and the CTL response (a) and pathology (b) during the course of acute infection. Parameters: T(0)=T0=106, I(0)=0, V(0)=103, Z(0)=Z0=1, d=0.5, β=5×10−8, α=0.1, p=103, c=3, k=102, s=2.5, h=10−3. We do not plot the dynamics of the free pathogen because of its high death rate V(t)≈p/cI(t). Maximum pathology during the infection is P*≈0.96 (f=0) and P*≈0.87 (f=1).
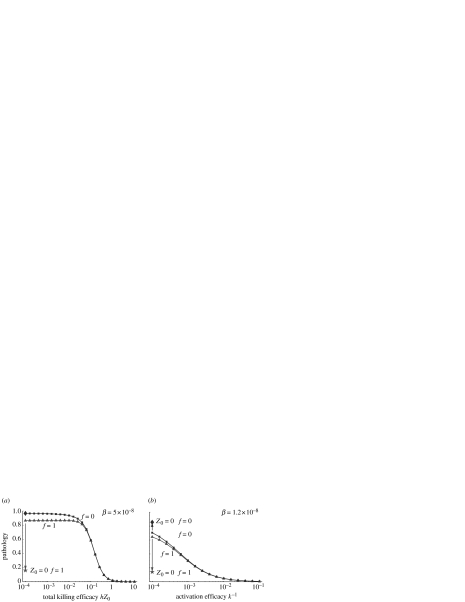
In figure 2 we plot how pathology depends on the properties of the host's immune response. These include the killing efficacy, h, activation efficacy k−1 and the initial CTL number Z0. As we show in the Electronic Appendix, pathology depends on the product hZ0; consequently, we discuss the effects of changing hZ0 and k−1. Increases in these two quantities are associated with stronger immune responses.
Figure 2.
Pathology during acute infections when the total killing efficacy hZ0 (a) or activation efficacy k−1 (b) of the CTL response is changed. Pathology is shown for f=1 (filled triangle, infected cells are fully functional) and f=0 (filled square, infected cells are non-functional). Other parameters are the same as in figure 1 except β=1.2×10−8 in panel b (for β=5×10−8 there is almost no change in pathology with the efficacy k−1). Pathology level in the absence of the immune response (i.e. at Z0=0) is shown for f=1 (filled star) and f=0 (filled diamond). The plots are qualitatively similar for non-cytolytic pathogens (with α=0) except that there is not pathology in the absence of the immune response at f=1.
Pathology generally decreases with the increasing product hZ0 (figure 2a). This reduction in pathology is most pronounced after hZ0 exceeds a critical threshold. This is because there is a time delay between the start of the infection and the time when the infection is controlled by the immune response. At low hZ0 this delay is long, and the pathogen and host target cells reach steady‐state levels before the immune response is able to control the infection. When hZ0 is larger, this delay is short, and the pathogen is controlled and cleared before it reaches this steady state. In this parameter regime faster clearance results in lower pathology. Pathology generally decreases with increasing activation efficacy k−1 (figure 2b), although changes for some parameter values may be much smaller than in the previous case. The reasons for the decrease in pathology with higher activation efficacy are similar to those given above.
The results for pathology during acute infections differ from those of the earlier studies of persistent infections when the efficacy of the immune response is low: during persistent infections there is very little pathology, whereas during acute infections there is high pathology. During a persistent infection with a weakly cytopathic/cytolytic pathogen, having a weak CTL response results in a low level of killing of infected cells (by CTLs or the pathogen) and consequently, in low pathology. During an acute infection, having a weak CTL response results in a large time delay before the response increases to a level sufficient to clear the pathogen. During this time most host target cells become infected and are subsequently killed by CTLs, resulting in high transient pathology. In light of these differences, it is interesting to note that if the CTL response could not be generated during an acute infection,1 pathology would become much lower for non-cytopathic pathogens (figure 2).
The predictions of models of acute and persistent infections have important implications for vaccination. Vaccination generally results in a large increase in the number of pathogen-specific cells (i.e. Z0), and a relatively modest change in the characteristics of these cells (i.e. k and h). Our model suggests that such vaccination will result in a decrease in pathology. By contrast, for persistent infections described by the earlier models, the steady state reached is independent of Z0 and is only dependent on the characteristics of pathogen-specific cells such as k and h. This suggests that the effect of vaccination against persistent infections (if it does not result in the clearance of such infections) will not be dependent on the magnitude of the response generated by vaccination, but on the changes in the efficacy of the cells generated by vaccination in comparison with those generated by natural infection.
We now bring the model into contact with experimental observations. The model makes a simple, robust prediction for the case of acute infections: increasing the efficacy of immune responses will result in decreasing pathology. This increase in efficacy could be experimentally obtained by increasing the number of pathogen-specific cells (either by immunization or adoptive transfer of such cells from immunized or transgenic hosts). Subsequent challenge of these mice should result in decreased pathology in mice with a higher number of pathogen-specific cells. This prediction is consistent with the data in a study by Ehl et al. (1998) for LCMV infections of mice. For the case of acute infections, the paper showed a decrease in the aspartate aminotransferase level (which corresponds to pathology) with the increasing number of adoptively transferred LCMV-specific CD8 T cells. However, more such studies are needed to examine the generality of this result for different initial doses as well as for infections with other pathogens.
Our study also suggests that care should be taken in interpreting whether the existing experiments on immunopathology are consistent with the predictions of models. In particular, care should be taken to compare how pathology changes with the efficacy of the immune response separately for acute and persistent infections, as the predictions are different for these two cases. We hope that this paper will stimulate the continued interaction between theoretical and experimental studies required for understanding immunopathology.
Acknowledgments
R.A. acknowledges support from the NIH. We thank Roland Regoes for helpful discussions.
Footnotes
On leave of absence from: Institute of Biophysics, Russian Academy of Sciences, Akademgorodok, Krasnoyarsk 660036, Russia.
This can be achieved by infecting, for example, severe combined immunodeficient (SCID) mice lacking T and B lymphocytes.
Supplementary Material
References
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- Antia R, Levin B.R, May R.M. Within-host population dynamics and the evolution and maintenance of microparasite virulence. Am. Nat. 1994;144:457–472. [Google Scholar]
- Antia R, Bergstrom C.T, Pilyugin S.S, Kaech S.M, Ahmed R. Models of CD8+ responses. 1. What is the antigen-independent proliferation program. J. Theor. Biol. 2003;221:585–598. doi: 10.1006/jtbi.2003.3208. [DOI] [PubMed] [Google Scholar]
- Buchmeier M, Zajac A. Lymphocytic choriomeningitis virus. In: Ahmed R, Chen I, editors. Persistent viral infections. John Wiley & Sons; New York: 1998. pp. 575–605. [Google Scholar]
- De Boer R.J, Oprea M, Antia R, Murali-Krishna K, Ahmed R, Perelson A.S. Recruitment times, proliferation, and apoptosis rates during the CD8(+) T-cell response to lymphocytic choriomeningitis virus. J. Virol. 2001;75:10 663–10 669. doi: 10.1128/JVI.75.22.10663-10669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Torre J, Oldstone M. Selective disruption of growth hormone transcription machinery by viral infection. Proc. Natl Acad. Sci. USA. 1992;89:9939–9943. doi: 10.1073/pnas.89.20.9939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehl S, Klenerman P, Zinkernagel R.M, Bocharov G. The impact of variation in the number of CD8(+) T-cell precursors on the outcome of virus infection. Cell Immunol. 1998;189:67–73. doi: 10.1006/cimm.1998.1344. [DOI] [PubMed] [Google Scholar]
- Finlay B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer D.C, Nowak M. T-cell induced pathogenesis in HIV: bystander effects and latent infection. Proc. R. Soc. B. 1999;266:1069–1075. doi: 10.1098/rspb.1999.0745. doi:10.1098/rsbl.2004.0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B.R, Antia R. Why we don't get sick: the within-host population dynamics of bacterial infections. Science. 2001;292:1112–1115. doi: 10.1126/science.1058879. [DOI] [PubMed] [Google Scholar]
- Wodarz D, Krakauer D.C. Defining CTL-induced pathology: implications for HIV. Virology. 2000;274:94–104. doi: 10.1006/viro.2000.0399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.