Abstract
Specialization to a particular environment is one of the main factors used to explain species distributions. Antarctic fishes are often cited as a classic example to illustrate the specialization process and are regarded as the archetypal stenotherms. Here we show that the Antarctic fish Pagothenia borchgrevinki has retained the capacity to compensate for chronic temperature change. By displaying astounding plasticity in cardiovascular response and metabolic control, the fishes maintained locomotory performance at elevated temperatures. Our falsification of the specialization paradigm indicates that the effect of climate change on species distribution and extinction may be overestimated by current models of global warming.
Keywords: cardiac scope, climate change, critical swimming speed, metabolism, plasticity
1. Introduction
In evolutionary theory, the principle of allocation (Levins 1968) predicts that animals living in environments with stable temperatures (stenotherms) should specialize to optimize performance at the prevalent narrow environmental temperature range. In contrast, species living in variable environments (eurytherms) can adjust or acclimatise physiologically to different temperature regimes (Wilson & Franklin 2002). Thermal specialization that optimizes performance at a particular temperature is thought to be accompanied by a trade-off so that the ability to respond to changing environments is lost (Huey & Hertz 1984; Somero et al. 1996). Inherent in this argument is the notion that the potential to respond to changing environments incurs a cost that is selected against. However, the prerequisite for a ‘generalist’ phenotype may simply require the genetic potential to upregulate or downregulate particular genes, or to express different isozymes of a rate-limiting enzyme. The possession of particular genes and the apparatus to control their expression may not be costly in itself.
Ectotherms in temporally or spatially varying environments often respond by compensating for thermal effects on performance traits such as muscle performance and locomotion (Johnston & Temple 2002) and metabolic performance, especially metabolic enzyme activity (Guderley & St Pierre 2002; Seebacher et al. 2003). Compensatory responses may occur phenotypically within individuals, particularly when thermal conditions fluctuate over weeks or months (Guderley 2004). For example, rainbow trout (Oncorhynchus mykiss) that naturally occur in strongly seasonal environments in northern America display marked phenotypic changes in metabolic performance in response to seasonally varying or artificially altered thermal conditions (St Pierre et al. 1998; Bouchard & Guderley 2003). On an evolutionary time-scale, populations or species may become genotypically distinct in response to differential selection pressures experienced at different climates (Crawford et al. 1999; West et al. 1999). A classic example of such adaptation is the Atlantic killifish (Fundulus spp.). Populations or closely related species occurring at different latitudes display genetically fixed differences in metabolic enzyme activities that correspond to the prevailing environmental temperatures (Crawford & Powers 1989; Pierce & Crawford 1997). In general, species living in relatively stable climates are considered to be adapted or specialized to their prevailing thermal conditions.
Antarctic fishes are regarded as the ultimate thermal specialist as a result of the very stable temperatures of Antarctic waters during the Quaternary, and selection pressures favouring specialization are thought to have been exacerbated by the extremely cold conditions experienced by Antarctic fishes during this period (Somero & DeVries 1967). However, the Antarctic climate has been characterized by pronounced fluctuations since the Palaeocene, and the present Antarctic climate is probably the coldest in the Tertiary/Quaternary periods (Clarke & Johnston 1996). Present water temperatures experienced by the Antarctic notothenioid fishes Pagothenia borchgrevinki range from −0.5 to −1.8 °C, and the fishes are thought to have evolved a performance peak in this range with a concomitant narrowing of their thermal tolerance range (Somero & DeVries 1967; Wilson et al. 2002; Johnston 2003). The fact that notothenioid fishes evolved in a highly fluctuating climate, and the questionable cost of thermal specialization, led us to test the hypothesis that Antarctic notothenioid fishes have in fact lost their ability to respond to environmental change.
2. Materials and methods
All experimentation was conducted at Scott Base, Antarctica. Fishes were caught in the wild and transferred into laboratory tanks, kept at either environmental water temperatures −1 °C (cold acclimation) or 4 °C (warm acclimation). After 4–5 weeks of acclimation, swimming performance was measured as the maximum critical swimming speed (Ucrit) in a purpose-designed swimming flume (Brett 1964). Ucrit was determined at increasing water temperatures: −1, 2, 4, 6 °C for cold-acclimated fishes (n=10), and also at 8 and 10 °C for warm-acclimated fishes (n=10). The same fishes were swum at the different temperatures with a minimum of 48 h between trials. None of the experimental fishes was fed during their period of captivity. As a control for habituation to the experimental situation and the effect of food deprivation on swimming performance, we repeatedly swam a third group of freshly caught fishes at the same temperature (−1 °C), and we swam the −1 °C-acclimated fishes again at −1 °C after completing experimental swimming trials. There was no effect of repeated swimming on swimming performance (repeated measures ANOVA, F2,29=0.50, p=0.62). Swimming performance of freshly caught fishes was not significantly different from fishes acclimated to −1 °C either at the end of the acclimation period and before swimming trials, or after swimming trials (F2,29=0.56, p=0.58), indicating that food deprivation did not affect swimming performance during the acclimation period. All experimental fishes were sacrificed at the conclusion of swimming trials and pectoral muscle samples collected. (Note that P. borchgrevinki are labriform swimmers relying primarily on pectoral fins for sustained locomotion.)
Cardiac scope was determined in different groups of P. borchgrevinki acclimated either to −1 °C (n=8) or 4 °C (n=8). Fishes were anaesthetised in MS222 (1 : 5000) and a single crystal Doppler flow probe was positioned around the ventral aorta. After 24 h recovery, cardiac output was measured at rest and after 5 min intensive exercise (to exhaustion) using a Doppler flow meter (Iowa University model 545C-4) connected to a Powerlab (ADInstruments, Australia). Fishes were tested in random order at −1, 2, 4, 6 and 8 °C, allowing 4 h recovery between temperatures. At completion of the test temperatures, fishes were euthanased with an overdose of MS222, a blood sample was taken and the bulbous arteriosus was cannulated. Blood was pumped (peristaltic pump) at known flow rates into the cannula and through the ventral aorta to calibrate the Doppler flow probe in situ (Axelsson et al. 1992). Factorial cardiac scope was determined by dividing maximal cardiac output by resting cardiac output.
Muscle myofibrillar ATPase activity, an indicator of muscular energy use, was measured using the EnzCheck Phosphate Assay Kit (Molecular Probes, The Netherlands) after preparing muscle tissue according to published protocols (Ball & Johnston 1996). Assays were conducted in duplicate in a temperature-controlled spectrophotometer (Biochrom, UK, Ultrospec 2100 Pro). Total protein concentration was determined by the bicinchoninic acid method (Pierce, USA). Assays of metabolic enzyme activities (lactate dehydrogenase, phosphofructokinase, citrate synthase and cytochrome c oxidase (CCO)) were conducted in the same spectrophotometer according to published methods (Thibault et al. 1997).
Data were analysed by ANOVAs with repeated measures when the same animals or tissues were assayed at different temperatures. Significant results in the ANOVAs were further analysed by Tukey post-hoc tests.
3. Results
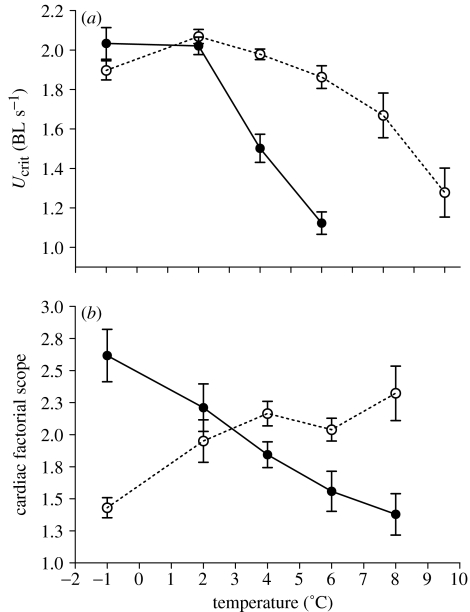
After acclimation to +4.0 °C, P. borchgrevinki significantly (F1,18=25.76, p<0.0001) shifted its temperature of maximum critical swimming speed (Ucrit) upwards by 5 °C (figure 1a). There were no significant differences in sustained swimming performance between the ‘cold’ group at −1 °C and the ‘warm’ group at +4 °C (paired t-test t18=−1.01, p=0.32).
Figure 1.
(a) Sustained aerobic swimming performance (maximal critical swimming speed, Ucrit, mean±s.e.) and (b) factorial cardiac scope (mean±s.e.) of Pagothenia borchgrevincki acclimated to natural temperatures (−1 °C; closed circles, solid lines) and to 4 °C (open circles, broken lines) for 4–5 weeks. BL, body length.
Acute increases in temperature caused a significant decline in cardiac scope in −1 °C-acclimated fishes (figure 1b), and potential for activity was severely limited (scope of 1.4) at 8 °C (F7,4=12.9, p<0.001). In an outstanding example of cardiovascular plasticity, +4 °C-acclimated fishes reversed this pattern and had a lower cardiac scope at −1 °C that increased with increasing temperature (figure 1b; F7,4=5.41, p<0.01). After acclimation to 4 °C, cardiac scope at 4 °C was not significantly different from cardiac scope of the control group at −1 °C (paired t-test; t16=2.0, p>0.05).
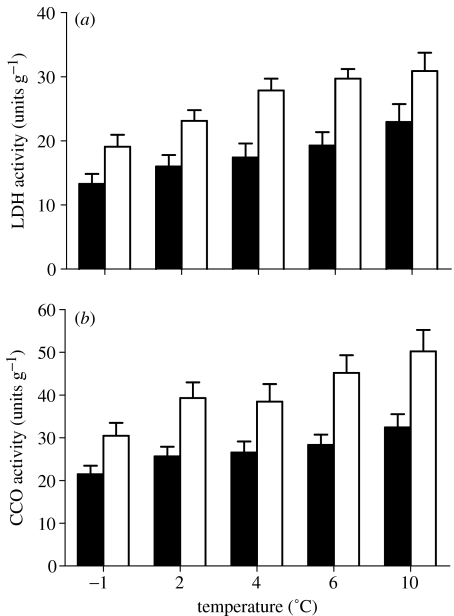
Muscle ATPase activity increased significantly with increasing temperature in both groups (F1,28=8.04, p<0.01), but there were no differences between acclimation groups (F1,28=1.86, p=0.18; table 1). In contrast, the activity of enzymes that control the main energy (ATP)-producing anaerobic and aerobic metabolic processes, lactate dehydrogenase (F1,18=11.78, p<0.01) and CCO (F1,18=9.99, p<0.01), respectively, were significantly increased in the warm-acclimated group (figure 2), thereby compensating for the additional energy demand at chronically increased temperatures. The activities of the glycolytic and Krebs Cycle enzymes, phosphofructokinase and citrate synthase, respectively, were not significantly different between acclimation groups (in both cases F1,18<2.19, p>0.16; data not shown).
Table 1.
Myofibrillar ATPase activity (mmol of inorganic phosphate produced min−1 mg protein−1; means±s.e.) in pectoral muscle of cold (−1 °C) and warm (+4 °C) acclimated Pagothenia borchgrevincki
| test temperature (°C) | cold-acclimated (−1 °C) | warm-acclimated (4 °C) |
|---|---|---|
| −1 | 3.17±1.03 | 1.83±0.57 |
| 4 | 4.48±1.14 | 4.03±0.87 |
Figure 2.
Activities of metabolic enzymes (units g wet tissue−1; mean+s.e.) primarily responsible for cellular energy (ATP) production, (a) lactate dehydrogenase (LDH) and (b) cytochrome c oxidase (CCO) increased significantly in the warm acclimated group of Pagothenia borchgrevincki (+4 °C, open bars) compared with the naturally acclimated fishes (−1 °C, closed bars).
4. Discussion
The shift in performance of P. borchgrevinki is reminiscent of textbook examples of thermal acclimation, as observed in typical temperate generalists living in seasonally variable environments. Living at higher temperatures incurs a greater energetic cost resulting from the increase in the rate of biochemical reactions with increasing temperature. Rather than downregulating energy-consuming processes, such as muscle function, P. borchgrevinki upregulated energy-producing metabolic processes, presumably to meet the higher maintenance costs that result from living at higher temperatures. Interestingly, the activities of glycolytic and Krebs Cycle control enzymes did not differ significantly between acclimation groups, and control of aerobic metabolic potential in response to environmental change appears to occur primarily during oxidative phosphorylation.
Clearly, P. borchgrevinki has not lost the capacity to respond to environmental change, and the species provides a definite falsification of the notion that animals living at thermal extremes must also trade-off their capacity to respond positively to environmental change (Somero & DeVries 1967; Somero 1991; Bennett & Lenski 1999). The fact that the acclimation response occurred at several levels of organization indicates that an obligatory trade-off in the loss of the ability to respond to environmental change does not exist, and that traits permitting life at extremely cold temperatures (DeVries 1984; Somero 1991) can evolve in addition to the capacity to respond positively to fluctuating environments.
Specialization to particular environments will occur only if evolutionary processes proceed at a faster rate than environmental change. Whether there exists an optimal response with respect to (reversible) phenotypic plasticity relative to genotypic (adaptive) change is debatable (Scheiner 1993; Bennett & Lenski 1999), and the case of P. borchgrevinki highlights the fact that the theory does not necessarily fit the observations. The theoretical position of species along the specialist/generalist trajectory needs to be verified by observation. A practical implication of these data lies in management of biodiversity in relation to global climate change. Anthropogenic climate warming is recognized as a major threat to global biodiversity (Thomas et al. 2004), and predictions of the effect of climate change on species distribution will be essential to manage the process of global warming. The example of P. borchgrevincki demonstrates that simple superimposition of climate model predictions (‘climate envelopes’) on present species distribution maps may overestimate the impact of climate change on species extinction. The phenotypic plasticity of each species will largely determine its responses to change and must be a factor in the management of biodiversity. However, phenotypic plasticity may in itself represent a cost, and upregulation of metabolism in response to maintain metabolic capacity will necessitate greater energy demands in the longer term. Hence, increasing temperatures may have an indirect negative effect, unless there is an increase in energy supply concomitant to the phenotypic response to warming. Nonetheless, the fact that an Antarctic fish has the capacity to compensate for chronic changes in temperature may call for a fresh look at the thermal plasticity of physiological processes in those species most likely to be affected by global warming.
Acknowledgments
The authors thank Antarctica New Zealand and the staff at Scott Base for support.
References
- Axelsson M, Davison W, Forster M.E, Farrell A.P. Cardiovascular responses of the red-blooded Antarctic fishes Pagothenia bernacchii and P. borchgrevinki. J. Exp. Biol. 1992;167:179–201. doi: 10.1242/jeb.167.1.179. [DOI] [PubMed] [Google Scholar]
- Ball B, Johnston I.A. Molecular mechanisms underlying the plasticity of muscle contractile properties with temperature acclimation in the marine fish Myoxocephalus scorpius. J. Exp. Biol. 1996;199:1363–1373. doi: 10.1242/jeb.199.6.1363. [DOI] [PubMed] [Google Scholar]
- Bennett A.F, Lenski R.E. Experimental evolution and its role in evolutionary physiology. Am. Zool. 1999;39:346–362. [Google Scholar]
- Bouchard P, Guderley H. Time course of the response of mitochondria from oxidative muscle during thermal acclimation of rainbow trout, Oncorhynchus mykiss. J. Exp. Biol. 2003;206:3455–3465. doi: 10.1242/jeb.00578. [DOI] [PubMed] [Google Scholar]
- Brett J.R. The respiratory metabolism and swimming performance of young sockeye salmon. J. Fish. Res. Board Can. 1964;21:1183–1226. [Google Scholar]
- Clarke A, Johnston I.A. Evolution and adaptive radiation of Antarctic fishes. Trends Ecol. Evol. 1996;11:212–218. doi: 10.1016/0169-5347(96)10029-x. [DOI] [PubMed] [Google Scholar]
- Crawford D.L, Powers D.A. Molecular basis of evolutionary adaptation at the lactate dehydrogenase-B locus in the fish Fundulus heteroclitus. Proc. Natl Acad. Sci. USA. 1989;86:9365–9369. doi: 10.1073/pnas.86.23.9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D.L, Pierce V.A, Segal J.A. Evolutionary physiology of closely related taxa: analyses of enzyme expression. Am. Zool. 1999;39:389–399. [Google Scholar]
- DeVries A.L. Role of glycopeptides and peptides in inhibition of crystallization of water in polar fishes. Phil. Trans. R. Soc. B. 1984;304:575–588. [Google Scholar]
- Guderley H. Metabolic responses to low temperature in fish muscle. Biol. Rev. 2004;79:409–427. doi: 10.1017/s1464793103006328. [DOI] [PubMed] [Google Scholar]
- Guderley H, St Pierre J. Going with the flow or life in the fast lane: contrasting mitochondrial responses to thermal change. J. Exp. Biol. 2002;205:2237–2249. doi: 10.1242/jeb.205.15.2237. [DOI] [PubMed] [Google Scholar]
- Huey R.B, Hertz P.E. Is a jack-of-all-temperatures a master of none? Evolution. 1984;38:441–444. doi: 10.1111/j.1558-5646.1984.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Johnston I.A. Muscle metabolism and growth in Antarctic fishes (suborder Notothenioidei): evolution in a cold environment. Comp. Biochem. Physiol. B. 2003;136:701–713. doi: 10.1016/s1096-4959(03)00258-6. [DOI] [PubMed] [Google Scholar]
- Johnston I.A, Temple G.K. Thermal plasticity of skeletal muscle phenotype in ectothermic vertebrates and its significance for locomotor behaviour. J. Exp. Biol. 2002;205:2305–2322. doi: 10.1242/jeb.205.15.2305. [DOI] [PubMed] [Google Scholar]
- Levins R. Princeton University Press; 1968. Evolution in changing environments. [Google Scholar]
- Pierce V.A, Crawford D.L. Phylogenetic analysis of glycolytic enzyme expression. Science. 1997;276:256–259. doi: 10.1126/science.276.5310.256. [DOI] [PubMed] [Google Scholar]
- Scheiner S.M. Genetics and evolution of phenotypic plasticity. Ann. Rev. Ecol. Syst. 1993;24:35–68. [Google Scholar]
- Seebacher F, Guderley H, Elsey R.M, Trosclair P.L., III Seasonal acclimatisation of muscle metabolic enzymes in a reptile (Alligator mississippiensis) J. Exp. Biol. 2003;206:1193–1200. doi: 10.1242/jeb.00223. [DOI] [PubMed] [Google Scholar]
- Somero G.N. Biochemical mechanisms of cold adaptation and stenothermality in Antarctic fish. In: diPrisco G, Maresca B, Tota B, editors. Biology of Antarctic fish. Springer; Berlin: 1991. pp. 232–247. [Google Scholar]
- Somero G.A, DeVries A.L. Temperature tolerance of some Antarctic fishes. Science. 1967;156:257–258. doi: 10.1126/science.156.3772.257. [DOI] [PubMed] [Google Scholar]
- Somero G.N, Dahlhoff E, Lin J.J. Stenotherms and eurytherms: mechanisms establishing thermal optima and tolerance ranges. Johnston I.A, Bennett A.F, editors. Animals and temperature: phenotypic and evolutionary adaptations. 1996 [Google Scholar]
- St Pierre J, Charest P.-M, Guderley H. Relative contribution of quantitative and qualitative changes in mitochondria to metabolic compensation during seasonal acclimatisation of rainbow trout Oncorhynchus mykiss. J. Exp. Biol. 1998;201:2961–2970. [Google Scholar]
- Thibault M, Blier P.U, Guderley H. Seasonal variation of muscle metabolic organization in rainbow trout (Oncorhynchus mykiss) Fish Physiol. Biochem. 1997;16:139–155. [Google Scholar]
- Thomas C.D, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- West J.L, et al. Activity levels of enzymes of energy metabolism in heart and red muscle are higher in north-temperate-zone than in Amazonian teleosts. Can. J. Zool. 1999;77:690–696. [Google Scholar]
- Wilson R.W, Franklin C.E. Testing the beneficial acclimation hypothesis. Trends Ecol. Evol. 2002;17:66–70. [Google Scholar]
- Wilson R.S, Kuchel L.J, Franklin C.E, Davison W. Turning up the heat on subzero fish: thermal dependence of sustained swimming in an Antarctic notothenioid. J. Therm. Biol. 2002;27:381–386. [Google Scholar]