Abstract
Relatively simple model organisms such as yeast, fruit-flies and the nematode, Caenorhabditis elegans, have proven to be invaluable resources in biological studies. An example is the widespread use of C. elegans to investigate the complex process of ageing. An important issue when interpreting results from these studies is the similarity of the observed C. elegans mortality pattern in the laboratory to that expected in its natural environment. We found that the longevity of C. elegans under more natural conditions is reduced up to 10‐fold compared with standard laboratory culture conditions. Additionally, C. elegans mutants that live twice as long as wild-type worms in laboratory conditions typically die sooner than wild-type worms in a natural soil. These results indicate that conclusions regarding extended longevity drawn from standard laboratory assays may not extend to animals in their native environment.
Keywords: ageing, senescence, Caenorhabditis elegans
1. Introduction
Caenorhabditis elegans is a popular model organism for use in ageing studies, largely because of the identification of numerous long-lived mutants (Johnson et al. 2000). Such mutants may help to identify genes specifically involved in ageing, and an extensive research effort is underway to elucidate the mechanisms by which these mutations extend longevity (Guarente & Kenyon 2000). In these studies, C. elegans' longevity is typically compared between strains grown either in liquid culture or, more commonly, on agar plates. Because C. elegans evolved as a soil‐inhabiting nematode, it is unclear whether results from longevity comparisons using laboratory culture conditions will be representative of those expected in a more natural environment. To study this issue, we compared the longevity of wild-type and a long-lived C. elegans mutant under both standard laboratory culture conditions and in soil and sand environments.
2. Material and methods
Soil from which C. elegans had previously been isolated (C. elegans strain TR403) was obtained and assayed for its native microfauna. An initial characterization of the soil fauna revealed a variety of soil invertebrates, including numerous microbivorous, fungivorous and plant‐feeding nematodes. To determine the survivorship pattern of C. elegans in this natural soil type, a known number of young adult C. elegans were added to soil samples. These samples were then assayed at regular intervals for C. elegans survivorship. To ensure that only individuals from the initial population of worms were recovered, we used C. elegans strains possessing a mutation affecting sperm motility. Such mutants grow, develop and age normally, but do not produce progeny when maintained at standard culture temperatures (Ward & Miwa 1978). Worm longevity of the strains placed in soil was compared with that of the same strains reared under typical laboratory culture conditions. To minimize the effects of microinvertebrate or other biotic predation that might affect C. elegans survival, we also assayed survivorship using soil which had been heat pasteurized to kill the native fauna prior to the addition of worms.
The fer-1 wv01 strain was considered the wild-type and daf-2 wv06 (a double mutant of daf-2 e1368 and fer-1 wv01) the long-lived strain. This daf-2 mutant is considered the most similar to wild-type in terms of growth, development and fecundity (Gems et al. 1998). Soil used in the study came from Madison, Wisconsin, USA. The native soil bacterial fauna was enriched with E. coli 2 days before adding worms. This was done to ensure that a bacterial food supply to which the worms were well adapted was present in the soil or sand environments. Heat‐pasteurized soil was sealed in a container and heated to 65 °C for 24 h prior to the addition of C. elegans. On day 0 of the experiment, approximately 300 1-day-old adult worms were added to a 2 ml soil or sand sample contained in a 15 ml plastic centrifuge tube. A sub-population of these worms was placed on 3.5 cm Petri dishes containing nematode growth medium (NGM) agar inoculated with E. coli (Brenner 1974), and assayed for longevity. The E. coli strain NA22 was used as a supplemental food source for the worms in both the soil and sand environments. This E. coli strain was used for the food source in these environments because the E. coli strain commonly used to grow C. elegans in the laboratory, OP50, requires an external source of uracil to grow well. It is unlikely that the soil would have a readily available source of uracil. Longevity assays of the worms were also conducted on NGM plates spotted with OP50. This E. coli strain was used to allow the worms to be more easily tracked on less dense bacterial lawns, and with allow the comparison of longevity data from these experiments with previous longevity assays conducted in the laboratory on C. elegans. It was determined that the survivorship of C. elegans on agar plates was essentially identical irrespective of whether they were grown on OP50 or NA22 as a food source (see Electronic Appendix).
Groups were maintained at 23 °C and worms from six soil or sand samples were extracted at each time point using a colloidal silica method (Donkin & Dusenbery 1993). Soil extraction 3 hours after addition of the worms recovered around 80% of the initial population, of which more than 95% were alive. A worm was scored as dead when it was not moving and did not respond to mechanical stimulus.
Soil is a complex environment in which many factors aside from predation (e.g. the production of toxic compounds by soil bacteria) can influence C. elegans' longevity. To minimize the impact of such biological factors, we examined the longevity of C. elegans in autoclaved, acid‐washed sand. To ensure food availability, the soil or sand samples were supplemented with E. coli. To minimize the biological activity of the E. coli, it was heat killed at 65 °C before being placed in the sand. To ensure that E. coli was not limiting as a food source, an amount far in excess of that which would be eaten by the several hundred worms was added to each sample. This is analogous to the conditions to which C. elegans are subjected during standard longevity assays in the laboratory, in which worms are on agar plates with many times their body mass of E. coli. We also assayed the longevity of a C. elegans mutant that typically lives around twice as long as the wild-type in laboratory conditions (Gems et al. 1998) to determine if it was also relatively long-lived in a soil environment.
3. Results
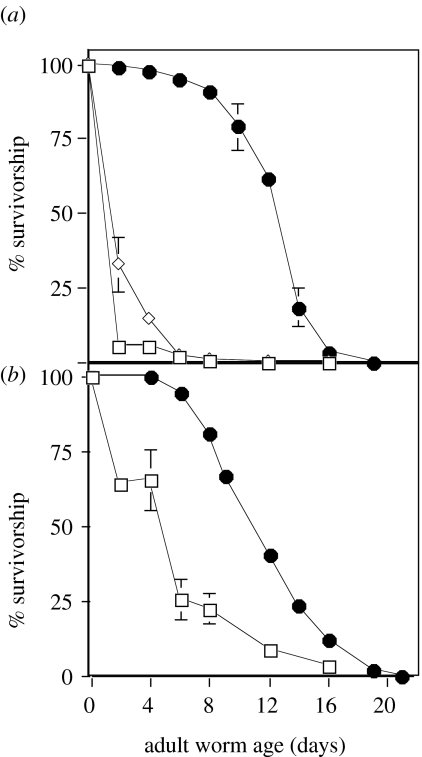
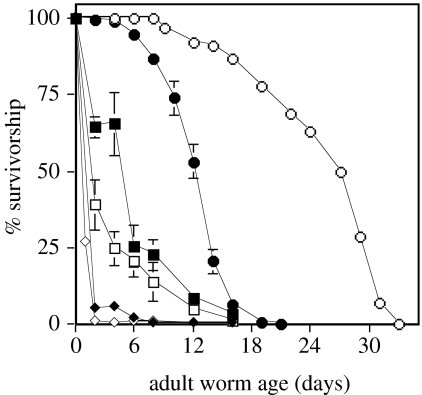
The median longevity of wild-type C. elegans is reduced by an order of magnitude in soil compared with worms maintained on agar plates (figure 1a). This large reduction does not appear to be caused by interactions with other soil microfauna because the longevity of wild-type C. elegans in pasteurized soil is the same or less than in non-pasteurized soil (figure 1a). Worm populations in sterilized sand lived longer than worms in soil, but only about one-third as long as the comparable wild-type strain on agar (figure 1b). While the nominally long-lived mutant daf-2 lived significantly longer than the wild-type when assayed on agar, daf-2 was shorter-lived than wild-type in both the sand and soil environments (figure 2).
Figure 1.
Longevity of wild-type Caenorhabditis elegans is reduced in a natural soil or sand environment compared with agar plates. (a) Survivorship of C. elegans added to a natural unheated (open diamond) or heat-pasteurized (open square) soil. The median adult survivorship in unheated soil was 1.5 days and 1.0 day for worms in heat‐treated soil. The median adult survivorship of wild-type worms on agar (filled circle) was 12 days. (b) Survivorship of C. elegans in acid‐washed, autoclaved sand. Median adult survivorship in sand (open square) was 4.5 days and wild-type on agar (filled circle) was 11.5 days. Median survivorship was estimated as the time at which 50% of the population remained alive. Data for the sand and soil treatments are the means and standard errors for six samples at each time point. Data of survivorship on agar are plotted for 151 and 115 worms for a, b, respectively. To determine if the sand/soil environments could support a mixed-age population, a fertile wild-type strain of C. elegans (N2) was added to the sand/soil environment. These worms were able to maintain reproducing populations for at least two weeks in the sand/soil environments used in the longevity assays.
Figure 2.
Longevity of a long-lived Caenorhabditis elegans mutant is less than wild-type in a natural soil or sand environment. The median adult survivorship of daf-2 worms was 0.8 days in heat-treated soil (open diamond), 1.8 days in sand (open square) and 27 days on agar (open circle).The median adult survivorship of wild-type was 1.0 days in heat-treated soil (filled diamond), 4.5 days in sand (filled square) and 12 days on agar (filled-circle). The survivorship in sand of wild-type worms is significantly greater than that of the long-lived mutant for the 2 and 4 day samples (two-tailed t-test, p=0.05 and p=0.002, respectively, d.f.=5). Wild-type survivorship data on agar are combined from figure 1a,b. Survivorship data for daf-2 agar are plotted for 104 worms.
4. Discussion
The reason for the relatively rapid mortality of C. elegans in a soil environment is unclear, although it appears unlikely that the worms are dying of starvation or other factors such as soil pH levels (see Electronic Appendix). Although the relatively short lifespan of C. elegans in soil compared with laboratory conditions may appear surprising, the shortened lifespan of worms in soil is more than sufficient for C. elegans to maintain viable populations. The short generation time, high rate of progeny production, and self fertilizing mode of reproduction allows even a single C. elegans to quickly found a large population (Wood 1988; Venette & Ferris 1998). Because C. elegans can produce almost all of its progeny within the first 2 days of its adult life, additional longevity adds little to its lifetime reproductive output (Byerly et al. 1976; Hirsh et al. 1976).
The relatively short longevity of C. elegans in soil makes it unlikely that C. elegans would have evolved a signalling mechanism inversely coupling longevity to its reproductive output. Although it has been proposed that such a mechanism may exist in C. elegans (Kenyon 2001), this hypothesis is based on longevity assays done on agar plates. Under such conditions, even wild-type C. elegans typically live several times longer than their reproductive period. From an evolutionary viewpoint, this extended post-reproductive longevity is puzzling. A non-reproducing worm is essentially evolutionarily irrelevant and there should be minimal selection for extended post-reproductive survivorship (Rose 1991). The high mortality rate of C. elegans under more natural conditions in our study suggests that few individuals would survive for long enough to benefit from any increase in late-life fecundity. Selection should favour rapidly reproducing types over survivors, as previously observed in C. elegans, where mutants that produce more total progeny are eventually displaced by wild-type worms, which have a shorter generation time (Hodgkin & Barnes 1991). The apparent short longevity of C. elegans in nature could also help to explain why biological processes such as protein biosynthesis and turnover rates are tightly regulated during C. elegans development but not post-reproductively (Herndon et al. 2002).
Among the mechanisms postulated to be responsible for the extended longevity of long-lived C. elegans mutants is an increased ability to tolerate stress (Lithgow & Kirkwood 1996; Martin et al. 1996). Alternatively, this extended longevity has been proposed to result from a reduction in metabolic rate (Van Voorhies & Ward 1999). Factors that reduce metabolic rate also generally increase the ability of the organism to withstand stress (Sohal et al. 2000). Although the daf-2 mutant studied here had a mean longevity twice that of wild-type under laboratory conditions on agar, they were shorter-lived than wild-type under more natural conditions. This indicates that any increased ability of such mutants to tolerate laboratory-induced stresses does not extend to the stresses encountered in a more natural environment. Results from similar experiments showed that under optimized laboratory conditions, populations of a long-lived C. elegans mutant persisted as well as wild-type worms, but were displaced by the wild-type under more stringent environmental conditions (Walker et al. 2000). The relatively poor survivorship of long-lived mutants under more natural conditions may also explain why such mutations are not known to occur in natural populations of C. elegans.
The factors responsible for C. elegans mortality in a natural environment appear to be different from those responsible for mortality in the laboratory. While C. elegans remains a valuable organism for the study of ageing, it is critical to consider its natural history when interpreting results from such studies.
Acknowledgements
We thank R. Parker and S. Ward for manuscript review and research discussions, P. Anderson for supplying the soil used in the study and the Caenorhabditis Genetics Center for C. elegans strains. This research was supported by a grant from the National Institutes of Aging.
Supplementary Material
References
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L, Cassada R.C, Russell R.L. The life cycle of the nematode Caenorhabditis elegans. Dev. Biol. 1976;51:23–33. doi: 10.1016/0012-1606(76)90119-6. [DOI] [PubMed] [Google Scholar]
- Donkin S.G, Dusenbery D.B. A soil toxicity test using the nematode Caenorhabditis elegans and an effective method of recovery. Arch. Environ. Contam. Toxicol. 1993;25:145–151. [Google Scholar]
- Gems D, Sutton A.J, Sundermeyer M.L, Albert P.S, King K.V, Edgley M.L, Larsen P.L, Riddle D.L. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Herndon L.A, Schmeissner P.J, Dudaronek J.M, Brown P.A, Listner K.M, Sakano Y, Paupard M.C, Hall D.H, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Hirsh D, Oppenheim D, Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev. Biol. 1976;49:200–219. doi: 10.1016/0012-1606(76)90267-0. [DOI] [PubMed] [Google Scholar]
- Hodgkin J, Barnes T.M. More is not better: brood size and population growth in a self-fertilizing nematode. Proc. R. Soc. B. 1991;246:19–24. doi: 10.1098/rspb.1991.0119. [DOI] [PubMed] [Google Scholar]
- Johnson T.E, Cypser J, de Castro E, de Castro S, Henderson S, Murakami S, Rikke B, Tedesco P, Link C. Gerontogenes mediate health and longevity in nematodes through increasing resistance to environmental toxins and stressors. Exp. Gerontol. 2000;35:687–694. doi: 10.1016/s0531-5565(00)00138-8. [DOI] [PubMed] [Google Scholar]
- Kenyon C. A conserved regulatory system for aging. Cell. 2001;105:165–168. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- Lithgow G.J, Kirkwood T.B.L. Mechanisms and evolution of aging. Science. 1996;273:80. doi: 10.1126/science.273.5271.80. [DOI] [PubMed] [Google Scholar]
- Martin G.M, Austad S.N, Johnson T.E. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nature Genetics. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- Rose M. Oxford University Press; 1991. The evolutionary biology of aging. [Google Scholar]
- Sohal R.S, Mockett R.J, Orr W.C. Current issues concerning the role of oxidative stress in aging: a perspective. In: Hekimi S, editor. The molecular genetics of aging. Springer; Berlin: 2000. pp. 45–65. [DOI] [PubMed] [Google Scholar]
- Van Voorhies W.A, Ward S. Genetic and environmental conditions that increase longevity in Caenorhabditis elegans decrease metabolic rate. Proc. Natl Acad. Sci. USA. 1999;96:11 399–11 403. doi: 10.1073/pnas.96.20.11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venette R, Ferris H. Influence of bacterial type and density of population growth of bacterial-feeding nematodes. Soil Biol. Biochem. 1998;30:949–960. [Google Scholar]
- Walker D.W, McColl G, Jenkins N.L, Harris J, Lithgow G.J. Evolution of lifespan in C. elegans. Nature. 2000;405:296–297. doi: 10.1038/35012693. [DOI] [PubMed] [Google Scholar]
- Ward S, Miwa J. Characterization of a temperature-sensitive fertilization-defective mutant of the nematode Caenorhabditis elegans. Genetics. 1978;88:285–303. doi: 10.1093/genetics/88.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W.B, editor. The nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.