Abstract
Arandaspids are the earliest skeletonizing vertebrates known from articulated remains. Despite a wealth of data, their affinity remains questionable because they exhibit a random mixture of primitive and derived characteristics. We constrain the affinity of arandaspids by providing the first detailed characterization of their dermoskeleton which is revealed to be three-layered, composed of a basal laminated, cancellous middle and tubercular superficial layers. All three layers are composed of acellular bone but the superficial layer also includes dentine and enameloid, comprising the tubercles. As such, the composition of the arandaspid dermoskeleton is common to heterostracans and astraspids, supporting existing hypotheses of early vertebrate phylogeny. This emphasizes the peculiarity of existing interpretations of aranadaspid anatomy and there is need for a complete reappraisal of the existing anatomical data.
Keywords: gnathostome, vertebrate, histology, dermoskeleton, dentine, enameloid
1. Introduction
Sacabambaspis janvieri, from the Llanvirn and Caradoc (Ordovician) of South America (Gagnier et al. 1986; Albanesi et al. 1995) and Australia (Young 1997), is the oldest skeletonizing vertebrate known from articulated remains; it is also the earliest undisputable vertebrate to appear in the stratigraphic record (Janvier 2003). As currently described, it exhibits an unusual combination of both primitive and derived characters, permitting contradictory hypotheses of affinity and complex patterns of character acquisition and loss in the assembly of the body plan of gnathostomes. These include paired and externally open nostrils, cellular dermal bone, sclerotic ossicles and ossified eye sclera, characters that are otherwise restricted to the clade, composed of living jawed vertebrates, as well as the extinct placoderms, osteostracans and galeaspids. At the same time, it exhibits apomorphies of heterostracans and vertebrate symplesiomorphies. This complex of characters is difficult to equate with the inferred phylogenetic position for S. janvieri (Gagnier 1993b, 1995; Janvier 1996a,b; Donoghue et al. 2000) and this has led to equivocation over the interpretations of its anatomy (Gagnier 1993a; Janvier 1996a; Donoghue et al. 2000). Resolution has not been aided by an absence of detailed histological data on the composition of the dermoskeleton, particularly given the pivotal significance of histological characters in resolving the interrelationships of early vertebrates (Donoghue et al. 2000). Based on new material, we provide the first detailed histological description of the dermoskeleton of S. janvieri and consider the relevance of these data to the question of the phylogenetic affinity of this problematic organism.
2. Material and methods
This study is based upon material from the Anzaldo Formation on a roadside section close to the village of Sacabambilla, Department of Cochabamba, Bolivia (for further details see Gagnier 1993a) and discrete plate fragments encountered in microfossil residues from the Sepulturas Formation, near Los Colorados, Cordillera Oriental of Jujuy Province, Argentina (Albanesi & Astini 2002). The Bolivian material has been subject to low-grade metamorphism, while in the Argentinian material most of the original phosphate has been replaced by pyrolusite and pyrite, the latter largely degraded to limonite. Nevertheless, sufficient detail is present for histological characterization and these data agree between material from both localities.
Specimens were embedded in polyester resin, and then processed as double polished thin sections, approximately 50 μm thick or prepared as ground and polished blocks for scanning electron microscope (SEM) analysis. SEM specimens were etched with 0.5% orthophosphoric acid for 10 min. Sections were also studied with a transmitted light microscope equipped with Nomarski optics.
Institutional abbreviations: BU—Lapworth Museum of Geology, University of Birmingham, UK; BRSUG—Geology Museum, University of Bristol, UK; CORD-MP—Museo de Paleontologia, Cordoba, Argentina; MHNC—Museo de Historia Natural Alcide d'Orbigny, Cochabamba, Bolivia; NRM—Swedish Museum of Natural History, Stockholm, Sweden.
3. Existing interpretation
Gagnier (1993a) described the gross structure of the dermoskeleton as three-layered, with a thin laminated basal layer, a vaulted or cancellous middle layer and a spongy superficial layer comprising the overlying ornamental ridges and tubercles. Gagnier (1993a, fig. 19) and Janvier (1996a, fig. 4.2F) depicted the vaulted walls as double-layered, hollow structures and described rare irregular voids within the walls of the middle and basal layers as cell spaces, interpreting the tissue as cellular bone. Nothing more has been discerned due to the diagenetic alteration of the tissues.
4. Histological description
We can confirm Gagnier's description of the gross structure of the dermoskeleton. It is composed of a laminated basal layer (figure 1a,c), (irregularly) vaulted middle layer in which the vertical struts circumscribe large cancellae (figure 1a,b), and a superficial layer permeated by anastomosing canals in its lower region and compact in the upper layers that comprise the surface ornamental ridges (figure 1a,b,d–f).
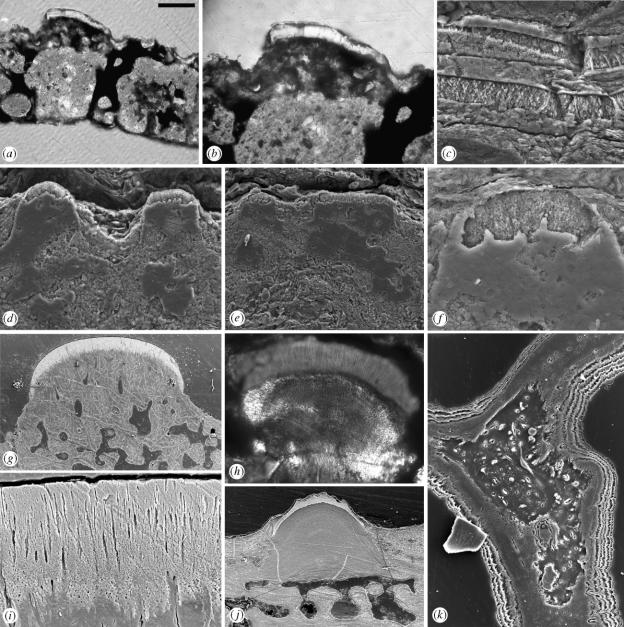
Figure 1.
(a–f) Sacabambaspis janvieri Gagnier, Blieck and Rodrigo. (a) and (b) Optical section through tesserae (CORD-MP 9101) illustrating, from the base upwards, the fragmented basal layer, cancellous middle layer and cap of enameloid on the upper surface. (b) Pervasive vascularization immediately under the enameloid cap and the fine tubules penetrating into the enameloid. (c–f) Etched section of S. janvieri (MHNC 13261) showing (c) the laminated basal layer. (d) and (e) overviews of adjacent enameloid capped tubercles, (f) the fine crystalline nature of the enameloid cap and tubules penetrating the lower part of the tissue. (g) and (i) Etched section (BRSUG 27184) through the dermoskeleton of Tesseraspis tessellata Wills showing the fine crystalline and tubular nature of the enameloid cap. (h,j) Optical (BU 4440) and etched (NRM P11114) sections (respectively) through the dermoskeleton of Astraspis desiderata (Walcott), (h) showing fine calibre tubules penetrating through the enameloid layer. (k) Etched section (BRSUG 27189) through the dermoskeleton of Loricopteraspis dairydinglensis (White) showing the presence of fibre-spaces within the vaulted walls of the cancellar (middle) layer. Relative scale bar: (a) 125 μm, (b) 73 μm, (c) 18 μm, (d) 35 μm, (e) 62 μm, (f) 13 μm, (g) 265 μm, (h) 63 μm, (i) 25 μm, (j) 157 μm, (k) 42 μm.
No spaces are present within the tissues comprising the basal and middle layers and there is no evidence for a double-walled structure. Rather, the walls exhibit a compact structure (figure 1b,c). The basal layer is composed of laminae arranged parallel to the outer and inner surfaces of the dermoskeleton (figure 1c). The orientation of crystallites alternates between laminations (figure 1c).
The uppermost part of the superficial ornamental ridges is composed of a distinct tissue layer, approximately 15–20 μm in thickness, which is translucent and highly birefringent in transmitted light (figure 1a,b). This capping layer is sharply delineated from the underlying vascularized tissues of the superficial layer by a convoluted junction and is localized to capping the raised oak-leaf shaped surface ridges (figure 1a,b,d–f). SEM analyses reveal a microcrystalline structure in which individual crystallites are orientated predominately perpendicular to the upper and lower surfaces of the cap (figure 1f). This corresponds to a similarly aligned optical fabric observed using Nomarski DIC (figure 1b). Fine calibre tubules penetrate into the cap, emanating from the underlying tissue and terminating below the outer surface of the capping tissue (figure 1b,d–f).
5. Histological interpretation
The gross structure of the dermoskeleton conforms to that seen in other stem-gnathostomes such as heterostracans and osteostracans. The laminated basal layer (figure 1c) compares favourably to the structure of the basal layer in heterostracans where it is interpreted as isopedin, as type of acellular bone (Gross 1956, 1961; cf Wang et al. in press). The vaulted cancellous middle layer compares to the honey-comb structure of heterostracans (e.g. Donoghue & Sansom 2002). The putative cell spaces identified by Gagnier (1993a) throughout the dermoskeleton in Sacabambaspis are indistinguishable from the fibre spaces that permeate the acellular bone in heterostracans (figure 1k; Donoghue & Sansom 2002).
Structurally and topologically, the tissue forming the superficial caps of Sacabambaspis tubercles is closely comparable to the enameloid capped dentine tubercles comprising the superficial layer in other pteraspidomorph taxa such as Astraspis (figure 1h,j and Sansom et al. 1997) and Tesseraspis (figure 1g,i), both in terms of the size and orientation of the crystallites, the presence of fine tubules permeating into the enameloid from the underlying tissue.
Topologically, the spongy layer beneath the superficial enameloid caps in Sacabambaspis occupies a position in the dermoskeleton where dentine would be expected. Indeed, the fine tubules in the enameloid in comparable taxa (e.g. Astraspis, figure 1h and Tesseraspis, figure 1i) are odontoblast cell processes that permeate from underlying dentine and the coarser calibre canals within the superficial layer beneath the tubercles in Sacabambaspis (figure 1b) are directly comparable to the pulp canals in these histologically better preserved taxa (figure 1g). Furthermore, the presence of an enameloid cap in itself suggests that the underlying tissue is dentine because the formation of enameloid and all enamel-related tissues is contingent upon a cascade of inductive interactions involving dentine and its precursors (cf. Lumsden 1987; Donoghue 2002). No detail of the structure of the dentine is preserved, however, given the vestiges of pulp canals (figure 1d–f), it is likely that originally this tissue exhibited a tubular fabric.
6. Implications and concluding remarks
Histological analysis reveals the dermoskeleton of S. janvieri to be composed of superficial tubercles composed of dentine and enameloid, and underlain by a cancellous layer of acellular bone, and this is in turn underlain by a basal layer of laminated acellular bone, equivalent to the isopedin layer in galeaspids, osteostracans, placoderms and, primitively, in osteichthyans (Wang et al. in press; figure 2). Sacabambaspis janvieri is the best characterized of the arandaspids and, in the absence of contradictory evidence, we can take these data as representative of the clade. On the basis of our observations on the histology of Sacabambaspis, arandaspids possess a dermoskeleton compositionally equivalent to pteraspidomorphs and, in possessing a cancellous middle layer, exhibiting closest comparison to heterostracans sensu Janvier (1996a).
Figure 2.
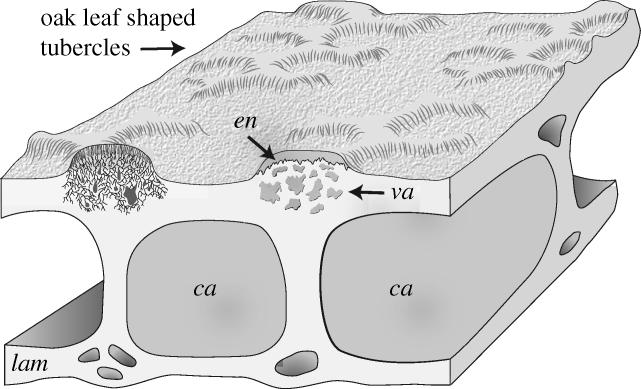
Three dimensional reconstruction of the histology of Sacabambaspis based upon the material illustrated in figure 1(a–f). Reconstructed tubercle on the left includes dentine tubules based upon the interpretation of the heavily vascularized area immediately beneath the enameloid caps and comparison with Astraspis and Tesseraspis. Abbreviations: ca, cancellous middle layer; en, enameloid; lam, laminated basal layer; va, vascularized region underlying tubercles.
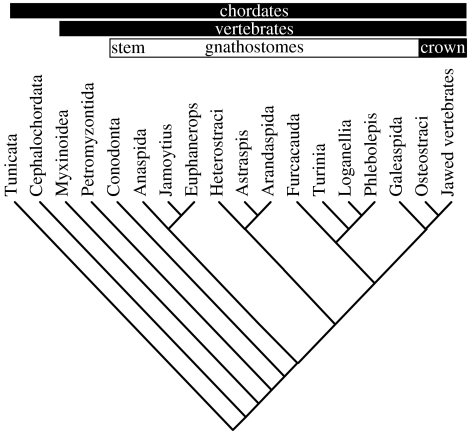
In comparison to previous histological interpretations of arandaspids, our conclusions are entirely congruent with the distribution of histological characters in existing cladograms of early vertebrates which consistently place the arandaspids as a sister group to the heterostracans sensu stricto (Forey & Janvier 1993; Gagnier 1993b; Forey 1995; Janvier 1996b; Donoghue et al. 2000; Donoghue & Smith 2001). This is reinforced by our parsimony analysis of the cladistic dataset compiled by Donoghue & Smith (2001; and revised in line with our reinterpretation of arandaspid dermoskeletal histology) which resolves arandaspids as the sister-group to Astraspis, rather than heterostracans sensu stricto (figure 3).
Figure 3.
Revised chordate phylogeny based upon the new histological data for arandaspids presented herein. Dataset of Donoghue & Smith (2001) updated to included codings for arandaspids (66: 1; 67: 0; 68: 1; 69: ?; 70: 2; 73: 1) and corrected coding for Heterostraci (75: 0). 100 replicate random sequence addition heuristic search yielded 3 optimal trees at 192 steps. The inclusion of the new data resolves the arandaspids as the sister group to Astraspis.
The unusual mixture of primitive and derived morphological characters exhibited by arandaspids remains unparsimonious, which, on the basis of this hypothesis of affinity, suggests that the derived characters have been acquired independently in the lineage leading to pteraspidomorphs and jawed vertebrates, or else these are all vertebrate symplesiomorphies that have been secondarily lost in other taxa. However, existing interpretations of these contentious characters have been questioned repeatedly and a thorough reconsideration of the anatomy of arandaspids is essential for an understanding of the polarity of character acquisition in the assembly of the body plan of jawed vertebrates.
Acknowledgments
We thank Ricardo Astini (CONICET, Cordoba), Ramiro Suarez Soruco and Ricardo Cespedes (Cochabamba), Philippe Janvier (MNHN, Paris) and Dr Rob Ixer (Birmingham). This research was funded by NERC (NE/B503576/1 to I.J.S. and GT5/99/ES/5 to P.C.J.D.) and CONICET (G.A.).
References
- Albanesi G.L, Astini R. Fauna de conodontes y Sacabambaspis janvieri (vertebrata) en el Ordovícico Medio de la Cordillera Oriental Argentina. Implicancias estratigráficas y paleogeográficas. In: Anzo´tegui. L.A, Lutz A.I, Gallego O.F, editors. VIII Congreso Argentino de Paleontologı´a y Bioestratigrafı´a, Corrientes, Chile, 7–10 October 2002. Resumen; 2002. p. 17a. [Google Scholar]
- Albanesi G.L, Benedetto J.L, Gagnier P.-Y. Sacabambaspis janvieri (Vertebrata) y conodontes del Llandeiliano temprano en la Formacion La Cantera, Precordillera de San Juan, Argentina. Boletin de la Academia Nacional de Ciencias, Córdoba, Argentina. 1995;60:519–543. [Google Scholar]
- Donoghue P.C.J. Evolution of development of vertebrate teeth and scales: unravelling concepts, regulatory theories and homologies. Paleobiology. 2002;28:474–507. [Google Scholar]
- Donoghue P.C.J, Sansom I.J. Origin and early evolution of vertebrate skeletonization. Microsc. Res. Tech. 2002;59:352–372. doi: 10.1002/jemt.10217. [DOI] [PubMed] [Google Scholar]
- Donoghue P.C.J, Smith M.P. The anatomy of Turinia pagei (Powrie) and the phylogenetic status of the Thelodonti. Trans. R. Soc. Edinb. (Earth Sci.) 2001;92:15–37. [Google Scholar]
- Donoghue P.C.J, Forey P.L, Aldridge R.J. Conodont affinity and chordate phylogeny. Biol. Rev. 2000;75:191–251. doi: 10.1017/s0006323199005472. [DOI] [PubMed] [Google Scholar]
- Forey P.L. Agnathans recent and fossil, and the origin of jawed vertebrates. Rev. Fish Biol. Fish. 1995;5:267–303. [Google Scholar]
- Forey P.L, Janvier P. Agnathans and the origin of jawed vertebrates. Nature. 1993;361:129–134. [Google Scholar]
- Gagnier P.-Y. Sacabambaspis janvieri, vertébré Ordovicien de Bolivie. I. Analyse morphologique. Ann. Paléontol. 1993a;79:19–69. [Google Scholar]
- Gagnier P.-Y. Sacabambaspis janvieri, vertébré Ordovicien de Bolivie. II. Analyse phylogénétique. Ann. Paléontol. 1993b;79:119–166. [Google Scholar]
- Gagnier P.Y. Ordovician vertebrates and agnathan phylogeny. Bulletin du Muséum national d'Histoire naturelle, Paris. 1995;17:1–37. [Google Scholar]
- Gagnier P.Y, Blieck A, Rodrigo G. First Ordovician vertebrate from South America. Geobios. 1986;19:629–634. [Google Scholar]
- Gross W. Über Crossopterygier und Dipnoer aus dem baltischen Oberdevon im Zusammenhang einer vergleichenden Untersuchung des Porenkanalsystems paläozoischer Agnathen und Fische. Kungliga Svenska Vetenskapsakademiens Handlingar. 1956;5:1–140. [Google Scholar]
- Gross W. Aufbau des Panzers obersilurischer Heterostraci und Osteostraci Norddeutschlands (Geschiebe) und Oesels. Acta Zool. (Stockholm) 1961;42:73–150. [Google Scholar]
- Janvier P. Oxford monographs on geology and geophysics. vol. 33. Oxford University Press; 1996a. Early vertebrates. [Google Scholar]
- Janvier P. The dawn of the vertebrates: characters versus common ascent in the rise of current vertebrate phylogenies. Palaeontology. 1996b;39:259–287. [Google Scholar]
- Janvier P. Vertebrate characters and the Cambrian vertebrates. C. R. Palevol. 2003;2:523–531. [Google Scholar]
- Lumsden A.G.S. The neural crest contribution to tooth development in the mammalian embryo. In: Maderson P.F.A, editor. Developmental and evolutionary aspects of the neural crest. Wiley; New York: 1987. pp. 261–300. [Google Scholar]
- Sansom I.J, Smith M.P, Smith M.M, Turner P. Astraspis—the anatomy and histology of an Ordovician fish. Palaeontology. 1997;40:625–643. [Google Scholar]
- Wang, N.-Z., Donoghue, P. C. D, Smith, M. M. & Sansom, I. J. In press. The histology of the galeaspid dermoskeleton and endoskeleton, and the origin of cranial endoskeletons from the neurocranium. J. Vertebr. Paleontol.
- Young G.C. Ordovician microvertebrate remains from the Amadeus Basin, central Australia. J. Vertebr. Paleontol. 1997;17:1–25. [Google Scholar]