Abstract
The remarkable X-linked colour vision polymorphism observed in many New World primates is thought to be maintained by balancing selection. Behavioural tests support a hypothesis of heterozygote advantage, as heterozygous females (with trichromatic vision) exhibit foraging benefits over homozygous females and males (with dichromatic vision) when detecting ripe fruit on a background of leaves. Whilst most studies to date have examined the functional relevance of polymorphic colour vision in the context of foraging behaviour, alternative hypotheses proposed to explain the polymorphism have remained unexplored. In this study we examine colour vision polymorphism, social group composition and breeding success in wild red-bellied tamarins Saguinus labiatus. We find that the association of males and females within tamarin social groups is non-random with respect to colour vision genotype, with identified mating partners having the greatest allelic diversity. The observed distribution of alleles may be driven by inbreeding avoidance and implies an important new mechanism for maintaining colour vision polymorphism. This study also provides the first preliminary evidence that wild trichromatic females may have increased fitness compared with dichromatic counterparts, as measured by breeding success and longevity.
Keywords: colour vision, heterozygote advantage, inbreeding avoidance, opsin, polymorphism, tamarin
1. Introduction
The majority of New World primate species exhibit a remarkable intraspecific variation in colour vision (Mollon et al. 1984). In these species, all males have dichromatic colour vision (similar to red–green colour blind humans), whereas females may be dichromats or trichromats (resembling humans with normal colour vision). This variation has a simple genetic basis, where a polymorphic X-linked locus encodes photopigments (opsins) sensitive in the green–red part of the spectrum. Males and homozygous females produce a single red–green opsin, which together with the invariant autosomally encoded blue opsin leads to dichromacy. Heterozygous females have two different X-linked red–green opsins, leading to trichromacy. In the majority of New World monkey species there are three alleles at this X-linked locus, giving three dichromatic phenotypes and three trichromatic phenotypes (Williams et al. 1992; Jacobs et al. 1993). These alleles have been maintained across different species for many millions of years (Surridge & Mundy 2002).
The evolutionary processes that led to the origin and subsequent maintenance of colour vision polymorphism are intriguing, but remain poorly understood (reviewed in Surridge et al. 2003). Most discussion to date has focused on selective forces acting differentially on dichromats and trichromats. In particular, trichromats are expected to be better at detecting ripe fruit than dichromats (Caine & Mundy 2000; Smith et al. 2003a), whereas a possible benefit for dichromats is in detecting red–green camouflaged objects (Caine et al. 2003), such as cryptic insects. This provides a mechanism whereby balancing selection operates on the locus. A combination of selection for trichromats with widely spaced photopigments and selection for dichromats with longer wavelength photopigments (Osorio et al. 2004) probably contributes to the maintenance of the three alleles, albeit at unequal frequencies (Cropp et al. 2002; Surridge et al. in press).
Alternative hypotheses proposed to explain the maintenance of polymorphic colour vision have remained largely unexplored. For example, the importance of mate choice and social group composition has rarely been considered in this context. Generally, there is only one breeding female in tamarin groups, which contain both related and unrelated individuals. The female may mate with more than one male (Sussman & Garber 1987). If females mate disassortatively with respect to opsin genotype (e.g. if a trichromatic female mates with a male with the allele that the female is lacking) this would help to retain variation at this locus, since individuals with rare alleles would have an advantage finding mates. It has also been proposed that group success might be dependent on individuals possessing a range of colour vision phenotypes (Mollon et al. 1984). Additionally, it is not known if the differential foraging abilities of trichromats and dichromats observed in captivity translate into differences in fitness in the wild.
To date, information on the distribution of opsin alleles within wild New World monkey groups is limited. In the present study we genotyped the colour vision alleles of wild individuals of red-bellied tamarins (Saguinus labiatus) from eight adjacent social groups in order to ask the following questions. (i) Is there disassortative mating, or disassortative association of males and females by opsin genotype? (ii) Do adult males and females associate according to each other's phenotype? (iii) Is there evidence for higher fitness in trichromatic than dichromatic females?
2. Material and methods
Eight neighbouring groups of red-bellied tamarins (S. labiatus) were trapped (Garber et al.1993; Savage et al. 1993) as part of an 18 month field study (July 1999–December 2000) at the Tahuamanu Biological Station in northwestern Bolivia (S. Suárez, unpublished data). Hair samples for genetic analysis were plucked from a total of 47 animals. Animals were classified into age categories based on developmental stage, dental eruption and dental wear (Neyman 1977; Garber et al. 1993).
DNA was extracted from hairs using a DNA minikit (Qiagen) according to the manufacturer's protocol. Genotyping of the three alleles at the tamarin X-linked opsin locus (543, 556 and 563 nm wavelengths) was performed by PCR amplification and direct sequencing of exons 3, 4 and 5 on both strands. PCR primers and detailed methods are described elsewhere (Surridge & Mundy 2002). Non-random distributions of alleles and genotypes were assessed using randomization tests (10 000 replicates) that randomly placed adults into a new set of groups while retaining the observed male:female group composition. Tests on contingency tables were performed using a Monte Carlo simulation in RxC (Miller 1997).
3. Results
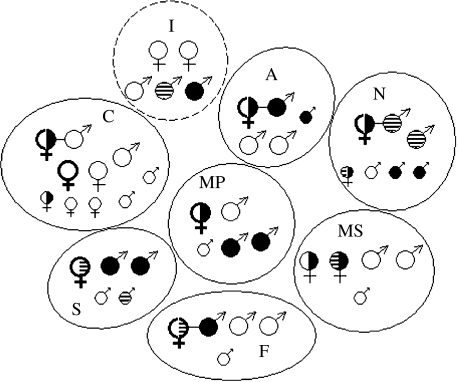
The distribution of opsin alleles among individuals in the eight groups is shown in figure 1. The 543 nm and 563 nm alleles were present in individuals in all eight groups, and the 556 nm allele was present in monkeys in five out of the eight groups. Seven out of the eight groups contained trichromatic females, and the total number of colour vision phenotypes in each group varied from two to five (out of a maximum number of six). There was no evidence that adults associated in a way that maximized the phenotypic diversity in the group.
Figure 1.
Distribution of opsin genotypes among eight tamarin groups. Black, 543 nm opsin allele; stripes, 556 nm allele; white, 563 nm allele. Large circles, adults; small circles, infants, juveniles and subadults. Identified breeding pairs are shown joined by a line and females that were confirmed breeders during the study are shown with thick lines. Group ranges are shown in approximate geographic position. Group I (dotted line) was unstable over the course of the study. Since the individuals shown were not all present at the same time, and several adults were not captured, this group was excluded from randomization analyses.
Adult males and females were non-randomly distributed among groups with respect to the opsin alleles they possessed. In particular, there was a significant excess of alleles that were specific to either adult females or adult males within a group (i.e. alleles that were absent from one sex within a group were present in the other sex; randomization test, p<0.02). The randomization was repeated, since one adult male was not trapped from group S. The result held (p<0.05) when an extra male with a genotype drawn from a population with the allele frequencies found in the present population was added to this group. The composition of Group I changed during the course of this study (see below) and was excluded from this analysis.
There was a trend for older females to be trichromats—the five oldest females sampled were all trichromats (contingency table, p<0.09). Four breeding pairs were identified by additional microsatellite genotyping (S. Suárez, unpublished data), and all of these cases involved trichromatic females mating with a male with the most divergent opsin phenotype possible in the group. Two of these cases involved females mating with males with the third allele that they did not possess. The group which did not contain any trichromatic females (Group I) was unstable and breeding did not occur during the course of the study. Group composition changed frequently once a dichromatic female was forcibly ejected by two immigrating females.
4. Discussion
(a) Disassortative mating by opsin genotype
Strikingly, this study finds that the association of males and females within wild tamarin groups is non-random with respect to opsin alleles. Since females generally breed with a male from the same group (S. Suárez, unpublished data), this will lead to disassortative mating by opsin genotype. The opsin genotypes of the small number of breeding pairs identified in this study confirm this. The simplest explanation for these results is that they arise from a general inbreeding avoidance mechanism, rather than any mechanism specific to the opsin locus. Indeed, there is evidence for inbreeding avoidance in this population from variation at neutral microsatellite loci (S. Suárez, unpublished data).
There are several interesting consequences expected from disassortative mating by opsin allele. First, the frequency of heterozygous female trichromats in the population will be higher than expected. A slight excess of heterozygous females is indeed found in this population (observed female heterozygosity=0.63, expected female heterozygosity=0.56), but this is not significant. Since trichromats have a foraging advantage over homozygous dichromats (at least for fruit; Smith et al. 2003b), this is a potential mechanism to increase fitness of mating pairs.
Second, disassortative mating could help to maintain the opsin polymorphism. If an opsin allele became rare, individuals with that allele would have a wider choice of mates, a higher mating success and produce a larger proportion of heterozygous offspring. In the absence of such a mechanism, inbreeding within small groups could lead rapidly to loss of opsin alleles (Hiramatsu et al. in press). Most discussion of the maintenance of the polymorphism to date has focused on selective advantages of either trichromats or dichromats. While selection is probably the major evolutionary force maintaining the polymorphism, disassortative mating could make an important contribution. For example, it could help to explain how alleles are maintained at low frequencies (the estimated frequency of the 556 nm allele in this population is 0.13, and this allele appears to be generally rare in tamarins (Saguinus) and lion tamarins (Leontopithecus; Surridge & Mundy 2002; Surridge et al. in press), and how three alleles have been maintained in most lineages over very long periods of evolutionary time (Surridge & Mundy 2002). As inbreeding avoidance has been documented in other marmosets and tamarins (Nievergelt et al. 2000; Faulkes et al. 2003; Huck et al. 2005), the pattern found here may be general for callitrichids.
(b) Fitness advantages for trichromatic females
Our data provide the first preliminary evidence that trichromatic females might have long-term fitness advantages over dichromatic females in the wild, both in terms of longevity and breeding success. In particular, all of the older cohort of female tamarins are trichromats and only groups containing trichromats show current breeding success. Larger sample sizes are required to confirm these results.
In conclusion, this study shows that wild tamarin group composition is non-random with respect to colour vision genotype. While this might be driven by factors that do not directly involve the opsin locus, it will act to promote and maintain opsin genetic diversity. We find no evidence that individuals of either sex are grouping according to each other's phenotypes, which might be expected if group success is dependent on phenotypic diversity. Non-random mating by opsin genotype will increase the proportion of trichromatic females in tamarin groups. These females appear to have an increased breeding success compared with dichromatic females.
Acknowledgments
This work was funded by the BBSRC (98/S11498 to H.M.B.-S. and N.I.M.), the J. William Fulbright Foreign Scholarship Board, the Wenner–Gren Foundation (grant no: 6560), the L.S.B. Leakey Foundation and the American Society of Primatologists. All field and laboratory methods were approved by the corresponding international authorities, including the Bolivian CITES authority, the US Fish and Wildlife Service and the Animal Welfare authority of New York University. Special thanks to Stephanie Dammermann, Leeann Haggerty, Rina Aviram, Laura Miller, Edilio Nacimiento and Rafael Suárez for fieldwork assistance.
References
- Caine N.G, Mundy N.I. Demonstration of a foraging advantage for trichromatic marmosets (Callithrix geoffroyi) dependent on food colour. Proc. R. Soc. B. 2000;267:439–444. doi: 10.1098/rspb.2000.1019. doi:10.1098/rspb.2000.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine N.G, Surridge A.K, Mundy N.I. Dichromatic and trichromatic Callithrix geoffroyi differ in relative foraging ability for red–green color camouflaged and non-camouflaged food. Int. J. Primatol. 2003;24:1163–1175. doi:10.1023/B:IJOP.0000005985.18112.25 [Google Scholar]
- Cropp S, Boinski S, Li W.-H. Allelic variation in the squirrel monkey X-linked color vision gene: biogeographical and behavioral correlates. J. Mol. Evol. 2002;54:734–745. doi: 10.1007/s00239-001-0073-2. doi:10.1007/s0023901-0073-2 [DOI] [PubMed] [Google Scholar]
- Faulkes C.G, Arruda M.F, Cruz A.O.M.d. Matrilineal genetic structure within and among populations of the cooperatively breeding common marmoset, Callithrix jacchus. Mol. Ecol. 2003;12:1101–1108. doi: 10.1046/j.1365-294x.2003.01809.x. doi:10.1046/j.1365-294X.2003.01809.x [DOI] [PubMed] [Google Scholar]
- Garber P, Encarnación F, Moya L, Pruetz J.D. Demographic and reproductive patterns in moustached tamarin monkeys (Saguinus mystax): implications for reconstructing platyrrhine mating systems. Am. J. Primatol. 1993;29:235–254. doi: 10.1002/ajp.1350290402. doi:10.1002/ajp.1350290402 [DOI] [PubMed] [Google Scholar]
- Hiramatsu C., Tsutsui, T., Matsumoto, Y., Aureli, F., Fedigan, L. M., Kawamura, S. In press. Color vision polymorphism in wild capuchins (Cebus capucinus) and spider monkeys (Ateles geoffroyi) in Costa Rica. Am. J. Primatol [DOI] [PubMed]
- Huck M, Löttker P, Böhle U.R, Heymann E.W. Paternity and kinship patterns in polyandrous moustached tamarins (Saguinus mystax) Am. J. Phys. Anthropol. 2005;127:449–464. doi: 10.1002/ajpa.20136. doi:10.1002/ajpa.20136 [DOI] [PubMed] [Google Scholar]
- Jacobs G.H, Neitz J, Neitz M. Genetic basis of polymorphism in the color vision of platyrrhine monkeys. Vision Res. 1993;33:269–274. doi: 10.1016/0042-6989(93)90083-9. doi:10.1016/0042-6989(93)90083-9 [DOI] [PubMed] [Google Scholar]
- Miller M.P. Northern Arizona University; Flagstaff, AZ: 1997. RxC. A program for the analysis of contingency tables. [Google Scholar]
- Mollon J.D, Bowmaker J.K, Jacobs G.H. Variations in colour vision in a New World primate can be explained by polymorphism of retinal photopigments. Proc. R. Soc. B. 1984;222:373–399. doi: 10.1098/rspb.1984.0071. [DOI] [PubMed] [Google Scholar]
- Neyman P.E. Aspects of the ecology and social organization of free-ranging cotton-top tamarins (Saguinus oedipus) and the conservation status of the species. In: Kleiman D, editor. The biology and conservation of the Callitrichidae. Smithsonian Press; Washington, DC: 1977. pp. 3971–3972. [Google Scholar]
- Nievergelt C.M, Digby L.J, Ramiakrishnan U, Woodruff D.S. Genetic analysis of group composition and breeding system in a wild common marmoset (Callithrix jacchus) population. Int. J. Primatol. 2000;21:1–20. doi:10.1023/A:1005411227810 [Google Scholar]
- Osorio D, Smith A.C, Vorobyev M, Buchanan-Smith H.M. Detection of fruit and the selection of primate visual pigments for color vision. Am. Nat. 2004;164:696–708. doi: 10.1086/425332. doi:10.1086/425332 [DOI] [PubMed] [Google Scholar]
- Savage A, Giraldo L.H, Blumer E.S, Soto L.H, Burger W, Snowdon C.T. Field techniques for monitoring cotton-top tamarins (Saguinus oedipus oedipus) in Colombia. Am. J. Primatol. 1993;31:189–196. doi: 10.1002/ajp.1350310304. doi:10.1002/ajp.1350310304 [DOI] [PubMed] [Google Scholar]
- Smith A.C, Buchanan-Smith H.M, Surridge A.K, Mundy N.I. Leaders of progressions in wild mixed-species troops of saddleback (Saguinus fuscicollis) and moustached tamarins (S. mystax), with emphasis on colour vision and sex. Am. J. Primatol. 2003a;61:145–157. doi: 10.1002/ajp.10117. doi:10.1002/ajp.10117 [DOI] [PubMed] [Google Scholar]
- Smith A.C, Buchanan-Smith H, Surridge A.K, Osorio D, Mundy N.I. The effect of colour vision status on the detection and selection of fruits by tamarins (Saguinus spp.) J. Exp. Biol. 2003b;206:3159–3165. doi: 10.1242/jeb.00536. doi:10.1242/jeb.00536 [DOI] [PubMed] [Google Scholar]
- Surridge A.K, Mundy N.I. Trans-specific evolution of opsin alleles and the maintenance of trichromatic vision in Callitrichine primates. Mol. Ecol. 2002;11:2157–2169. doi: 10.1046/j.1365-294x.2002.01597.x. doi:10.1046/j.1365-294X.2002.01597.x [DOI] [PubMed] [Google Scholar]
- Surridge A.K, Osorio D, Mundy N.I. Evolution and selection of trichromatic vision in primates. Trends Ecol. Evol. 2003;18:198–205. doi:10.1016/S0169-5347(03)00012-0 [Google Scholar]
- Surridge, A. K., Suarez, S., Buchanan-Smith, H. M., Smith, A. C. & Mundy, N. I. In press. Colour vision pigment frequencies in wild tamarins (Saguinus spp.). Am. J. Primatol [DOI] [PubMed]
- Sussman R.W, Garber P.A. A new interpretation of the social organisation and mating system of the Callitrichidae. Int. J. Primatol. 1987;8:73–92. [Google Scholar]
- Williams A.J, Hunt D.M, Bowmaker J.K, Mollon J.D. The polymorphic photopigments of the marmoset: spectral tuning and genetic basis. EMBO J. 1992;11:2039–2045. doi: 10.1002/j.1460-2075.1992.tb05261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]