Abstract
Infection density is among the most important factors for understanding the biological effects of Wolbachia and other endosymbionts on their hosts. To gain insight into the mechanisms of infection density regulation, we investigated the adzuki bean beetles Callosobruchus chinensis and their Wolbachia endosymbionts. Double-infected, single-infected and uninfected host strains with controlled nuclear genetic backgrounds were generated by introgression, and infection densities in these strains were evaluated by a quantitative polymerase chain reaction technique. Our study revealed previously unknown aspects of Wolbachia density regulation: (i) the identification of intra-specific host genotypes that affect Wolbachia density differently and (ii) the suppression of Wolbachia density by co-infecting Wolbachia strains. These findings shed new light on symbiont–symbiont and host–symbiont interactions in the Wolbachia–insect endosymbiosis and strongly suggest that Wolbachia density is determined through a complex interaction between host genotype, symbiont genotype and other factors.
Keywords: Wolbachia, multiple infection, symbiont–symbiont interaction, host–symbiont interaction, infection density
1. Introduction
Many insects harbour symbiotic micro-organisms in their gut, body cavity or cells (Buchner 1965). Some symbionts are mutualistic and contribute to the fitness of their hosts, while other symbionts are rather parasitic and tend to have negative effects on their hosts. Infections with these symbionts are, in general, maintained through host generations by vertical transmission from mothers to their offspring and often cause considerable effects on various biological aspects of the host insects (O'Neill et al. 1997).
The infection density of the symbionts is one of the most important factors for understanding their biological effects. Reduced infection density may result in imperfect vertical transmission and consequent loss of infection. Excessive infection density may lead to pathology and hence negative effects on the fitness of their hosts. On account of the selective pressures acting on the both partners, it is expected that some mechanisms should have evolved to control infection density within an appropriate range, which leads to the idea that both symbiont genotype and host genotype may contribute to the regulation of infection density (Mouton et al. 2003).
In addition, symbiont–symbiont interactions may substantially affect the infection density. Endosymbiotic associations with multiple micro-organisms are commonly found in a wide array of insect groups (Buchner 1965). Being confined in the same host body must encourage various interactions between the coexisting symbionts. The symbionts may be in competition for available resources and space in the host body or, on the other hand, they may share resources and niches by regulating their own exploitation so as not to damage the net performance of the symbiotic system (cf. Koga et al. 2003).
Endosymbionts of the genus Wolbachia are very commonly present in diverse insect groups, being estimated to infect over 20% of all insect species (e.g. Werren & Windsor 2000). Researchers have paid much attention to cytoplasmic incompatibility (CI) and other reproductive phenotypes caused by Wolbachia infection (O'Neill et al. 1997). Meanwhile, it has also been recognized that Wolbachia–insect associations provide a model system for investigating the mechanisms that regulate the infection density of endosymbionts (Ikeda et al. 2003; Mouton et al. 2003, 2004). Not only single infections but also multiple infections with different Wolbachia strains have frequently been detected in natural and laboratory insect populations (reviewed in Kikuchi & Fukatsu 2003). Using experimental techniques such as microinjection, antibiotic therapy and introgression, different combinations of Wolbachia infections can be generated under controlled genetic backgrounds of host insects. Using quantitative polymerase chain reaction (PCR) techniques, infection densities of respective Wolbachia strains coexisting in the same hosts can be strictly and efficiently evaluated. Our current knowledge of this issue from previous works is summarized below.
Effect of symbiont genotype. Single infections with different Wolbachia strains showed remarkably different infection densities in the same host genotypes (McGraw et al. 2002; Ikeda et al. 2003; Mouton et al. 2004; Veneti et al. 2004). Different Wolbachia strains exhibited remarkably different infection densities and dynamics in the same double- or triple-infected host insects (Ijichi et al. 2002; Kondo et al. 2002a; Ikeda et al. 2003; Mouton et al. 2003, 2004). These studies suggested that symbiont genotypes had significant effects on infection density.
Effect of host genotype. Interspecific transfer experiments revealed that the same Wolbachia strains showed remarkably different infection densities in different host species (between drosophilid flies (Boyle et al. 1993; Poinsot et al. 1998) and between pyralid moths (Ikeda et al. 2003)). Cytological examination of introgressed strains of Wolbachia-infected Drosophila simulans suggested that, although the data were rather qualitative, intra-specific genetic variations of the host may affect the infection density (Clark et al. 2003).
Effect of symbiont–symbiont interaction. Previous studies on a pyralid moth and parasitic wasps demonstrated that infection densities of a specific Wolbachia strain were almost constant in the presence or absence of co-infecting other Wolbachia strains (Ikeda et al. 2003; Mouton et al. 2003, 2004). These results suggested that, at least in these insect systems, infection densities of coexisting Wolbachia strains are regulated not collectively but independently and effects of symbiont–symbiont interactions are, if any, negligible.
In this study, we report previously unknown aspects of Wolbachia density regulation: (i) identification of host genotypes that affect Wolbachia density differently and (ii) suppression of Wolbachia density with co-infecting Wolbachia strains.
2. Methods
(a) Wolbachia infection in Callosobruchus chinensis
The adzuki bean beetle C. chinensis (Coleoptera: Bruchidae) is universally infected with two Wolbachia strains, wBruCon (hereafter Con) causing complete CI, and wBruOri (hereafter Ori) causing moderate CI. Initially, a third Wolbachia strain, wBruAus, was also identified on the basis of wsp gene sequences (Kondo et al. 2002a), but subsequent detailed analyses revealed that wBruAus is not a microbial entity but represents a Wolbachia genome fragment located on the X-chromosome of the host insect (Kondo et al. 2002b). Specimens of C. chinensis from Japanese populations exhibited infection frequencies of Con and Ori of 100% and 96%, respectively, corresponding to 96% double infection with Con and Ori and 4% of single infection with Con (Kondo et al. 2002a).
(b) Insect materials
Adult insects of C. chinensis were collected in adzuki (Vigna angularis) and sasage bean (Vigna unguiculata) fields in Japan and were maintained as isofemale lines in the laboratory as described previously (Kondo et al. 2002a).
To generate insect strains with different Wolbachia infection types in an identical genetic background, we started with three field-collected isofemale lines of C. chinensis: the strain COk (or k10 collected at Kasukabe, Saitama) double-infected with Con and Ori; the strain Cs (or s7 collected at Tsukuba, Ibaraki) single-infected with Con; and the strain Cg (or g19 collected at Mino, Gifu) single-infected with Con. The strain Uk, generated by tetracycline treatment of the strain COk, was genetically identical to the strain COk but lacked the Wolbachia infection. The strain Ck(s) was generated by introgressing the k10 nuclear genome into the strain Cs. After ten generations of backcrosses between Cs females and Uk males, 99.9% of nuclear genes of the strain Ck(s) were expected to be replaced by those of the strain Uk. The strain Ck(g) was generated by introgressing the k10 nuclear genome into the strain Cg in the same way.
These insect strains are listed in table 1. In short, nuclear genomes of the strains COk, Ck(s), Ck(g) and Uk were substantially identical. The wsp sequences (547 bp) of Con from the strains COk, Ck(s) and Ck(g) were 100% identical to each other, although these Wolbachia endosymbionts originated from different host populations. Note that all of the C. chinensis strains used in this study possess the transferred Wolbachia genome fragment, wBruAus, on their chromosome.
Table 1.
Strains of Callosobruchus chinensis used in this study.
| Wolbachi1a genotype | |||||
|---|---|---|---|---|---|
| strain code | infection typea | nuclear genotype | Con | Ori | descriptionb |
| COk | CO | k10 | k10 | k10 | An isofemale line originating from kkC98 population; infected with Con and Ori; nuclear background k10 |
| Cs | C | s7 | s7 | — | An isofemale line originating from skC98 population; infected with Con; nuclear background s7 |
| Cg | C | g19 | g19 | — | An isofemale line originating from mgC98 population; infected with Con; nuclear background g19 |
| Ck(s) | C | k10 | s7 | — | An introgressed line created from crosses between Uk males and Cs females; infected with Con; nuclear background k10 |
| Ck(g) | C | k10 | g19 | — | An introgressed line created from crosses between Uk males and Cg females infected with Con; nuclear background k10 |
| Uk | U | k10 | — | — | An antibiotic-treated line derived from COk strain; no infection; nuclear background k10 |
C, infected with Con (=wBruCon); O, infected with Ori (=wBruOri); U, no infection.
Geographical information of the populations was described in Kondo et al. (2002a).
(c) Quantitative PCR
Adult insects were preserved in acetone within 24 hours of emergence and individually subjected to DNA extraction by using a QIAamp tissue mini kit (Qiagen). To eliminate possible density effects, only insects that had emerged from beans with one or two eggs were sampled. The purified DNA was eluted with 200 μl of TE buffer. The concentration of total insect DNA was measured by a spectrophotometer on the basis of OD260. Bacterial titres of Con and Ori in the DNA samples were quantified by using the TaqMan PCR and ABI Prism 7700 Sequence Detection System (PE Applied Biosystems) in terms of wsp gene copies as previously described (Kondo et al. 2002a).
3. Results
(a) Host genotype affected the density of Con
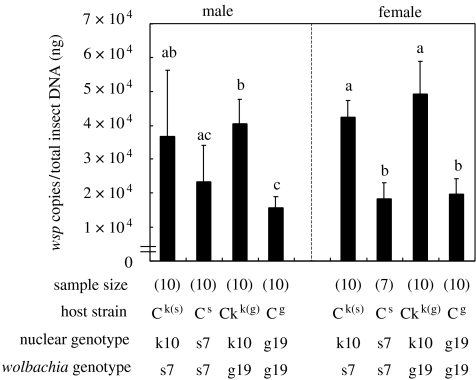
Adult insects of the single-infected strains, Cs, Cg, Ck(s) and Ck(g), were examined for bacterial titres of Con. The densities of Con reflected the host nuclear genotype rather than the symbiont genotype. Namely, Con was significantly more abundant in the nuclear genotype k10 than in the other genotypes s7 and g19, irrespective of the Wolbachia genotype and the sex of the host insect (figure 1).
Figure 1.
Infection densities of Con in single-infected strains of C. chinensis. Sample size, host strain, nuclear genotype and Wolbachia genotype are shown at the bottom. Different letters indicate statistically significant differences (Bonferonni test by using p-values with median test, p<0.05). Males and females were subjected to the statistical analysis separately. Error bars, +1 standard deviation.
(b) Co-infection with Ori suppressed the density of Con
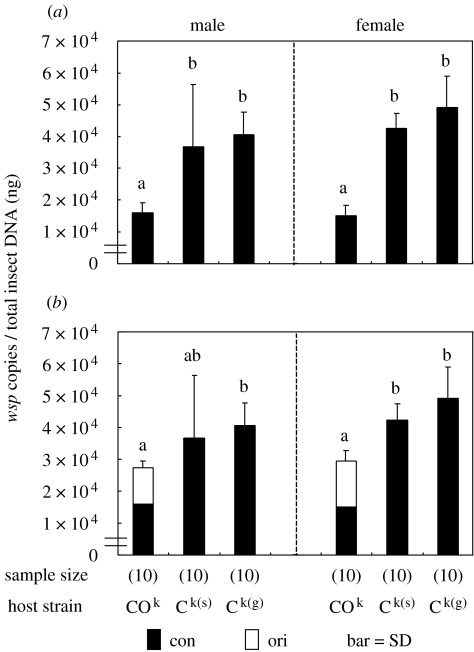
Adult insects of the double-infected strain COk and the single-infected strains Ck(s) and Ck(g), whose nuclear genomes were introgressed to become substantially identical, were examined for bacterial titres of Con and Ori, respectively. In the double-infected strain, the densities of Con were less than a half of those in the single-infected strains. Moreover, the total Wolbachia densities, including both Con and Ori, were significantly lower in the double-infected strain than in the single-infected strains. These patterns were observed in both sexes of the host insect (figure 2).
Figure 2.
Infection densities of Con and Ori in double- and single-infected strains of C. chinensis. (a) Densities of Con. (b) Total Wolbachia densities. Nuclear genotypes of these strains were introgressed to be identical to the k10 genotype. Different letters indicate statistically significant differences (Bonferonni test by using p-values with median test, p<0.05). Males and females were subjected to the statistical analysis separately.
4. Discussion
We have demonstrated that infection densities of Con were strongly affected by nuclear genotypes of C. chinensis: there were significantly higher densities in the k10 genotype than in the s7 and g19 genotypes (figure 1). The nuclear genotypes of the Con-infected strains, k10, s7 and g19, originated from different natural populations of C. chinensis, kkC98, skC98 and mgC98 (see table 1). Therefore, it is expected that considerable genetic variations in this trait may exist in these and other host populations, which would provide interesting opportunities for investigating the diversity and evolution of Wolbachia–insect interactions.
Multiple infections with different Wolbachia strains are commonly found among diverse insect taxa (Kikuchi & Fukatsu 2003). In the same host body, various interactions are expected to occur between the coexisting symbionts that would influence their infection densities. We demonstrated here that infection densities of Con were significantly suppressed by co-infecting Ori (figure 2a). Moreover, total infection densities were lower in the double infection than in the single infections (figure 2b). To our knowledge, this study is the first to directly detect a suppressive interaction between co-infecting Wolbachia endosymbionts. In a previous study, when Wolbachia densities were quantified in different tissues of a double-infected strain of C. chinensis, both Con and Ori were detected in all the tissues while their relative abundance differed between the tissues (Ijichi et al. 2002). The competitive interaction between Con and Ori appears to agree with their general co-occurrence in the host tissues.
Our findings with the beetle contrast with results from studies of wasps and moths, where infection densities of coexisting Wolbachia strains were independently controlled (Ikeda et al. 2003; Mouton et al. 2003, 2004). The seemingly discrepant results suggest that different conditions in different Wolbachia–insect systems may lead to different evolutionary consequences of density regulation, which should reflect complex symbiont–symbiont and host–symbiont interactions. It is of great interest to determine what factors favour the evolution of independent or collective control over the densities of coexisting endosymbionts in the same host.
Acknowledgments
A part of this research was supported by a PROBRAIN grant from the Bio-Oriented Technology Research Advancement Institution. N.K. was supported by the JSPS Research Fellowship for Young Scientists.
References
- Boyle L, O'Neill S.L, Robertson H.M, Karr T.L. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science. 1993;260:1796–1799. doi: 10.1126/science.8511587. [DOI] [PubMed] [Google Scholar]
- Buchner P. Interscience; New York: 1965. Endosymbiosis of animals with plant microorganisms. [Google Scholar]
- Clark M.E, Veneti Z, Bourtzis K, Karr T.L. Wolbachia distribution and cytoplasmic incompatibility during sperm development: the cyst as the basic cellular unit of CI expression. Mech. Dev. 2003;120:185–198. doi: 10.1016/s0925-4773(02)00424-0. [DOI] [PubMed] [Google Scholar]
- Ijichi N, Kondo N, Matsumoto R, Shimada M, Ishikawa H, Fukatsu T. Internal spatiotemporal population dynamics of infection with three Wolbachia strains in the adzuki been beetle, Callosobruchus chinensis (Coleoptera: Bruchidae) Appl. Environ. Microbiol. 2002;68:4074–4080. doi: 10.1128/AEM.68.8.4074-4080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Ishikawa H, Sasaki T. Regulation of Wolbachia density in the Mediterranean flour moth, Ephestia kuehniella, and the almond moth, Cadra cautella. Zool. Sci. 2003;20:153–157. doi: 10.2108/zsj.20.153. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Fukatsu T. Diversity of Wolbachia endosymbionts in heteropteran bugs. Appl. Environ. Microbiol. 2003;69:6082–6090. doi: 10.1128/AEM.69.10.6082-6090.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga R, Tsuchida T, Fukatsu T. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc. R. Soc. B. 2003;270:2543–2550. doi: 10.1098/rspb.2003.2537. doi:10.1098/rspb.2003.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo N, Ijichi N, Shimada M, Fukatsu T. Prevailing triple infection with Wolbachia in Callosobruchus chinensis (Coleoptera: Bruchidae) Mol. Ecol. 2002;11:167–180. doi: 10.1046/j.0962-1083.2001.01432.x. [DOI] [PubMed] [Google Scholar]
- Kondo N, Nikoh N, Ijichi N, Shimada M, Fukatsu T. Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect. Proc. Natl Acad. Sci. USA. 2002;99:14 280–14 285. doi: 10.1073/pnas.222228199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw E.A, Merritt D.J, Droller J.N, O'Neill S.L. Wolbachia density and virulence attenuation after transfer into a novel host. Proc. Natl Acad. Sci. USA. 2002;99:2918–2923. doi: 10.1073/pnas.052466499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton L, Henri H, Bouletreau M, Vavre F. Strain-specific regulation of intracellular Wolbachia density in multiply infected insects. Mol. Ecol. 2003;12:3459–3465. doi: 10.1046/j.1365-294x.2003.02015.x. [DOI] [PubMed] [Google Scholar]
- Mouton L, Dedeine F, Henri H, Bouletreau M, Profizi N, Vavre F. Virulence, multiple infections and regulation of symbiotic population in the Wolbachia–Asobara tabida symbiosis. Genetics. 2004;168:181–189. doi: 10.1534/genetics.104.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill S.L, Hoffmann A.A, Werren J.H. Oxford University Press; New York: 1997. Influential passengers: inherited microorganisms and arthropod reproduction. [Google Scholar]
- Poinsot D, Bourtzis K, Markakis G, Savakis C, Merçot H. Wolbachia transfer from Drosophila melanogaster into D. simulans: host effect and cytoplasmic incompatibility relationships. Genetics. 1998;150:227–237. doi: 10.1093/genetics/150.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneti Z, Clark M.E, Karr T.L, Savakis C, Bourtzis K. Heads or tails: host–parasite interactions in the Drosophila–Wolbachia system. Appl. Environ. Microbiol. 2004;70:5366–5372. doi: 10.1128/AEM.70.9.5366-5372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren J.H, Windsor D.M. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc. R. Soc. B. 2000;267:1277–1285. doi: 10.1098/rspb.2000.1139. doi:10.1098/rspb.2000.1139 [DOI] [PMC free article] [PubMed] [Google Scholar]