Abstract
Thermogenesis, in which cellular respiratory activity is considerably stimulated, requires mitochondrial uncoupling protein (UCP) in mammals and an alternative oxidase (AOX) in plants. Here, we show that the genes for both proteins are expressed in thermogenic plants, but the type correlates with the respiratory substrate. A novel gene termed PsUCPa encoding a variant of UCP was specifically expressed in thermogenic flowers of Philodendron selloum, which uses lipids as substrates. However, a gene termed DvAOX encoding for AOX protein was expressed in thermogenic flowers of Dracunculus vulgaris, which presumably uses carbohydrates as substrates. These findings suggest that cellular metabolism is a major determinant in selective expression of appropriate thermogenic genes in plants.
Keywords: thermogenic plants, Philodendron selloum, Dracunculus vulgaris, respiratory substrates, uncoupling protein, alternative oxidase
1. Introduction
Aerobic respiration—a mitochondrial process common to almost all eukaryotic organisms—involves the controlled oxidation of reduced organic substrates to carbon dioxide and water. Numerous compounds can serve as substrates for respiration, including carbohydrates, lipids, proteins and organic acids. Respiration releases a large amount of free energy not only to phosphorylate ADP (Mitchell 1961), but also to support other biological processes such as transport and metabolism (Fernie et al. 2004).
Interestingly, dissipation of mitochondrial electrochemical potential by uncoupling protein (UCP) or redox potential by alternative oxidase (AOX) increases futile respiration, leading to an increase in temperature of the thermogenic tissue or organ (Sluse & Jarmuszkiewicz 2002). This is demonstrated in some mammals by UCP1-based non-shivering thermogenesis within brown adipose tissue (Nicholls & Locke 1984) and in some plants by AOX-mediated thermogenesis as shown in the voodoo lily (Sauromatum guttatum; Raskin et al. 1987; Rhoads & McIntosh 1992). It is reasonable to assume that mammals use UCP for adaptive thermoregulation, because they lack the AOX gene. It is questionable, however, whether plants that have both AOX and UCP genes (Berthold et al. 2000) use only the AOX gene during thermogenesis.
In the present study, two arum lilies, Philodendron selloum and Dracunculus vulgaris, were chosen for examination of the expression profiles for UCP and AOX genes in relation to respiratory substrate. Measurements of respiratory exchange ratios near 1.0 have shown that carbohydrates are the respiratory substrates in Arum maculatum (James & Beevers 1950; ap Rees et al. 1976, 1977) and Symplocarpus foetidus (Seymour & Blaylock 1999; Ito et al. 2003a,b). However, thermogenic Philodendron selloum switches to lipids, because its respiratory exchange ratio becomes 0.83 (Seymour et al. 1984) and 13C : 12C ratio in respired CO2 decreases (Walker et al. 1983). In this species, the lipids are in the form of glyoxysome-like bodies in the cytoplasm of the thermogenic sterile male florets (Walker 1980), and calorimetric studies show that the energy for thermogenesis is not imported during flowering (Seymour et al. 1983). Although the respiratory exchange ratio has not been measured in D. vulgaris, calorimetric studies show that it imports substrates into its thermogenic organs (Seymour & Schultze-Motel 1999). Because hydrophobic lipids are scarcely translocated through xylem sap (Fisher 2000), we assume that soluble carbohydrates support thermogenesis in this species.
2. Material and methods
(a) Plants and thermometry
Experiments were performed during the flowering season of Philodendron selloum and Dracunculus vulgaris in suburban Adelaide, South Australia in November 2001. Thermal images were obtained using an infrared colour LSD camera (TVS-600; Nippon Avionics Ltd, Tokyo, Japan) equipped with a 35 mm lens (Ito et al. 2003a,b; Seymour et al. 2003).
(b) cDNA cloning and expression analysis
For the isolation of cDNAs for AOXs and UCPs, total RNAs were first isolated from flash-frozen, pulverized thermogenic tissues according to the previous reports (Ito et al. 1999; Ito 1999). Oligo (dT)-primed first-strand cDNAs were prepared from 1 μg samples of total RNAs using the Advantage RT-for-PCR Kit (Clontech Laboratories Inc., Palo Alto, CA) according to the manufacturer's instructions. Detailed procedures for the polymerase chain reaction (PCR) cloning and expression analyses are described in the electronic supplementary material.
3. Results
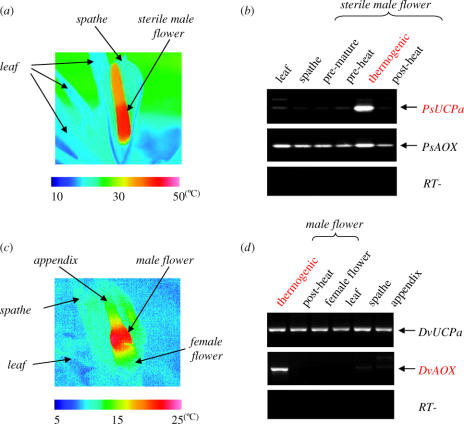
The thermogenic sterile male flowers of P. selloum reached 43 °C when the ambient temperature was 15 °C (figure 1a). Expression analysis of isolated cDNAs encoding UCP and AOX, termed PsUCPa and PsAOX, revealed that the expression of PsUCPa was specifically detected only in thermogenic sterile male flowers (figure 1b), whereas that of PsAOX was found in all tissues examined, including pre-thermogenic, post-thermogenic and thermogenic sterile male flowers. Control experiments without a reverse-transcriptase reaction confirmed that there was no genomic DNA contamination in either RNA (figure 1b).
Figure 1.
Thermogenesis and expression patterns of genes for uncoupling protein (UCP) and alternative oxidase (AOX) in Philodendron selloum and Dracunculus vulgaris. (a) Infrared thermal analysis of thermogenesis in a sterile male flower of P. selloum. (b) Expression patterns of PsUCPa and PsAOX from P. selloum in leaves, spathes and pre-thermogenic, thermogenic and post-thermogenic sterile male flowers. (c) Infrared thermal analysis of male flower-specific thermogenesis in D. vulgaris. (d) Expression patterns of DvUCPa and DvAOX from D. vulgaris in thermogenic and post-thermogenic male flowers, female flowers, leaves, spathes and appendices. RT-PCR products using gene-specific primers were visualized by Southern hybridization with probes of full-length UCP or AOX for each species. Reactions without reverse transcriptase (RT−) were also carried out to confirm the absence of DNA contamination in each RNA sample.
In D. vulgaris, the temperature of the thermogenic male flowers increased to as high as 23 °C, even when the ambient air temperature was 8 °C (figure 1c). cDNA cloning of the expressed genes for UCP and AOX led to their identification, termed DvUCPa and DvAOX, respectively. Expression of DvAOX was specific to the thermogenic male flowers, whereas that of DvUCPa was ubiquitous in thermogenic and post-thermogenic male flowers, female flowers, leaves, spathes and appendices (figure 1d). Again, it was confirmed that there was no genomic DNA contamination in any of the RNA (figure 1d). These results suggest the significance of AOX gene expression during thermogenesis in plants.
The activities of AOX have been shown to be affected by redox modulation and α-keto acid activation (Vanlerberghe et al. 1998). A cysteine residue termed CysI, which has been shown to be required for such a regulation (Berthold et al. 2000), was conserved in the amino acid sequences in both DvAOX and PsAOX (data not shown). PsUCPa, however, lacked the sixth transmembrane spanning region and the purine nucleotide-binding domain (PNBD; supplementary figure 1), which potentially shows an inhibitory effect on its uncoupling activity (Bouillaud et al. 1994). On the contrary, like the UCPs of other plants and mammals, DvUCPa contained six transmembrane domains and a conserved PNBD (supplementary figure 1).
4. Discussion
Our results clearly undermine the common dichotomy that thermogenesis depends on UCPs in mammals and AOXs in plants. P. selloum and D. vulgaris, two thermogenic plants used in this study, apparently express both UCP and AOX genes in their thermogenic tissues simultaneously. However, the specificity of the expression patterns of UCP and AOX seems to depend on the substrates for thermogenesis; that is, PsUCPa for lipids and DvAOX for carbohydrates.
Because free fatty acids (FFA) activate UCPs in plant mitochondria (Sluse et al. 1998), the lipid-based thermogenesis found in P. selloum seems to confer the advantage of using its metabolites including FFA to activate UCPs within the cells. This idea is also supported by the fact that mammalian non-shivering thermogenesis, which is stimulated by FFA-mediated UCP1 activities, uses lipids as a major respiratory substrate in brown adipocytes (Lowell & Spiegelman 2000). Furthermore, our finding of a novel gene encoding for PsUCPa, which lacks both the sixth transmembrane region and the purine-nucleotide binding domain, suggests that PsUCPa would exert its uncoupling function without any negative control by the purine nucleotides. It should also be noted that UCP3S, a mammalian homologue of PsUCPa, which also lacks both the sixth transmembrane region and the purine-nucleotide binding domain, has been shown to exhibit more active uncoupling activities than those of UCP3L, the long type of UCP3, in a yeast heterologous expression system (Hinz et al. 1999). Therefore, it is probable that PsUCPa, a short variant of the UCP family, supports the high level of mitochondrial respiration in the sterile male flowers of P. selloum.
In our study, the thermogenic tissue-specific expression of the AOX gene was found in D. vulgaris, which is consistent with the previously proposed view of the importance of AOX gene expression in thermogenic tissues in plants (Rhoads & McIntosh 1992). However, expression of a gene encoding for DvUCPa, which contains six transmembrane-spanning regions and the purine-nucleotide binding domain, was found to be ubiquitous. Because similar ubiquitous expression of a gene encoding HmUCPa, which belongs to a longer type of the UCP family, has been found in the thermogenic dead horse arum Helicodiceros muscivorus (Ito et al. 2003a,b; Seymour et al. 2003), it seems that UCPs with six transmembrane regions are not primarily involved in tissue-specific thermogenesis in plants.
Our present results further suggest that carbohydrate-based thermogenesis, which requires specific AOX gene expression, has been developed to use its metabolic intermediates such as pyruvate to stimulate AOX activity (McDonald et al. 2002). Because pyruvate is synthesized as an end-product of glycolysis and further oxidized in the TCA cycle within mitochondria, pyruvate would be a one of the ideal activators of AOX when glycolysis is a major metabolic pathway during thermogenesis.
The two species of this study exhibit increasing respiration rates with decreasing ambient and tissue temperatures, a pattern termed ‘thermoregulation’ (Nagy et al. 1972; Seymour et al. 1984; Seymour & Schultze-Motel 1999). Clear upregulation of UCP and AOX suggests that these enzymes may play a crucial role in thermoregulation in plants. However, the two species exhibit a distinct diversity in their respiratory substrates and specificity of thermogenic genes, so it is important to elucidate the detailed molecular mechanisms of tissue- and substrate-specific gene expression in thermogenic plants.
Acknowledgments
This study was supported by Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN), a Grant-in-Aid for the 21st Century COE Programme from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Australian Research Council. We thank Y. Abe, T. Sato and S. D. Johnston for assistance in gene expression analysis, and M. Brew for growing the Dracunculus.
Supplementary Material
References
- ap Rees T, Fuller W.A, Wright B.W. Pathways of carbohydrate oxidation during thermogenesis by the spadix of Arum maculatum. Biochim. Biophys. Acta. 1976;437:22–35. doi: 10.1016/0304-4165(76)90344-5. doi:10.1016/0304-4165(76)90344-5 [DOI] [PubMed] [Google Scholar]
- ap Rees T, Wright B.W, Fuller W.A. Measurements of starch breakdown as estimates of glycolysis during thermogenesis by the spadix of Arum maculatum L. Planta. 1977;134:53–56. doi: 10.1007/BF00390094. [DOI] [PubMed] [Google Scholar]
- Berthold D.A, Andersson M.E, Nordlund P. New insight into the structure and function of the alternative oxidase. Biochim. Biophys. Acta. 2000;1460:241–254. doi: 10.1016/s0005-2728(00)00149-3. doi:10.1016/S0005-2728(00)00149-3 [DOI] [PubMed] [Google Scholar]
- Bouillaud F, Arechaga I, Petit P.X, Raimbault S, Levi-Meyrueis C, Laurent C.L, Rial M, Ricquier D. A sequence related to a DNA recognition element is essential for the inhibition by nucleotides of proton transport through the mitochondrial uncoupling protein. EMBO J. 1994;13:1990–1997. doi: 10.1002/j.1460-2075.1994.tb06468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie A.R, Carrari F, Sweetlove L. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004;7:1–8. doi: 10.1016/j.pbi.2004.03.007. doi:10.1016/j.pbi.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Fisher D.B. Long-distance transport. In: Buchanan B.B, Gruissem W, Jones R.L, editors. Biochemistry & molecular biology of plants. American Society of Plant Physiologists; Maryland: 2000. pp. 730–784. [Google Scholar]
- Hinz W, Grüninger S, Pover A.D, Chiesi M. Properties of the human long and short isoforms of the uncoupling protein-3 expressed in yeast cells. FEBS Lett. 1999;462:411–415. doi: 10.1016/s0014-5793(99)01568-9. doi:10.1016/S0014-5793(99)01568-9 [DOI] [PubMed] [Google Scholar]
- Ito K. Isolation of two distinct cold-inducible cDNAs encoding plant uncoupling proteins from the spadix of skunk cabbage (Symplocarpus foetidus) Plant Sci. 1999;149:167–173. doi:10.1016/S0168-9452(99)00159-4 [Google Scholar]
- Ito K, Kusano T, Tsutsumi K. A cold-inducible bZIP protein gene in radish root regulated by calcium- and cycloheximide-mediated signals. Plant Sci. 1999;142:57–65. doi:10.1016/S0168-9452(99)00159-4 [Google Scholar]
- Ito K, Abe Y, Johnston S.D, Seymour R.S. Ubiquitous expression of a gene encoding for uncoupling protein isolated from the thermogenic inflorescence of the dead horse arum Helicodiceros muscivorus. J. Exp. Bot. 2003a;54:1113–1114. doi: 10.1093/jxb/erg115. doi:10.1093/jxb/erg115 [DOI] [PubMed] [Google Scholar]
- Ito K, Onda Y, Sato T, Abe Y, Uemura M. Structural requirements for the perception of ambient temperature signals in homeothermic heat production of skunk cabbage (Symplocarpus foetidus) Plant Cell Environ. 2003b;26:783–788. doi: 10.1046/j.1365-3040.2003.00989.x. doi:10.1046/j.1365-3040.2003.00989.x [DOI] [PubMed] [Google Scholar]
- James W.O, Beevers H. The respiration of Arum spadix. A rapid respiration, resistant to cyanide. New Phytol. 1950;49:353–374. [Google Scholar]
- Lowell B.B, Spiegelman B.M. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. doi:10.1038/35007527 [DOI] [PubMed] [Google Scholar]
- McDonald A.E, Sieger S.M, Vanlerberghe G.C. Methods and approaches to study plant mitochondrial alternative oxidase. Physiol. Plant. 2002;116:135–143. doi: 10.1034/j.1399-3054.2002.1160201.x. doi:10.1034/j.1399-3054.2002.1160201.x [DOI] [PubMed] [Google Scholar]
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Nagy K.A, Odell D.K, Seymour R.S. Temperature regulation by the inflorescence of Philodendron. Science. 1972;178:1195–1197. doi: 10.1126/science.178.4066.1195. [DOI] [PubMed] [Google Scholar]
- Nicholls D.G, Locke R.M. Thermogenic mechanisms in brown fat. Physiol. Rev. 1984;64:1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Raskin I, Ehmann A, Melander W.R, Meeuse B.J.D. Salicylic acid: a natural inducer of heat production in Arum lilies. Science. 1987;237:1601–1602. doi: 10.1126/science.237.4822.1601. [DOI] [PubMed] [Google Scholar]
- Rhoads D.M, McIntosh L. Salicylic acid regulation of respiration in higher plants: alternative oxidase expression. Plant Cell. 1992;4:1131–1139. doi: 10.1105/tpc.4.9.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour R.S, Blaylock A.J. Switching off the heater: influence of ambient temperature on thermoregulation by eastern skunk cabbage Symplocarpus foetidus. J. Exp. Bot. 1999;50:1525–1532. doi:10.1093/jexbot/50.338.1525 [Google Scholar]
- Seymour R.S, Schultze-Motel P. Respiration, temperature regulation and energetics of thermogenic inflorescences of the dragon lily Dracunculus vulgaris (Araceae) Proc. R. Soc. B. 1999;266:1975–1983. doi:10.1098/rspb.1999.0875 [Google Scholar]
- Seymour R.S, Bartholomew G.A, Barnhart M.C. Respiration and heat production by the inflorescence of Philodendron selloum Koch. Planta. 1983;157:336–343. doi: 10.1007/BF00397405. [DOI] [PubMed] [Google Scholar]
- Seymour R.S, Barnhart M.C, Bartholomew G.A. Respiratory gas exchange during thermogenesis in Philodendron selloum Koch. Planta. 1984;161:229–232. doi: 10.1007/BF00982917. [DOI] [PubMed] [Google Scholar]
- Seymour R.S, Gibernau M, Ito K. Thermogenesis and respiration of inflorescences of the dead horse arum Helicodiceros muscivorus, a pseudo-thermoregulatory aroid associated with fly pollination. Funct. Ecol. 2003;17:886–894. doi:10.1111/j.1365-2435.2003.00802.x [Google Scholar]
- Sluse F.E, Jarmuszkiewicz W. Uncoupling proteins outside the animal and plant kingdoms: functional and evolutionary aspects. FEBS Lett. 2002;510:117–120. doi: 10.1016/s0014-5793(01)03229-x. doi:10.1016/S0014-5793(01)03229-X [DOI] [PubMed] [Google Scholar]
- Sluse F.E, Almeida A.M, Jarmuszkiewicz W, Vercesi A.E. Free fatty acids regulate the uncoupling protein and alternative oxidase activities in plant mitochondria. FEBS Lett. 1998;433:237–240. doi: 10.1016/s0014-5793(98)00922-3. doi:10.1016/S0014-5793(98)00922-3 [DOI] [PubMed] [Google Scholar]
- Vanlerberghe G.C, McIntosh L, Yip J.Y.H. Molecular localization of a redox-modulated process regulating plant mitochondrial electron transport. Plant Cell. 1998;10:1551–1560. doi: 10.1105/tpc.10.9.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, D.B. 1980 Structural and histochemical study in the heat-generating, sterile male florets in Philodendron selloum (Conference Abstr.) Botany 80, Vancouver, B.C.
- Walker D.B, Gysi J, Sternberg L, DeNiro M.J. Direct respiration of lipids during heat production in the inflorescence of Philodendron selloum. Science. 1983;220:419–421. doi: 10.1126/science.220.4595.419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.