Abstract
Most seabirds are visual hunters and are thus strongly affected by light levels. Dependence on vision should be problematic for species wintering at high latitudes, as they face very low light levels for extended periods during the Polar night. We examined the foraging rhythms of male great cormorants (Phalacrocorax carbo) wintering north of the Polar circle in West Greenland, conducting the first year-round recordings of the diving activity in a seabird wintering at high latitudes. Dive depth data revealed that birds dived every day during the Arctic winter and did not adjust their foraging rhythms to varying day length. Therefore, a significant proportion of the dive bouts were conducted in the dark (less than 1 lux) during the Polar night. Our study underlines the stunning adaptability of great cormorants and raises questions about the capacity of diving birds to use non-visual cues to target fish.
Keywords: vision, foraging, year-round recording, data loggers, arctic, seabird
1. Introduction
Seabirds and marine mammals operating at high latitudes exploit some of the most productive zones of the world's oceans (Hensen 1892). These abundant resources are nonetheless highly seasonal, becoming scarce or less accessible during the winter months. Polar winters are also characterized by very low temperature and light levels, causing a potential handicap to endothermic visual hunters such as diving birds and mammals. If some species are highly adapted to such drastic constraints (e.g. seals), others are apparently not designed for this challenge. Among them the great cormorant (Phalacrocorax carbo), a diving, piscivorous bird, is probably the worst candidate on the Polar over-wintering team because (i) it is small compared to marine mammals, and therefore cannot minimize thermoregulatory costs via a mass effect, (ii) it does not accumulate fat layers, and its plumage is partly wettable (Grémillet et al. 2005). Great cormorants therefore lose large amounts of heat to the water when diving and their metabolic costs of diving in cold water are the highest so far measured in a diving endotherm (Grémillet et al. 2001), (iii) it is assumed to be a visual hunter (Strod et al. 2004).
Despite these handicaps, a small population of great cormorants breeds and winters along the west coast of Greenland, north of the Polar circle (Boertmann & Mosbech 1997; Lyngs 2003). Great cormorants avoid flying long distances over the open sea, and it seems that this population is confined to Greenland because the two potential wintering quarters (Newfoundland and Iceland) are too far away. During the Arctic winter great cormorants are exposed to very low light levels for extended periods (the Polar night, when the sun remains below the horizon). This disadvantage is likely to affect their foraging performance, with potential impact on their survival.
We studied great cormorants wintering in Greenland to understand the behavioural responses of a visually hunting marine predator to the strongly varying light levels which occur during the Polar winter. To this end we used miniature electronic devices which allowed us to record the diving activity of free-ranging individuals for a period of 6–12 months (i.e. throughout the Polar night).
2. Material and methods
In June 2002 miniature data loggers (60×24×7 mm; 20 g i.e. less than 0.6% of body mass, see Woakes et al. 1995) were placed into the abdominal cavity of 10 male great cormorants breeding on Disko Island, West Greenland (69°30′ N, 54°05′ W) under gas anaesthesia (Stephenson et al. 1986). These electronic tags were programmed to record dive depth at 2 s intervals every second day for a period of 1 year and were calibrated before and after use (depth accurate to ±0.05 m). Great cormorants were observed to breed normally after being fitted with the devices. Nine data loggers were recovered during the subsequent breeding season in June 2003, and one in June 2004. All 10 birds bred successfully after removal of the internal units. Seven data loggers collected 90 Mio. data points throughout the winter of 2002–2003 while three recorded no or partial data sets. Recorded depth data were analysed using Multitrace (Jensen Software Systems, Laboe, Germany) in order to determine the timing of each foraging trip and the total time spent in the water per day. Great cormorants only stay in the water when they are actively diving; hence time in the water was defined as the time period between the beginning of the first dive (sharp increase of recorded pressure values) and the end of the last dive in a dive bout. Total time in the water per day was calculated as the sum of the durations of all dive bouts performed during that period.
All experiments conformed to the British Home Office regulations for avoiding unnecessary suffering to the animals. They were performed by trained veterinary personnel under permits of the Ethics committees of the French Polar Institute, The Danish Polar Center, The Science Board of the Arctic Station, The Danish Veterinary Administration, and the Greenland Homerule Government.
3. Results
The male great cormorants studied in West Greenland dived on every recorded day (i.e. every second day throughout the Polar winter), strongly suggesting that these birds dive every day, year-round. During the non-breeding period (September to April) they swam 60±20 min d−1 on average (range 18–131 min d−1) in waters of 12.8±2.8 m depth (range 8.0–18.0 m). Cormorants are benthic feeders; therefore these dive depths correspond to water depths. Time in the water increased significantly with decreasing day length (χ12=49.7, p<0.001), to reach maximum values of up to 100 min d−1 during the Polar night (December and January). Birds spent less time in the water to catch their daily ration of fish during the December full moon than during the January new moon (50±21 and 84±50 min d−1, respectively). This difference was nonetheless not statistically significant (F1,6=3.11, p=0.103).
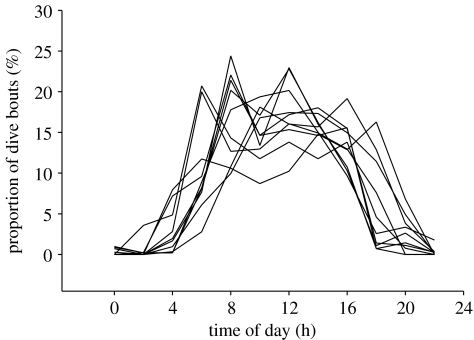
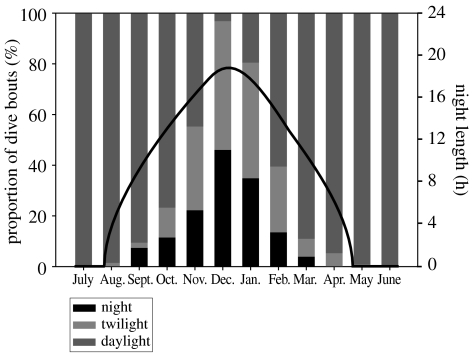
Most surprisingly, birds did not change their daily rhythms according to fluctuating light levels. Diving activities mainly took place between 05.00 and 20.00 h but the timing of dive bouts did not shift towards midday as winter progressed (figure 1; χ102=2.953, p=0.966). Consequently, the proportion of dive bouts performed in the dark increased gradually as day length decreased, with 46% of dive bouts taking place at night (sun more than 6° below the horizon) in December (figure 2).
Figure 1.
Proportion of dive bouts (in %) conducted by Greenland great cormorants at different times of the 24 h cycle between July and the following April (one line per month, seven birds, 4764 foraging trips).
Figure 2.
Proportion of dive bouts (in %) performed by Greenland great cormorants during daylight, twilight (sun 0–6° below horizon), and night hours (sun more than 6° below horizon) throughout the year (seven birds, 4764 foraging trips). The continuous line shows night length.
4. Discussion
Our study is the first, to our knowledge, to record the diving behaviour of a seabird wintering at high latitude continuously. It shows that great cormorants, contrary to that expected, do not modify their foraging rhythms according to light levels, and manage to feed in the dark during the Polar night. A recent study has shown that diving animals foraging at depths of approximately 10 m at night (sun more than 6° below the horizon) experience an illuminance of less than 1 lux (McCafferty et al. 2004), which is equivalent to starlight.
Many species of seabirds, especially petrels, are known to forage at night (Martin & Prince 2001). However, little information is available on how light levels affect the foraging tactics and the foraging performance of these marine predators. In an extensive study concerning five penguin species Wilson and colleagues (1993) showed that these birds had lower foraging activity and lower foraging success at night, indicating that light intensity is a major constraint, even for penguin eyes (Martin & Young 1984). Similar light-limited foraging patterns were also recorded in blue-eyed shags (Phalacrocorax atriceps), and European shags (Phalacrocorax aristotelis) (Wanless et al. 1999). During the day, deep diving species such as king penguins (Aptenodytes patagonicus) experience very low light levels (less than 1 lux) at their foraging depths (more than 300 m) which could seriously impede prey detection (Kooyman et al. 1992; Pütz & Bost 1994). It has been suggested that birds counter this problem by foraging on bioluminescent myctophid fish (Martin 1999). In the Arctic, two studies based upon direct observations conducted over limited time periods investigated the behavioural strategies of diving seabirds wintering at high latitudes (70° N). They indicated that Steller's eiders (Polysticta stelleri) and great cormorants wintering in northern Norway shifted their foraging times towards midday as winter progressed, in order to forage during twilight hours (Johansen et al. 2001; Systad & Bustnes 2001).
These different examples show that seabird species studied to date respond to diel light levels, either through a shift of their foraging rhythms, or via specific prey choice. None of these strategies are apparent in great cormorants wintering in Greenland, which keep the same daily rhythms (figure 1), and the same diet (sculpins Myoxocephalus, which are not bioluminescent; Grémillet et al. 2001) throughout the winter phase.
Some aspects of the behaviour of great cormorants wintering in Greenland do change during the Arctic winter: they spend more time in the water during the darkest months of the year than in the autumn and the spring. This change might have two different causes, which are not mutually exclusive. (i) Birds achieve lower foraging performance at low light levels, and therefore require longer foraging periods to gather their daily load of fish. (ii) Birds diving in waters at sub-zero temperature have very high thermoregulatory costs, and therefore compensate by eating more fish. Gathering this additional amount of food might also lengthen foraging sessions.
After observing Greenland great cormorants foraging at the end of the Polar winter (March), we proposed that birds adapt to Arctic winter conditions by a sharp increase in their foraging efficiency (Grémillet et al. 2001). We speculated that this tactic could allow them to minimize time spent in cold water, therefore allowing them to balance their energy budget in a thermally hostile environment. This hypothesis, which might be true during the Arctic spring, does not hold for the Polar night. At this time, great cormorants spend more time in the water every day than during the breeding season (up to 100 min d−1 during the Polar night versus 45 min d−1 when rearing young chicks on Disko Island, see Grémillet et al. 2001), suggesting substantial daily energy expenditure and/or reduced foraging efficiency.
Great cormorants are considered strict visual hunters, and their underwater visual acuity can be affected by even minor levels of turbidity (Strod et al. 2004). It is therefore perplexing how they catch fish under the very poor light conditions of the Polar night. According to local Inuit hunters most of the cormorants' foraging areas are covered by ice during this period. Birds have to find open water maintained by tidal currents where they catch fish along the ice edge. We suggest that they then switch from visual to tactile and/or acoustic foraging strategies. Such alternative mechanisms are also suspected in night-active petrels foraging on epipelagic prey (Martin & Prince 2001). Great cormorants could for instance scan the seabed with their beaks until they hit sculpins (their main prey) which are sluggish at low temperatures.
The hypothesis that diving avian predators such as cormorants can efficiently catch fish underwater using non-visual cues remains to be tested.
Acknowledgments
This study was funded by the French Polar Institute and CNRS. We warmly thank the crews of the vessels Porsild and Maya, the staffs of the Arktisk Station and of the French Polar Institute. Grateful thanks are also due to Cécile Devred, Emeline Pettex and Gry Bastholm for their extensive help during the field sessions, to Frédéric Sardon, Delphine Haas and Aurélie Girard for their participation in data analysis, and to Graham Martin, Warren P. Porter, Sascha Hooker, David Boertmann, Manfred Enstipp, and Rory P. Wilson for constructive comments.
References
- Boertmann D, Mosbech A. Breeding distribution and abundance of the great cormorant Phalacrocorax carbo carbo in Greenland. Pol. Res. 1997;16:93–100. [Google Scholar]
- Grémillet D, Wanless S, Carss D.N, Linton D, Harris M.P, Speakman J.R, Le Maho Y. Foraging energetics of arctic cormorants and the evolution of diving birds. Ecol. Lett. 2001;4:180–184. [Google Scholar]
- Grémillet D, Chauvin C, Wilson R.P, Le Maho Y, Wanless S. Unusual feather structure allows partial plumage wettability in diving great cormorants. J. Avian Biol. 2005;36:1–7. [Google Scholar]
- Hensen V. Lipsius & Tischer Verlag; Kiel und Leipzig: 1892. Ergebnisse der (in den Atlantischen Ozean von Mitte Juli bis Anfang November 1889) Plankton Expedition der Humboldt Stiftung. [Google Scholar]
- Johansen R, Barrett R.T, Pedersen T. Foraging strategies of Great Cormorants Phalacrocorax carbo carbo wintering north of the arctic circle. Bird Study. 2001;48:59–67. [Google Scholar]
- Kooyman G.L, Cherel Y, Le Maho Y, Croxall J.P, Thorson P.H, Ridoux V. Diving behavior and energetics during foraging in king penguins. Ecol. Monogr. 1992;62:143–163. [Google Scholar]
- Lyngs P. Migration and winter ranges of birds in Greenland. An analysis of ringing recoveries. Dansk Ornithologisk Forenings Tidsskrift. 2003;97:18–21. [Google Scholar]
- Martin G.R. Eye structure and foraging in King Penguins Aptenodytes patagonicus. Ibis. 1999;141:444–450. [Google Scholar]
- Martin G.R, Prince P.A. Visual fields and foraging in procellariiform seabirds: sensory aspects of dietary segregation. Brain Behav. Evol. 2001;57:33–38. doi: 10.1159/000047224. [DOI] [PubMed] [Google Scholar]
- Martin G.R, Young S.R. The eye of the Humboldt penguin, Spheniscus humboldti: visual fields and schematic optics. Proc. R. Soc. B. 1984;223:197–222. doi: 10.1098/rspb.1984.0090. [DOI] [PubMed] [Google Scholar]
- McCafferty D.J, Walker T.R, Boyd I.L. Using time-depth-light recorders to measure light levels experienced by a diving marine mammal. Mar. Biol. 2004;146:191–199. [Google Scholar]
- Pütz K, Bost C.A. Feeding behaviour of free-ranging king penguins (Aptenodytes patagonicus) Ecology. 1994;75:489–497. [Google Scholar]
- Stephenson R, Butler P.J, Woakes A.J. Diving behaviour and heart rate in tufted ducks (Aythya fuligula) J. Exp. Biol. 1986;126:341–359. doi: 10.1242/jeb.126.1.341. [DOI] [PubMed] [Google Scholar]
- Strod T, Arad Z, Izhaki I, Katzir G. Cormorants keep their power: visual resolution in a pursuit-diving bird under amphibious and turbid conditions. Curr. Biol. 2004;14:R376–R377. doi: 10.1016/j.cub.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Systad G.H, Bustnes J.O. Coping with darkness and low temperatures: foraging strategies in Steller's eiders, Polystica stelleri, wintering at high latitudes. Can. J. Zool. 2001;79:402–406. [Google Scholar]
- Wanless S, Finney S.K, Harris M.P, McCafferty D.J. Effect of the diel light cycle on the diving behaviour of two bottom feeding marine birds: the blue-eyed shag Phalacrocorax atriceps and the European shag P-aristotelis. Mar. Ecol. Progr. Ser. 1999;188:219–224. [Google Scholar]
- Wilson R.P, Pütz K, Bost C.A, Culik B.M, Bannasch R, Reins T, Adelung D. Diel dive depth in penguins in relation to diel vertical migration of prey—whose dinner by candlelight? Mar. Ecol. Progr. Ser. 1993;94:101–104. [Google Scholar]
- Woakes A.J, Butler P.J, Bevan R.M. Implantable data logging system for heart rate and body temperature: its application to the estimation of field metabolic rates in Antarctic predators. Med. Biol. Eng. Comput. 1995;33:145–151. doi: 10.1007/BF02523032. [DOI] [PubMed] [Google Scholar]