Abstract:
What are the mechanisms that prevent partners from cheating in potentially cooperative interactions between unrelated individuals? The cleaner fish Labroides dimidiatus and client reef fish both benefit from an interaction as long as the cleaner eats ectoparasites. However, the cleaner fish prefers some client mucus, which constitutes cheating. Field observations suggested that clients control such cheating by using punishment (chasing the cleaner) or by switching partners (fleeing from the cleaner). Here, we tested experimentally whether such client behaviours result in cooperative cleaner fish. Cleaners were allowed to feed from Plexiglas plates containing prawn items and fish flake items. A lever attached to the plates allowed us to mimic the behaviours of clients. As cleaners showed a strong preference for prawn over flakes, we taught them that eating their preferred food would cause the plate to either chase them or to flee, while feeding on flakes had no negative consequences. We found a significant shift in cleaner fish foraging behaviour towards flake feeding after six learning trials. As punishment and terminating an interaction resulted in the cleaners feeding against their preferences in our experiment, we propose that the same behaviours in clients improve the service quality of cleaners under natural conditions.
Keywords: mutualism, cooperation, punishment, partner choice, biological market, Labroides dimidiatus
1. Introduction
The iterated prisoner's dilemma (IPD) game, with all its many theoretical extensions (Dugatkin 1997; Hammerstein 2003), does not appear to provide a general framework for the evolution and maintenance of cooperation between unrelated individuals. Few examples of intraspecific cooperation fulfil its assumptions (Hammerstein 2003), and no example of interspecific mutualism is thought to solve the IPD (Bergstrom et al. 2003). Instead, a variety of alternative control mechanisms have been proposed, based either on game theoretic modelling or on empirical results. Such alternative mechanisms include pseudoreciprocity (Conner 1986), the threat of terminating an interaction (Johnstone & Bshary 2002), ‘passive partner choice’ usually called ‘sanctions’ (Kiers et al. 2003), active partner switching (Bshary & Schäffer 2002; Ferrière et al. 2002) and punishment (Clutton-Brock & Parker 1995). The mutualism between the cleaner wrasse Labroides dimidiatus and its client reef fish seems to be a good model system to study some of these control mechanisms (Bshary & Noë 2003; Bshary & Bronstein 2004). While a variety of studies support the mutualistic nature of cleaning interactions in general (Grutter 1999; Grutter & Lester 2002; Cheney & Côté 2003), there is experimental evidence for an important conflict: the cleaner fish prefers some mucus over ectoparasites (Grutter & Bshary 2003). Mucus is important for a client's health (Ebran et al. 1999 and references therein), so its loss should be costly. Hence, clients face the challenge of how to make cleaners feed against their preference in order to receive a high service quality during interactions.
Predatory clients could counter any mucus feeding by what Bshary & Bronstein (2004) termed the ‘threat of reciprocity’: predators can try to cheat in return and eat a cheating cleaner. The threat of reciprocity differs from tit-for-tat like reciprocity as cheating by a predator terminates the game, while tit-for-tat may alter the cheater's behaviour in the future. The threat of reciprocity may account for an almost unconditional cooperative behaviour of cleaners towards predators (Bshary 2001). The vast majority of clients, however, do not prey on cleaners (Bshary 2001) and therefore have no means to counter-exploit a cheating cleaner fish. Therefore, the game between cleaners and these non-predatory clients is asymmetric in that clients have no means to cheat (gain from exploiting the partner) a cleaner in response to its cheating (as IPD based strategies like tit-for-tat do). Field observations suggest that these clients use mainly two alternative control mechanisms: visiting clients with access to several cleaning stations respond with immediate flight and visit another cleaning station (partner switching) for their next inspection (Bshary & Schäffer 2002), while resident clients with access to only one cleaning station respond to cleaner fish cheating with aggressive chasing (punishment) of the cleaner (Bshary & Grutter 2002). Note that client aggression also terminates the interaction. Any benefits of the aggression are delayed to future interactions (Bshary & Grutter 2002) as required by the definition of punishment (Clutton-Brock & Parker 1995). Here, we test experimentally whether apparent punishment or partner switching could cause cleaner fishes to feed against their preference, the essential requirement for a mutually beneficial outcome of cleaning interactions (Grutter & Bshary 2003).
2. Methods
The study was carried out in March/April 2003 at the Lizard Island Research Station, Great Barrier Reef, Australia. We caught 24 adult cleaner fish (6.2–8.9 cm total length) of unknown sex from the reef surrounding the island. The fishes were kept individually in aquaria ranging in size from 69×25×30 cm to 95×35×35 cm with direct seawater flow through and a small pipe for shelter. The fishes were fed daily with mashed prawn flesh or with a mixture of mashed prawn flesh and fish flakes (referred to as ‘flakes’). The flakes mixture was prepared fresh every day with one-third volume of flakes and two-third volume of prawn. The food was spread on plastic (Plexiglas) plates (Grutter & Bshary 2004). Cleaners learned to feed from the plates within 1–3 days of exposure. The plates had a variety of uniform colours, grey, beige or white. Each cleaner was exposed to all different colours to become accustomed to the presentation of unfamiliar stimuli (to avoid potentially neophobic cleaners). In the experiments, half of the cleaners were confronted first with a plate of pink and black patterns to test for their initial food preference, and then with a plate with beige and yellow patterns during the learning phase and the final experiment (see below). The other half of the cleaners interacted with the two types of plates in the opposite order. The different plates were used to possibly make it easier for the cleaners to realize that the situation has changed (i. e. the pink and black plate does not accept that the cleaner eats its preferred food while previously the beige and yellow plate had not responded). The experiments began after the fishes had been in captivity for at least 20 days.
The experiment consisted of three phases, namely an initial preference test, subsequent learning phase and the final foraging test. The plates used in the experiment were attached to a 40 cm long lever that allowed the experimenter to simulate the behaviour of the client fishes (fleeing or chasing the cleaner, or just leaving after the cleaner finished foraging).
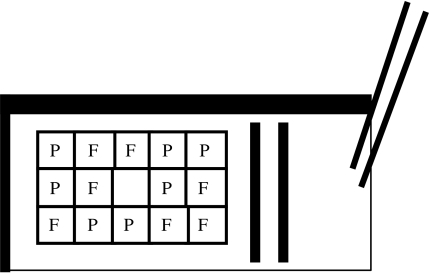
The initial preference test: we offered the cleaners an unfamiliar plate with seven prawn items and seven flake items. The items were placed within a 5×3 grid, each grid cell being 1×1 cm in size. The sequence of the 14 items (prawn or flake) placed in the grid cells was determined by using tables of random sequences of 0 and 1 s, where 0 represented prawn and 1 represented flake. The central grid cell was always left empty (figure 1). The cleaners could eat all items but plates were removed once a cleaner stopped feeding with items still remaining. After three trials that allowed cleaners to become familiar with the plates, we conducted the initial preference test. We offered the plate three times to each cleaner and scored the first seven items eaten. This meant that we could possibly find a 100% preference for either prawn or flakes. We used the total of 21 food items eaten in the three trials to calculate the degree of preference of each cleaner for one food as expressed in percentage of items eaten of that food type.
The learning phase: we split the 24 cleaner fishes into three experimental groups of eight individuals each. Each cleaner was subjected to six learning trials separated by 120 min intervals. In the first group (‘control’), cleaners were allowed to continue to eat as they chose. The plate was only removed once the cleaners stopped foraging. In the second group (‘client flees’), cleaners were trained such that eating the less preferred food items had no consequences while eating a preferred item led to the immediate and rapid removal of the plate (‘fleeing’). In each trial, the plate was offered to the fishes again after 60 s (with the remaining food items on it but the already eaten ones not replaced) until the cleaner ate a second preferred food item. That led to the removal of the plate until the next learning trial 120 min later. In the third group (‘client chases’), cleaners were trained that whenever a preferred item was eaten, the plate would chase them in the aquarium for about 1–2 s, without touching the cleaners. The cleaner was then allowed to forage again until it had eaten its preferred food for a second time, which led to first chasing and then the plate being removed until the next trial 120 min later. To improve the probability that cleaners might learn what the consequences were of eating either their preferred or less preferred food, we offered two preferred and 12 non-preferred items during the first three learning periods (also in the control group). This ensured that cleaners ate also from their less preferred food and therefore could learn that there were no negative consequences of doing so. After another three learning trials with the standard 7 : 7 distribution of food items, the learning phase was terminated.
Final foraging experiment: Each cleaner was allowed to interact once with the plate that did not respond to the cleaner's foraging behaviour. In other words, eating a preferred food item had no negative consequences. Again, we scored the first seven items eaten, allowing for the possibility of a 100% bias for either food.
Figure 1.
Experimental Plexiglas plate. The stripes on the plates that are illustrated in black had two different colouration patterns, either pink and black or yellow and beige. The initial preference test was conducted with one plate type, and the teaching trials and the final experiment with the other plate type. Fourteen food items were offered in each trial. Grid cells were filled from the upper left to the lower right, according to a random, but balanced, sequence from a random table. Once one food type had been selected seven times, the remaining grid cells were filled with the other food type. Each random sequence was used only once in each of the three treatment groups; the sequence shown in the figure is just one possibility. P, prawn item (corresponding to a 0 in the random sequence); F, flake item (corresponding to a 1 in the random sequence). On the right of the plate is the lever (40 cm long) that allowed the experimenter to react to cleaner fishes' foraging behaviour according to the treatment group (no reaction, fleeing, or chasing in response to prawn feeding).
The time schedule for each cleaner was the following, with 120 min intervals between trials: day 1: three introductory trials plus the three preference tests. day 2: four rounds of learning trials. day 3: two rounds of learning trials followed by the experimental trials. All items were similar in size, weighing about 0.0002 g each. Trials were scheduled so that cleaners received about 0.005–0.01 g of food per day. We tested individuals of all three groups simultaneously. Thus, individuals of all three groups were tested with the same batch of flake food.
Wilcoxon matched pair tests were used to evaluate whether each of the three different behaviours of plates during the learning phase had a significant influence on cleaner fish foraging behaviour in the final experiment as compared to the initial preference tests.
3. Results
In the initial preference test, all 24 cleaners ate more prawn items than flake items. The least extreme preference observed was 15 prawn items to six flake items. Thus our cleaners showed a highly significant preference for prawn (Wilcoxon-test, n=24, T=0, p<0.0001). On average, 91% of the first seven items eaten were prawn items. Therefore, all cleaners could be trained that feeding on flakes had no consequences but feeding on prawn led to either chasing or fleeing.
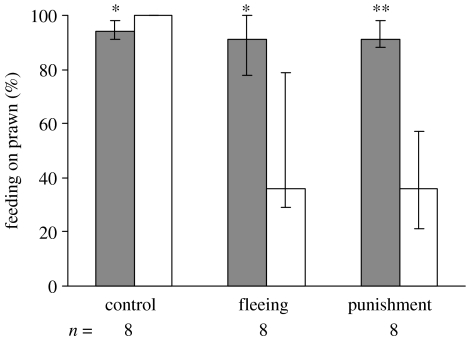
As the plates did not ‘respond’ to cleaner fishes' foraging behaviour in the final experiment, individuals of all three groups had the opportunity to eat the seven prawn items first and then the seven flake items if still hungry. We found that individuals of the control group significantly increased their initial preference for prawn during the learning trials, ending with a 100% preference for prawn as opposed to a 92% preference for prawn during the initial preference tests (Wilcoxon-test, n=8, two ties, resulting n=6, T=0, p=0.032; figure 2). In contrast, the other two experimental groups altered their foraging behaviour significantly in the opposite direction. Cleaners that had been exposed to plates being removed (fleeing) after they had eaten prawn ate significantly less prawn items during the final experiment than during the initial preference tests (Wilcoxon-test, n=8, one tie, resulting n=7, T=0, p=0.016; figure 2). Similarly, cleaners that had been exposed to plates chasing (punishing) them if they had eaten prawns ate significantly less prawn items during the final experiment than during the initial preference tests (Wilcoxon-test, n=8, T=0, p=0.008, figure 2).
Figure 2.
The percentage of prawn items eaten in the three treatment groups during the initial preference test (grey columns) and during the final experiment after the teaching period (white columns). Shown are the median and the interquartiles for n=8 individual cleaners for each treatment.
4. Discussion
Our experiment was based on the following chain of assumptions. First, clients benefit from cleaning interactions if cleaners eat ectoparasites but pay a cost if cleaners feed on mucus as the latter is important for a client's health (Ebran et al. 1999). Second, cleaners prefer mucus over parasites in a choice experiment (Grutter & Bshary 2003). Third, clients therefore have to control cleaner fishes' behaviour in a way that makes cleaners feed against their preference. Fourth, field observations indicated that either client aggression (chasing) towards the cleaner or client fleeing (and visiting a different cleaner for their next inspection) may be such control mechanisms that promote cleaners to feed mostly against their preference (Grutter & Bshary 2003).
In our simple learning experiment, we offered the cleaners the same key stimuli that they also receive under natural conditions (Bshary & Grutter 2002): they were offered a choice between a preferred food type and a less preferred food type. Eating the less preferred food type had no negative consequences whereas eating the preferred food type terminated the interaction by either the immediate fleeing of the food source (as clients with access to several cleaning stations do) or by the food source chasing the cleaner before swimming off (as resident clients with access to only one cleaning station do). These stimuli, provided with the help of a lever attached to a Plexiglas plate, resulted in significant changes in cleaner foraging behaviour after only six learning trials. Both fleeing and aggression resulted in increased consumption by the cleaners of the less preferred food items. As the control group increased their preference for the preferred prawn food during the learning trials, the previous result is not due to a sequence effect and a preference shift towards flakes. Thus, fleeing and aggression may result in cleaners feeding against their preference, though we do not know how our experimental punishment compares in magnitude to the aggression of clients under natural conditions. Nevertheless, we propose that the fleeing plus partner switching and the aggression used by clients under natural conditions serve the same purpose, causing the cleaners to feed on ectoparasites against their preference for mucus. Our results thus provide the first experimental evidence that terminating an interaction (and subsequent partner switching) and punishment are partner control mechanisms used by animals to promote cooperative behaviour of their partners.
As we observed a significant shift towards prawn feeding after only six learning trials, cleaners therefore learned this task very rapidly. This may seem surprising at first as primates often fail to choose optimally in a similar task: when asked to point at either a small amount or a large amount of food, most species point at the large amount even if they receive always the food they had not pointed to (and hence pointing at the smaller food item would yield the larger benefit, summarized by Genty et al. 2004). We propose that cleaners learned this task very rapidly in our experiment because they often may have to forage against their preferences in the wild. As cleaners have more than 2000 interactions per day (Grutter 1997), they receive feedback about the consequences of their actions over 2000 times a day. Under such circumstances, instrumental conditioning could produce seemingly cognitively demanding behaviour (Heyes 1998). We, therefore, propose that cleaners are likely to have learned to vary their own behaviour according to client responses under natural conditions. In our experiment, they only had to apply their knowledge to a new combination of food items (flake and prawn) and to a new form of clients (Plexiglas plates). That may be the reason why the behaviour of cleaners in the fleeing treatment was very similar to the behaviour of cleaners in the punishment treatment. One might have expected a stronger effect of punishment as aggression is added to the fact that interactions are terminated (Bshary & Grutter 2002). In the field, however, cleaners treat punishing clients and partner switching clients very similarly (Bshary 2001). There is experimental evidence that cleaners can recognize individual clients (Tebbich et al. 2002), which is a cognitive prerequisite to link their own behaviour to the response of particular clients so that punishment may yield future benefits to the client. Our study also suggests that the cognitive demands for partner control mechanisms like fleeing or punishment may not be so high that the costs constrain the evolution of these mechanisms, at least for vertebrates.
Acknowledgments
We thank the Lizard Island Research Station for their continuous support and friendship, Wolfgang Wickler for his support and for discussions on this topic, and two anonymous referees for very valuable comments. Funding was provided by NERC (R.B.) and the Australian Research Council (A.S.G.). The research was carried out with the permit number G04/12405.1
References
- Bergstrom C, et al. Interspecific mutualism—puzzles and predictions. In: Hammerstein P, editor. Genetic and cultural evolution of cooperation. MIT Press; Cambridge, MA: 2003. pp. 241–256. [Google Scholar]
- Bshary R. The cleaner fish market. In: Noë R, van Hooff J.A.R.A.M, Hammerstein P, editors. Economics in nature. Cambridge University Press; 2001. pp. 146–172. [Google Scholar]
- Bshary R, Bronstein J.L. Game structures in mutualisms: what can the evidence tell us about the kind of models we need? Adv. Stud. Behav. 2004;34:59–101. [Google Scholar]
- Bshary R, Grutter A.S. Asymmetric cheating opportunities and partner control in a cleaner fish mutualism. Anim. Behav. 2002;63:547–555. [Google Scholar]
- Bshary R, Noë R. Biological markets: the ubiquitous influence of partner choice on cooperation and mutualism. In: Hammerstein P, editor. Genetic and cultural evolution of cooperation. MIT Press; Cambridge, MA: 2003. pp. 167–184. [Google Scholar]
- Bshary R, Schäffer D. Choosy reef fish select cleaner fish that provide high service quality. Anim. Behav. 2002;63:557–564. [Google Scholar]
- Cheney K.L, Côté I.M. Habitat choice in adult longfin damselfish: territory characteristics and relocation times. J. Exp. Mar. Biol. Ecol. 2003;287:1–12. [Google Scholar]
- Clutton-Brock T.H, Parker G.A. Punishment in animal societies. Nature. 1995;373:209–215. doi: 10.1038/373209a0. [DOI] [PubMed] [Google Scholar]
- Connor R.C. Pseudo-reciprocity: investing in altruism. Anim. Behav. 1986;34:1562–1566. [Google Scholar]
- Dugatkin L.A. Oxford University Press; 1997. Cooperation among animals: an evolutionary perspective. [Google Scholar]
- Ebran N, Julien S, Orange N, Saglio P, Lemaitre C, Molle G. Pore-forming properties and antibacterial activity of proteins extracted from epidermal mucus of fish. Comp. Biochem. Physiol. A. 1999;122:181–189. doi: 10.1016/s1095-6433(98)10165-4. [DOI] [PubMed] [Google Scholar]
- Ferrière R, Bronstein J.L, Rinaldi S, Law R, Gauduchon M. Cheating and the evolutionary stability of mutualisms. Proc. R. Soc. B. 2002;269:773–780. doi: 10.1098/rspb.2001.1900. doi:10.1098/rspb.2001.1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty E, Palmier C, Roeder J.-J. Learning to suppress responses to the larger of two rewards in two species of lemurs, Eulemur fulvus and E. macaco. Anim. Behav. 2004;67:925–932. [Google Scholar]
- Grutter A.S. Spatio-temporal variation and feeding selectivity in the diet of the cleaner fish Labroides dimidiatus. Copeia. 1997;1997:346–355. [Google Scholar]
- Grutter A.S. Cleaners really do clean. Nature. 1999;398:672–673. [Google Scholar]
- Grutter A.S, Bshary R. Cleaner wrasse prefer client mucus: support for partner control mechanisms in cleaning interactions. Proc. R. Soc. B. 2003;270(Suppl. 2):242–244. doi: 10.1098/rsbl.2003.0077. doi:10.1098/rsbl.2003.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutter A.S, Bshary R. Cleaner fish Labroides dimidiatus diet preferences for different types of mucus and parasitic gnathiid isopods. Anim. Behav. 2004;68:583–588. [Google Scholar]
- Grutter A.S, Lester R.J.G. Cleaner fish Labroides dimidiatus reduce Argothona macronema (Corallanidae) isopod infections on the coral reef fish Hemigymnus melapterus. Mar. Ecol. Prog. Ser. 2002;234:247–255. [Google Scholar]
- Hammerstein P. Why is reciprocity so rare in animals? A protestant appeal. In: Hammerstein P, editor. Genetic and cultural evolution of cooperation. MIT Press; Cambridge: 2003. pp. 83–94. [Google Scholar]
- Heyes C.M. Theory of mind in non human primates. Behav. Brain Sci. 1998;21:101–148. doi: 10.1017/s0140525x98000703. [DOI] [PubMed] [Google Scholar]
- Johnstone R.A, Bshary R. From parasitism to mutualism: partner control in asymmetric interactions. Ecol. Let. 2002;5:634–639. [Google Scholar]
- Kiers E.T, Rousseau R.A, West S.A, Denison R.F. Host sanctions and the legume-rhizobium mutualism. Nature. 2003;425:78–81. doi: 10.1038/nature01931. [DOI] [PubMed] [Google Scholar]
- Tebbich S, Bshary R, Grutter A.S. Cleaner fish Labroides dimidiatus recognise familiar clients. Anim. Cogn. 2002;5:139–145. doi: 10.1007/s10071-002-0141-z. [DOI] [PubMed] [Google Scholar]