Abstract
Growth of fast myotomal muscle in teleosts involves the continuous production of muscle fibres until some genetically pre-determined length. The dwarf landlocked (Bleke) population of Atlantic salmon (Salmo salar L.) from Byglands-fjord, Southern Norway mature at about 25 cm fork length and reach a maximum size of only 30 cm in the wild. The maximum diameter (Dmax) of fast muscle fibres in 4-year-old Bleke salmon (25–28 cm fork length) was 118 μm and not significantly different from that found in immature migratory salmon of a similar size. In contrast no evidence for active fibre recruitment was found in the Bleke salmon, such that the maximum fibre number, FNmax, was only 21–30% of that reported in typical farmed and wild migratory populations, respectively. We hypothesise that, once established, the physiological consequences of the dwarf condition led to rapid selection for reduced fibre number, possibly to reduce the maintenance costs associated with ionic homeostasis.
Keywords: dwarfism, body size evolution, Salmonidae, skeletal muscle, growth, muscle fibre recruitment
1. Introduction
The normal life cycle of Atlantic salmon (Salmo salar L.) involves smoltification from a freshwater parr to a seawater stage that migrates to rich feeding grounds in the Arctic Ocean before becoming sexually mature and returning to its natal stream to spawn (Hansen & Quinn 1998). The Bleke salmon (figure 1a) is a fresh water Atlantic salmon population inhabiting the inner part of Byglands-fjord in Southern Norway. This slow-growing ice age relict was isolated from sea-migrating populations about 9000 years ago due to the barrier of the waterfall Vigelandsfoss (Dahl 1928). The population nearly went extinct in 1968–1971 and a limited number of individuals were maintained in a hatchery, and used for a restocking programme in 1979 (Barlaup et al. 2005). Female Bleke salmon become sexually mature after 4–5 years of freshwater life at about 25 cm fork length (FL; Barlaup et al. 2005) compared to 70–120 cm in ancestral migratory populations. Bleke salmon retain the paedomorphic characteristics of parr and females becoming sexually mature without undergoing smoltification (Nilsen et al. 2003). Dwarfism is common in other salmonidae including arctic charr (Salvelinus alpinus L.) (Skúlason & Smith 1995) and whitefish (Coregonus clupeaformis) (Trudel et al. 2001). The landlocked charr morphs from Thingvallvatn, Iceland are thought to have evolved as a consequence of divergent selection promoting differential trophic use (Skúlason & Smith 1995). Differences in ultimate body size between these charr morphs are largely maintained under common rearing conditions, consistent with rapid genetic differentiation of the gene networks regulating body size. Recently, we have shown that the dwarf morph stops recruiting fast myotomal muscle fibres at shorter body lengths and has around half the number of fibres of the larger morphs (Johnston et al. 2004). Here we report that fast fibre number is also reduced in the dwarf Bleke salmon, suggesting that the loss of fibre number is an early and ubiquitous response of salmonid genomes to a reduction in body size. On theoretical grounds it is suggested that there is a trade-off between the need to avoid diffusional constraints whilst minimizing the surface-to-volume ratio of muscle fibres and hence the energetic costs of ionic homeostasis. According to this hypothesis, there is an optimal fibre number associated with each body size which is modulated by factors that influence metabolic demand, principally temperature (Johnston et al. 2004). Thus we suggest that ecological factors may have promoted dwarfism in Bleke salmon, but once established the associated physiological consequences then acted as a powerful agent for selection and genetic differentiation.
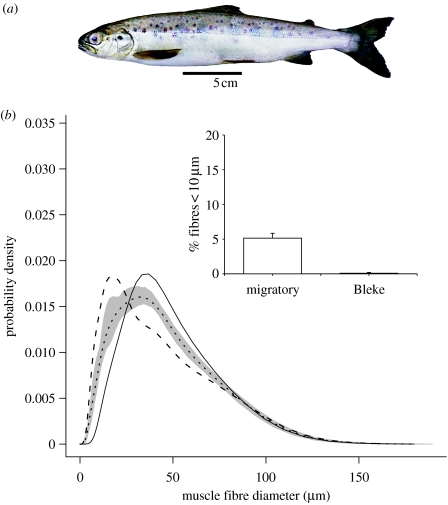
Figure 1.
(a) Bleke dwarf-Atlantic salmon (Salmo salar L.). (b) Distribution of muscle fibre size in Bleke salmon and a Scottish farmed salmon population of the same average body length. The dashed line is the average probability density function (PDF) for the farmed salmon, the solid line is the average PDF for the Bleke salmon, and the dotted line is the average PDF for the combined populations. Shaded area represents 100 bootstrap estimates of the combined populations of fibre diameter. Note the absence of the smallest size class of fibres in Bleke salmon indicating the absence of active muscle fibre recruitment. The inset shows the percentage of fibres <10 μm diameter.
2. Material and methods
Bleke salmon comprising 7 mature males (117.6±5.9 g body mass, Mb; 26.1±0.4 cm fork length, FL, mean±s.e.) and 8 spawning females (120.0±6.1 g Mb; 25.9±0.4 cm FL) were caught by gill netting during January 2005. A steak (0.5 cm) thick through the trunk was prepared with a sharp knife at the level of the first dorsal fin ray and photographed with a digital camera to determine the total cross-sectional area of fast myotomal muscle. A total of 4 blocks were prepared to sample the entire cross-section of fast myotomal muscle from one side of the fish. Blocks were frozen in 2-methyl butane cooled to its freezing point (−159 °C) in liquid nitrogen. Frozen sections were cut at 7 μm, stained with a slow muscle myosin antibody (S58) and counterstained with haematoxylin to differentiate between slow and fast muscle fibres (Johnston et al. 2004). The outlines of 200 randomly selected fast muscle fibres were digitized per block and fibre number and diameter estimated as previously described (Johnston et al. 1999). The muscle cellularity of male and female fish was similar and so the data were combined. Smooth nonparametric distributions were fitted to 1000 measurements of fibre diameter per fish using a kernel function (Johnston et al. 1999) with an average value of the smoothing coefficient h (Bowman and Azzalini 1997) of 0.105. The 97th percentile of fibre diameter, calculated from the smooth distributions, was used as an estimate of the maximum fibre diameter (Dmax). The data were compared with similar measurements from 14 Scottish farmed salmon representing an inbred line derived from a cross between the domesticated Norwegian “Mowi strain” and wild stock from the River Shin (Johnston et al. 2003a). The farmed salmon were slightly shorter (24.1±0.3 cm FL) but heavier (145.9±6.7 g body mass; mean±s.e., n=14). Bootstrap techniques were used to distinguish underlying structure in the distributions from random variation (Johnston et al. 1999). Since the computer programme requires an equal number of fish per group, one female Bleke was discarded at random. The Kolmogorov-Smirnov two-sample test statistic was used to test the null hypothesis that probability density functions of Bleke and farmed salmon were equal over all diameters.
3. Results
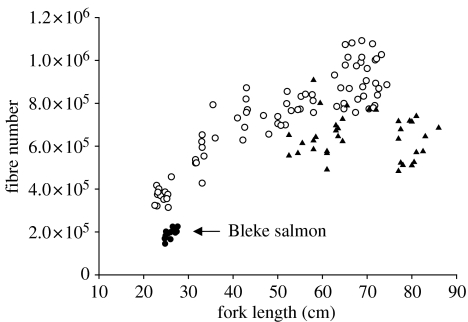
Maximum fibre diameter (Dmax) in the fast myotomal muscle of Bleke salmon was 118±8 μm (Mean±s.e., 14 fish) which was not significantly different from immature Scottish farmed salmon of similar body size (117±2 μm; mean±s.e., 14 fish) (one-way ANOVA with fork length as co-variate). The fast myotomal muscle of the Bleke salmon showed no fibres in the smallest size class (4–7 μm) and fewer than 0.1% fibres <10 μm diameter, consistent with no active fibre recruitment (figure 1b). In contrast, the percentage of fibres <10 μm diameter was around 5% in the immature farmed salmon (figure 1b). The probability density functions (PDFS) of fibre diameter in Bleke and Scottish farmed salmon fell outside that for 100 bootstrap estimates of the combined populations of fibre diameter (figure 1b). The PDFS were significantly different using a Kolmogorov-Smirnov test (p<0.01), demonstrating the different patterns of muscle cellularity between the two salmon strains. The relationship between the number of fast fibres per myotomal cross-section and fork length for several different salmon populations is shown in figure 2. Since there was no evidence for active fibre recruitment in the dwarf Bleke salmon, the measure fibre number was assumed to be the maximum (FNmax). FNmax for dwarf Bleke salmon was 195 300±5900 (mean±s.e., n=14) compared with 910 800±17 800 (mean±s.e., n=34) for the farmed Scottish salmon, 660 700±14 000 (mean±s.e., n=66) for a farmed Mowi strain from Northern Norway (Vieira et al. 2005) and 644 200±16 400 (mean±s.e., n=36) for a wild population of migratory salmon from the North Esk, Aberdeenshire (I. A. Johnston and V. L. A. Vieira, unpublished results). Thus the dwarf salmon had only around 21–30% of the number of fast muscle fibres found in the seawater stages of farmed and migratory salmon, respectively.
Figure 2.
Comparison of the number of fast muscle fibres in Bleke salmon in relation to previously studied wild and farmed populations. Scottish farmed salmon (Johnston et al. 2003a) (open circles); Norwegian farmed salmon (Vieira et al. 2005) (solid triangles); and Bleke salmon (solid circles).
4. Discussion
The maximum diameter (Dmax) of fast muscle fibre scales with body size until some limiting diameter is reached, and it is around 220–240 μm in farmed Atlantic salmon (Johnston et al. 2003a). It is likely that maximum diameter is set by diffusional constraints associated with oxygen delivery during recovery metabolism (Johnston et al. 2003a,b). The main determinates of Dmax are mean habitat temperature (which affects oxygen concentration at the fibre surface, and oxygen demand) and body size (which affects oxygen demand) (Johnston et al. 2004). The maximum diameter of fast fibres in Bleke salmon was 118 μm, which is comparable with that found in farmed salmon of the same body size (figure 1b; Johnston et al. 2003a). No active recruitment of fast muscle fibres was found in Bleke salmon of 24.7–27.6 cm fork length, indicating the maximum fibre number (FNmax) had been reached. FNmax at 195 300 per trunk cross-section was 21–30% of that reported in any migratory or farmed population of the same species (figure 2). The probability density function of fibre diameter was shifted to the right in Bleke compared with length-matched farmed salmon because of the absence of fibre recruitment (figure 1b). The results indicate that Bleke salmon have lost fast muscle fibres relative to the ancestral condition, and it is plausible that this represents an adaptation to dwarfism. Although FNmax can be influenced by egg incubation temperature (Johnston et al. 2000; 2003c), such developmental plasticity is an order of magnitude less than would be required to explain the current results. Furthermore, the difference in maximum body size between migratory and farmed salmon is maintained in the aquaculture situation (Bernt Olav Martinsen, personal communication). Bleke salmon show a much lower capacity to undergo smoltification, with a reduced ability to elevate gill Na+K+ ATPase activity on seawater transfer indicating genetic differentiation in the osmoregulatory system relative to the ancestral condition (Nilsen et al. 2003). Studies of recent introductions of sockeye salmon (Oncorhynchus nerka) indicate that reproductive isolation and genetic differentiation can occur after fewer than 13 generations (Hendry et al. 2000). Our results indicate that fibre number was under divergent selection in the Bleke population, possibly involving assortative mating mechanisms (Shine et al. 2001) and their reinforcement through hybrid inferiority (Butlin 1995).
Previously, we proposed the optimal fibre number hypothesis to account for the loss of fast fibres in dwarf arctic charr from Thingvallavatn (Johnston et al. 2004). In this case the large differences in body size and growth rate between the dwarf charr and large benthic and piscivorous morphs are maintained in the aquaculture situation when food is not limiting, indicating a large genetic component in these traits. Maintaining ionic homeostasis is thought to represent 20–40% of routine energy demand in teleosts (Jobling 1994). Fast muscle comprises at least 60% of body mass and is therefore quantitatively important tissue in determining the overall energy budget. Theoretically the cost of counteracting passive ion movements across the muscle sarcolemma would be expected to increase with the surface to volume ratio of the muscle fibres, i.e. increase with increasing fibre number. According to the optimal fibre number hypothesis there is a trade-off between requirements to avoid diffusional constraints whilst maximizing fibre diameter to minimize the energy costs of ionic homeostasis. The number of fast muscle fibres therefore needs to be sufficiently large to avoid an anoxic core in the centre of fibres but as small as possible to reduce maintenance costs. Without the marine feeding opportunities, Bleke salmon exhibit slow growth and reach sexual maturity earlier than the ancestral condition at a much reduced body size (Nilsen et al. 2003; Barlaup et al. 2005). Ecological factors related to energetics and feeding are almost certainly responsible for establishing dwarfism in the population, as was documented for Lake Whitefish populations (Trudel et al. 2001). However, once established we hypothesise that physiological factors related to scaling and the relatively high maintained costs of supporting an excess number of fibres for the body size would have acted as a powerful selective force for reducing fibre number. The largest Bleke salmon in the present study was 27.6 cm. Since 80% of fibres had a diameter of less than Dmax it is likely that under optimal feeding and environmental conditions the maximum body size could be considerably increased even with the reduced maximum fibre number. Indeed, Bleke of 250 g have been reported from the wild fishery (Barlaup et al. 2005) and in the aquaculture situation the maximum body size is around 2 kg (Bernt Olav Martinsen, personal communication).
Important and testable predictions of the ‘optimal fibre number hypothesis’ are that the number of ion pumps per unit volume of muscle and their contribution to the oxygen consumption of fibres should decrease with increasing fibre diameter. If correct, our hypothesis may help to explain much of the variation in muscle fibre number associated with changes in body size and temperature during the adaptive radiation of teleosts (Johnston et al. 2003b).
Acknowledgements
The S58 monoclonal antibody used was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA. We thank Bernt Olav Martinsen and Nils Børge Kile from Syrtveit Fiskeanlegg for collecting the Bleke salmon used in the study.
References
- Barlaup B.T, Kleiven E, Christensen H, Kile H, Martinsen B.O, Vethe A. Utredning 2005-3. Direktoratet for naturforvaltning; Trondheim, Norway: 2005. Bleka I Byglandsforden—bestandsstatus og tiltak for økt naturlig rekruttering; p. 72. English abstract. [Google Scholar]
- Bowman A.W, Azzalini A. The Kernel approach with S-plus illustrations. Oxford University Press; Oxford, UK: 1997. Applied smoothing techniques for data analysis. [Google Scholar]
- Butlin R.K. Reinforcement: an idea evolving. Trends Ecol. Evol. 1995;10:432–434. doi: 10.1016/s0169-5347(00)89173-9. doi:10.1016/S0169-5347(00)89173-9 [DOI] [PubMed] [Google Scholar]
- Dahl, K. 1928 The Blege or dwarf-salmon. Skr. Utg. Av Det Norske Videnskabsakad, 1927, Oslo.
- Hansen L.R, Quinn T.R. The marine phase of the Atlantic salmon (Salmo salar) life cycle, with comparisons to Pacific salmon. Can. J. Fish. Aquat. Sci. 1998;55(Suppl. 1):104–118. doi:10.1139/cjfas-55-S1-104 [Google Scholar]
- Hendry A.P, Wenburg J.K, Bentzen P, Volk E.C, Quinn T.P. Rapid evolution of reproductive isolation in the wild: evidence from introduced salmon. Science. 2000;290:516–518. doi: 10.1126/science.290.5491.516. doi:10.1126/science.290.5491.516 [DOI] [PubMed] [Google Scholar]
- Jobling M. Chapman & Hall; London: 1994. Fish bioenergetics. [Google Scholar]
- Johnston I.A, Strugnell G, McCracken M.L, Johnstone R.R. Muscle growth and development in normal-sex-ratio and all-female diploid and triploid Atlantic salmon. J. Exp. Biol. 1999;202:1991–2016. doi: 10.1242/jeb.202.15.1991. [DOI] [PubMed] [Google Scholar]
- Johnston I.A, McLay H.A, Abercromby M, Robins D. Phenotypic plasticity of early myogenesis and satellite cell numbers in Atlantic salmon spawning in upland and lowland tributaries of a river system. J. Exp. Biol. 2000;203:2539–2552. doi: 10.1242/jeb.203.17.2539. [DOI] [PubMed] [Google Scholar]
- Johnston I.A, Manthri S, Smart A, Campbell P, Nickell D, Alderson R. Plasticity of muscle fibre number in seawater stages of Atlantic salmon in response to photoperiod manipulation. J. Exp. Biol. 2003a;206:3425–3435. doi: 10.1242/jeb.00577. doi:10.1242/jeb.00577 [DOI] [PubMed] [Google Scholar]
- Johnston I.A, Fernandez D, Calvo J, Vieria V.L.A, North T.W, Abercromby M, Garland T., Jr Reduction in muscle fibre number during the adaptive radiation of Notothenioid fishes: a phylogenetic perspective. J. Exp. Biol. 2003b;206:2595–2609. doi: 10.1242/jeb.00474. doi:10.1242/jeb.00474 [DOI] [PubMed] [Google Scholar]
- Johnston I.A, Manthri S, Alderson R, Smart A, Campbell P, Nickell D, Robertson B, Paxton C.G.M, Burt M.L. Freshwater environment affects growth rate and muscle fibre recruitment in seawater stages of Atlantic salmon (Salmo salar) J. Exp. Biol. 2003c;206:1337–1351. doi: 10.1242/jeb.00262. doi:10.1242/jeb.00262 [DOI] [PubMed] [Google Scholar]
- Johnston I.A, Abercromby M, Vieira V.L.A, Sigursteindóttir R.J, Kristjánsson B.K, Sibthorpe D, Skúlason S. Rapid evolution of muscle fibre number in post-glacial populations of Arctic charr Salvelinus alpinus. J. Exp. Biol. 2004;207:4343–4360. doi: 10.1242/jeb.01292. doi:10.1242/jeb.01292 [DOI] [PubMed] [Google Scholar]
- Nilsen T.O, Lars O.E, Stefansson E.S.O. Smolting in andromous and landlocked strains of Atlantic salmon (Salmo salar) Aquaculture. 2003;222:71–82. doi:10.1016/S0044-8486(03)00103-0 [Google Scholar]
- Shine R, O'Connor D, Lemaster M.P, Mason R.T. Pick on someone your own size: ontogenetic shifts in mate choice by male garter snakes result in size-assortative mating. Anim. Behav. 2001;61:1133–1141. doi:10.1006/anbe.2001.1712 [Google Scholar]
- Skúlason S, Smith T.B. Resource polymorphism in vertebrates. Trends. Ecol. Evol. 1995;10:366–370. doi: 10.1016/s0169-5347(00)89135-1. doi:10.1016/S0169-5347(00)89135-1 [DOI] [PubMed] [Google Scholar]
- Trudel M, Tremblay A, Schetagne R, Rasmussen J.B. Why are dwarf fish so small? An energetic analysis of polymorphism in lakewhitefish (Coregonus clupeaformis) Can. J. Fish Aquat. Sci. 2001;58:394–405. doi:10.1139/cjfas-58-2-394 [Google Scholar]
- Vieira V.L.A, Johansen S.J.S, Bickerdike R, Johnston I.A. Impact of accelerated smoltification on muscle structure and fillet firmness at harvest in Atlantic salmon (Salmo salar) Aquaculture. 2005;246:197–208. doi:10.1016/j.aquaculture.2004.12.028 [Google Scholar]