Abstract
Humpback whales seasonally migrate long distances between tropical and polar regions. However, inter-oceanic exchange is rare and difficult to document. Using skin biopsy samples collected in the Indian Ocean and in the South Atlantic Ocean, and a genetic capture–recapture approach based on microsatellite genotyping, we were able to reveal the first direct genetic evidence of the inter-oceanic migration of a male humpback whale. This exceptional migration to wintering grounds of two different ocean basins questions traditional notions of fidelity to an ocean basin, and demonstrates how the behaviour of highly mobile species may be elucidated from combining genetics with long-term field studies. Our finding has implications for management of humpback whale populations, as well as for hypotheses concerning cultural transmission of behaviour.
Keywords: humpback whales, Megaptera novaeangliae, migration, philopatry
1. Introduction
Humpback whales (Megaptera novaeangliae) exhibit distinct patterns of seasonal distribution that range from the tropics, where they breed during winter months, to near-polar waters, where they feed during summer months. Some individuals may travel one-way distances exceeding 8000 km during the intervening migration (Stone et al. 1990). Movements of individuals between feeding and wintering migratory destinations within ocean basins have been extensively demonstrated through the hunting of marked whales, photographic capture–recapture, satellite tagging and genetic identification (Mackintosh 1942; Chittleborough 1965; Palsbøll et al. 1997; Mate et al. 1998).
Genetic studies have shown that maternally inherited fidelity to feeding grounds and presumed migratory routes can sustain long-term population structure in humpback whales (Baker et al. 1990), although in different years a small number of individuals may migrate to different wintering sites within the same ocean basin (Mattila & Clapham 1989; Darling & Cerchio 1993; Salden et al. 1999; Calambokidis et al. 2001). Many wide-ranging mammals do exhibit some degree of male dispersal due to polygyny and associated male competition (Greenwood 1980). However, inter-oceanic migration events appear to be very rare in humpback whales, and prior to our investigation had only been documented for two animals marked off eastern Australia in 1954–1955 and killed off western Australia in 1959 (Chittleborough 1965).
Here we use genetic samples collected from humpback whales in wintering regions of two different ocean basins in the Southern Hemisphere to document direct inter-oceanic movements of individuals by genetic capture–recapture of genotypes constructed from microsatellite markers, an approach applying firm resolution of unique genetic profiles to permit unambiguous identification of individuals (Palsbøll et al. 1997).
2. Material and methods
A total of 1202 skin samples were collected during the austral winter (July–September) from free-ranging humpback whales in the Southwestern Indian Ocean off the northeast coast of Madagascar (n=722) from 1996 to 2001, and in the eastern South Atlantic Ocean off the coast of Gabon (n=480) in 2001 and 2002 (Lambertsen 1987; table 1 in the electronic supplementary material). Genomic DNA was extracted, and the samples were sexed using ZFX/ZFY markers and genotyped using 11 cetacean microsatellite markers (see electronic supplementary material).
Table 1.
Microsatellite genotypes and mtDNA haplotypes for two matching samples collected in Madagascar (BA-00-S-200) and Gabon (GA-02-S-130), and for the individual associated with BA-00-S-200 (BA-00-S-201).
| alleles at each microsatellite locus (bp) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sample ID | H | GATA417 | GATA028 | GATA053 | TAA031 | 199/200 | 417/418 | 464/465 | EV1Pm | EV37Mn | EV94Mn | EV96Mn |
| BA-00-S-200 | HBA053 | 203–226 | 148–148 | 249–249 | 95–116 | 102–116 | 196–196 | 138–142 | 121–121 | 194–204 | 211–215 | 205–209 |
| GA-02-S-130 | HBA053 | 203–226 | 148–148 | 249–249 | 95–116 | 102–116 | 196–196 | 138–142 | 121–121 | 194–204 | 211–215 | 205–209 |
| BA-00-S-201 | HBA053 | 203–226 | 148–148 | 249–261 | 95–116 | 112–116 | 188–196 | 142–142 | 121–125 | 200–204 | 211–219 | 203–209 |
H indicates the mtDNA lineage (GenBank accession number DQ118384). Microsatellite loci are listed in the top row; numbers refer to basepair sizes of the two alleles at each locus. The genotypic phase is unknown.
To characterize maternal lineages we used a 486 bp mitochondrial DNA (mtDNA) fragment containing the majority of variable nucleotide positions in the mtDNA control region of humpback whales (Baker et al. 1993). Amplification protocols for this fragment and analyses of haplotype diversity are described in Rosenbaum et al. (2004).
Duplicate samples within each population were detected either from photographic identification or genotype identity using the Excel add-in Ms_Toolkit package (Park 2001), and were consequently eliminated. The average probability of different random individuals in the populations sharing the same genotype by chance (probability of identity, PI) was estimated using the software Api-Calc v. 1.0 (Ayres & Overall 2004). Since it is more likely that relatives, rather than random pairs, will share genotypes, for a specific case of a genotype match identified between Madagascar and Gabon we also calculated the more conservative PI for siblings (PIsib) and PI for parent–offspring (PIpof) (see electronic supplementary material). Although a relationship of full-siblings is very unlikely in humpback whales (Clapham & Palsbøll 1997), PIsib is more conservative than PIpof, which would be the next closest kin relationship.
Allele frequencies and measures of diversity such as mean number of alleles per locus (A), observed heterozygosity (Ho), and expected heterozygosity (He) under Hardy–Weinberg assumptions (Nei 1987) were computed using the Excel add-in MS_Toolkit package (Park 2001). Departure from Hardy–Weinberg equilibrium (HWE) and linkage disequilibrium (LD) between each pair of loci were evaluated using Genepop v. 3.4 (Raymond & Rousset 1995; see electronic supplementary material). Hypotheses of pedigree relationships between pairs of individuals were tested with the software Kinship v. 1.3.1 (Goodnight & Queller 1999; see electronic supplementary material).
To estimate migration rates between populations we used a maximum-likelihood framework based on coalescence theory, implemented in the program Migrate v. 2.0.3 (Beerli & Felsestein 1999; see electronic supplementary material).
3. Results
After removing duplicate samples from each population, detected either from photographic identification or genotype identity, a total of 972 identified individuals were included in the analyses. The mean numbers of alleles per locus (A) were 12.91 for Gabon and 13.09 for Madagascar. The largest number of alleles (21) was found at locus GATA417 for Madagascar; the smallest (4) was recorded at locus EV1Pm for both sampling sites. No significant differences were found between the observed heterozygosity (Ho=0.76±s.d.=0.01 for Gabon, Ho=0.75±s.d.=0.00 for Madagascar), and the heterozygosity expected under Hardy-Weinberg assumptions (He=0.76±s.d.=0.05 for Gabon, He=0.75±s.d.=0.05 for Madagascar). Deviation from HWE was rejected, and there was no evidence of LD (p<0.05) in the 110 pairwise tests performed.
The low estimate obtained for the average PI (PI=1.123×10−12) allowed us to conclude that any match recovered when searching genotype data for between-site matches would indicate inter-oceanic migration of an individual. One sample collected in 2000 in Antongil Bay, Madagascar, matched a sample collected two years later off Loango National Park, Gabon (figure 1, table 1; PIsib=3.114×10−5 and PIpof=5.382×10−9 for Madagascar, PIsib=2.811×10−5 and PIpof=5.640×10−9 for Gabon). Both samples were collected from a male individual and shared a locally rare mtDNA lineage (frequencies: p=0.008 in Madagascar and p=0.006 in Gabon, H. C. Rosenbaum et al., unpublished data). A photographic comparison has not yet been attempted for datasets from these two areas. However, a comparison of dorsal fin characteristics in photographs taken at the time of sampling confirmed the genetic match.
Figure 1.
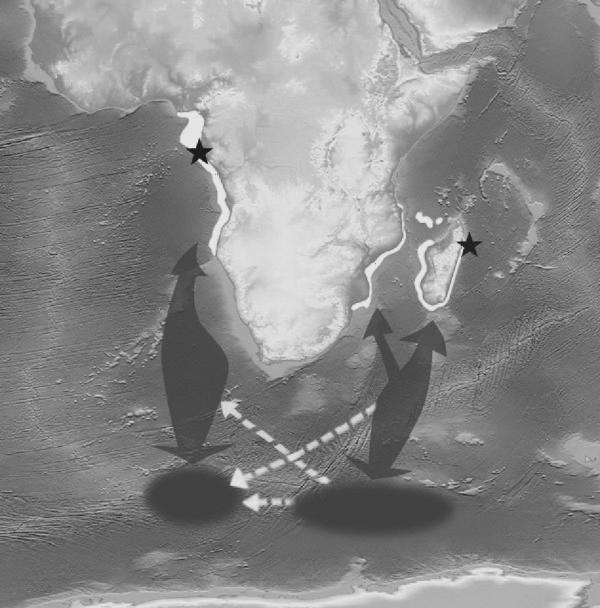
Humpback whale sampling areas and migration routes around Africa. Stars indicate the locations where the two matching samples were collected on July 17th 2000 in Antongil Bay (16°00′ S, 49°55′ E), Madagascar, and on August 13th 2002 off Loango National Park (1°51′ S, 9°20′ E), Gabon. Shading indicates humpback whale distribution in the wintering destinations (white) and in the feeding grounds (dark grey). Dark grey arrows indicate population seasonal migratory routes; white dotted arrows indicate three plausible alternative deviations from the main routes that the sampled individual might have undertaken during a migration from the Indian to the South Atlantic Ocean passing through the feeding grounds. (Map courtesy of the US National Geophysical Data Center.)
In Madagascar, this individual was accompanied by a female and analyses of genotypes putatively identified her as his mother (relationship log(ratio)=1.673, p<0.001; table 1; and see electronic supplementary material for kinship analyses). Other behavioural data show that humpback whale calves remain with their mother for their first year, and occasionally for their second year (Glockner-Ferrari & Ferrari 1984; Baker et al. 1987; Clapham & Mayo 1987). Therefore, when sighted in Gabon two years later, this individual could have been a three- or four-year-old juvenile, as the mean age at attainment of sexual maturity in male humpback whales is five years (Clapham 1992; Chittleborough 1955).
Gene flow estimates from Migrate based on seven sampled humpback whale populations indicated that the eastern South Atlantic and the southwestern Indian Oceans are expected to exchange approximately 35 migrants per generation (Nem) in each direction (95%CI=27.1–45.1). However, the same analysis estimated that only approximately one individual per generation (Nem=0.8, 95%CI=0.6–1.3) is expected to migrate from Madagascar to Gabon.
4. Discussion
The worldwide distribution of humpback whales mtDNA haplotypes seems to reflect historical interchange between the hemispheres (Baker et al. 1993). Inter-oceanic exchange is also expected to happen to some degree in the absence of geographic barriers, yet its documentation remains an exceptional event, and more so for the circumstances presented here. Since only a modest degree of migration is sufficient to confound structure between populations, dispersal events may be rare and therefore hard to identify. Previous documentation of migrations of this scale have centred on trans-equatorial movements (Stone et al. 1990), and the only other inter-oceanic migrants previously identified were the two animals killed off Australia (Chittleborough 1965). Although several statistical approaches permit an indirect estimate of gene flow between populations, it is often very difficult to distinguish between current exchange and retention of common ancestral genetic states, as both scenarios produce a similar pattern of allele sharing. Dispersal events in highly mobile species are therefore valuable when trying to distinguish between the above hypotheses. Given a generation time of 12–24 years (Roman & Palumbi 2003), our direct observation of one migrant over six and two years of sampling for Madagascar and Gabon, respectively, is consistent with the expectation (Nem=0.8, 95%CI=0.6–1.3), especially considering that Ne (effective population size) is smaller than N (population census size) and in light of the stochastic nature of one resighting.
Acoustic data on the radical replacement of humpback whale song in Australian populations suggested the immigration of adult males from the Indian into the Pacific Ocean (Noad et al. 2000). Cultural transmission seems to play an important role in maintaining song homogeneity over entire ocean basins (Guinee et al. 1983). Our finding is consistent with episodes of inter-oceanic cultural transmissions possibly occurring through direct movements of animals in addition to hypothesized contact on common feeding grounds or migration routes (Payne & Guinee 1983).
Even though this migrant whale was probably sexually immature, and therefore does not represent a direct vector of gene flow or song interchange, its behaviour is important proof of current inter-oceanic exchange between humpback whale wintering regions. Alternatively, given the young age of the animal, this inter-oceanic movement could be the result of navigational error due to lack of experience.
Despite genetic evidence for rejecting a single panmictic humpback whale population in the Southern Hemisphere, the relationship between populations of different ocean basins and the extent of potential gene flow merit further evaluation (Baker & Medrano-Gonzalez 2002). From a conservation perspective, an improved understanding of large whale migratory behaviour and population connectivity will help improve current definitions of population boundaries, evaluation of protected areas, and assessment of post-whaling recovery of populations. This is essential where revision of international management procedures by the International Whaling Commission may rely on accepted views or traditional notions that lack adequate scientific data.
Acknowledgments
The dedicated contributions of T. Collins, P. Ersts, Y. Razafindrakoto, S. Ngouessono and G.-P. Sounguet for the collection of field data and samples helped make this work possible. M. Leslie and M. Boyer provided invaluable assistance with laboratory analysis and graphics, respectively. We thank the Ministry of Forest Economy, the National Parks Council (CNPN) [Gabon], as well as the Ministry of Water and Forests, the National Association for the Management of Protected Areas (ANGAP) [Madagascar] for permission to conduct research. We also thank the WCS Gabon and Madagascar Country Programs, Operation Loango, and Aventures Sans Frontières for their support. Generous support for this work was provided by grants to C.P., H.C.R. and the Wildlife Conservation Society/American Museum of Natural History Cetacean Conservation and Research Program. R. DeSalle, G. Amato, T. Collins, S. Cerchio, P. Clapham and an anonymous reviewer offered helpful comments that improved this manuscript.
Supplementary Material
References
- Ayres K.L, Overall D.J. Api-Calc 1.0: a computer program for calculating the average probability of identity allowing for substructure, inbreeding and the presence of close relatives. Mol. Ecol. Notes. 2004;4:315–318. doi:10.1111/j.1471-8286.2004.00616.x [Google Scholar]
- Baker C.S, Medrano-Gonzalez L. World-wide distribution and diversity of humpback whale mitochondrial DNA lineages. In: Pfeiffer C.J, editor. Cell and molecular biology of marine mammals. Krieger Publishing Co.; New York: 2002. pp. 84–99. [Google Scholar]
- Baker C.S, Perry A, Herman L.M. Reproductive histories of female humpback whales (Megaptera novaeangliae) in the North Pacific. Mar. Ecol. Prog. Ser. 1987;41:103–114. [Google Scholar]
- Baker C.S, Palumbi S.R, Lambertsen R.H, Weinrich M.T, Calambokidis J, O'Brien S.J. Influence of seasonal migration on geographic distribution of mitochondrial DNA haplotypes in humpback whales. Nature. 1990;344:238–240. doi: 10.1038/344238a0. doi:10.1038/344238a0 [DOI] [PubMed] [Google Scholar]
- Baker C.S, et al. Abundant mitochondrial DNA variation and world-wide population structure in humpback whales. Proc. Natl Acad. Sci. USA. 1993;90:8239–8243. doi: 10.1073/pnas.90.17.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerli P, Felsestein J. Maximum-likelihood estimation of migration rates and effective population numbers in two populations using a coalescent approach. Genetics. 1999;152:763–773. doi: 10.1093/genetics/152.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calambokidis J, et al. Movements and population structure of humpback whales in the North Pacific. Mar. Mamm. Sci. 2001;17:769–794. [Google Scholar]
- Chittleborough R.G. Aspects of reproduction in the male humpback whale, Megaptera nodosa (Bonnaterre) Aust. J. Mar. Freshw. Res. 1955;6:1–29. [Google Scholar]
- Chittleborough R.G. Dynamics of two populations of humpback whales, Megaptera novaeangliae (Borowski) Aust. J. Mar. Freshw. Res. 1965;16:33–128. [Google Scholar]
- Clapham P.J. Age at attainment of sexual maturity in humpback whales. Can. J. Zool. 1992;70:1470–1472. [Google Scholar]
- Clapham P.J, Mayo C.A. Reproduction and recruitment in individually identified humpback whales (Megaptera novaeangliae) observed in Massachusetts Bay: 1979–1985. Can. J. Zool. 1987;65:2853–2863. [Google Scholar]
- Clapham P.J, Palsbøll P.J. Molecular analysis of paternity shows promiscuous mating in female humpback whales. Proc. R. Soc. B. 1997;264:95–98. doi: 10.1098/rspb.1997.0014. doi:10.1098/rspb.1997.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling J, Cerchio S. Movement of a humpback whale, Megaptera novaeangliae, between Japan and Hawaii. Mar. Mamm. Sci. 1993;9:84–89. [Google Scholar]
- Glockner-Ferrari D.A, Ferrari M.J. Reproduction in humpback whale (Megaptera novaeangliae) in Hawaiian waters. Rep. Int. Whaling Comm. Spec. Issue. 1984;6:237–242. [Google Scholar]
- Goodnight K.F, Queller D.C. Computer software for performing likelihood tests of pedigree relationship using genetic markers. Mol. Ecol. 1999;8:1231–1234. doi: 10.1046/j.1365-294x.1999.00664.x. [DOI] [PubMed] [Google Scholar]
- Greenwood P.J. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 1980;28:1140–1162. [Google Scholar]
- Guinee L.N, Chu K, Dorsey E.M. Changes over time in the songs of known individual humpback whales (Megaptera novaeangliae) In: Payne R.S, editor. Communication and behavior of whales. Westview Press; Boulder, CO: 1983. pp. 59–80. [Google Scholar]
- Lambertsen R.H. A biopsy system for large whales and its use for cytogenetics. J. Mammal. 1987;68:443–445. [Google Scholar]
- Mackintosh N.A. The southern stocks of whalebone whales. Discov. Rep. 1942;22:197–300. [Google Scholar]
- Mate B.R, Gisiner R, Mobley J. Local and migratory movements of Hawaiian humpback whales tracked by satellite telemetry. Can. J. Zool. 1998;76:863–868. doi:10.1139/cjz-76-5-863 [Google Scholar]
- Mattila D.K, Clapham P.J. Humpback whales Megaptera novaeangliae and other cetaceans on Virgin Bank and in the Northern Leeward Island Canada 1985 and 1986. Can. J. Zool. 1989;67:2201–2211. [Google Scholar]
- Nei M. Columbia University Press; New York: 1987. Molecular evolutionary genetics. [Google Scholar]
- Noad M.J, Cato D.H, Bryden M.M, Jenner M.-N, Jenner K.C.S. Cultural revolution in whale songs. Nature. 2000;408:537. doi: 10.1038/35046199. doi:10.1038/35046199 [DOI] [PubMed] [Google Scholar]
- Palsbøll P.J, et al. Genetic tagging of humpback whales. Nature. 1997;388:767–769. doi: 10.1038/42005. [DOI] [PubMed] [Google Scholar]
- Park S. Genetics Department, Trinity College; Dublin: 2001. MS_Toolkit: Excel add-in tool package for microsatellite data. [Google Scholar]
- Payne R.S, Guinee L.N. Humpback whale songs as an indicator of “stocks”. In: Payne R.S, editor. Communication and behavior of whales. Westview Press; Boulder, CO: 1983. pp. 333–358. [Google Scholar]
- Raymond M, Rousset F. GENEPOP (Version 1.1): population genetic software for exact tests and ecumenicism. J. Hered. 1995;86:248–249. [Google Scholar]
- Roman J, Palumbi S.R. Whales before whaling in the North Atlantic. Science. 2003;301:508–510. doi: 10.1126/science.1084524. doi:10.1126/science.1084524 [DOI] [PubMed] [Google Scholar]
- Rosenbaum, H. C., Pomilla, C., Leslie, M., Best, P. B., Findlay, K. P., Engel, M. H., Minton, G. & Collins, T. 2004 MtDNA diversity and population structure of humpback whales from their wintering areas in the Indian and South Atlantic Ocean (Breeding regions A, B, C and X). Paper SC/56/SH3 presented to the Scientific Committee of the International Whaling Commission.
- Salden D.R, Herman L.M, Yamaguchi M, Sato F. Multiple visits of individual humpback whales (Megaptera novaeangliae) between the Hawaiian and Japanese wintering grounds. Can. J. Zool. 1999;77:504–508. doi:10.1139/cjz-77-3-504 [Google Scholar]
- Stone G, Florez-Gonzalez L, Katona S.K. Whale migration record. Nature. 1990;346:705. doi:10.1038/346705a0 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


