Abstract
Objective
The associations between ozone concentrations measured outdoors and both morbidity and mortality may be partially due to indoor exposures to ozone and ozone-initiated oxidation products. In this article I examine the contributions of such indoor exposures to overall ozone-related health effects by extensive review of the literature as well as further analyses of published data.
Findings
Daily inhalation intakes of indoor ozone (micrograms per day) are estimated to be between 25 and 60% of total daily ozone intake. This is especially noteworthy in light of recent work indicating little, if any, threshold for ozone’s impact on mortality. Additionally, the present study estimates that average daily indoor intakes of ozone oxidation products are roughly one-third to twice the indoor inhalation intake of ozone alone. Some of these oxidation products are known or suspected to adversely affect human health (e.g., formaldehyde, acrolein, hydroperoxides, fine and ultrafine particles). Indirect evidence supports connections between morbidity/mortality and exposures to indoor ozone and its oxidation products. For example, cities with stronger associations between outdoor ozone and mortality tend to have residences that are older and less likely to have central air conditioning, which implies greater transport of ozone from outdoors to indoors.
Conclusions
Indoor exposures to ozone and its oxidation products can be reduced by filtering ozone from ventilation air and limiting the indoor use of products and materials whose emissions react with ozone. Such steps might be especially valuable in schools, hospitals, and childcare centers in regions that routinely experience elevated outdoor ozone concentrations.
Keywords: air exchange rates, aldehydes, indoor chemistry, inhalation intake, morbidity, mortality, secondary organic aerosols, surface chemistry, ultrafine particles
Many studies have reported associations between outdoor ozone concentrations and morbidity and mortality. Hubbell et al. (2005) systematically summarized this literature, including associations between ozone and respiratory-related hospital admissions, lost school days, restricted activity days, asthma-related emergency department visits, and premature mortality. Additionally, ozone has been associated with respiratory symptoms and the use of asthma medication for asthmatic school children using maintenance medication (Gent et al. 2003), and long-term exposure to ozone has been tentatively associated with the development of asthma in adult males (McDonnell et al. 1999). Since the submission of Hubbell et al. (2005), three independent meta-analyses have been published, indicating an increase of 0.87% in mortality per 10-ppb increase in daily ozone (Bell et al. 2005), an increase of 0.39% in mortality per 10-ppb increase in 1-hr daily maximum ozone (Ito et al. 2005), and an increase of 0.41% in mortality per 10-ppb increase in 1-hr daily maximum ozone (Levy et al. 2005); in most of the studies included in the meta analyses, same-day effects were larger than lagged effects. A study of 23 European cities found an increase of 0.66% in mortality per 10 ppb increase in 1-hr maximum ozone during the summer (Gryparis et al. 2004); a study in Genoa, Italy, found an increase of 4.0% in mortality per 25-ppb increase in ozone (Parodi et al. 2005); and a study in Shanghai found an increase of 0.45% in mortality per 5-ppb increase in 2-day average ozone (Zhang et al. 2006). Significantly, even when Bell et al. (2006) used data that included only days with average ozone levels lower than 15 ppb, outdoor ozone was significantly associated with premature mortality. For a more extended review of these and other studies, see the U.S. Environmental Protection Agency ozone criteria document (U.S. EPA 2006).
An increase in the concentration of outdoor ozone concomitantly produces an increase in the indoor concentrations of ozone and its reaction products (Weschler 2000). Thus, some of the associations between outdoor ozone and both morbidity and mortality are likely due to outdoor ozone transported into various indoor environments (e.g., residences, workplaces, schools, hospitals, motor vehicles) where subsequent exposures occur. Although indoor ozone concentrations tend to be smaller than corresponding outdoor concentrations, this is somewhat counterbalanced by the much larger fraction of time that most people spend indoors. Moreover, excepting nitrogen dioxide, total concentrations of ozone reaction products are anticipated to be larger indoors than outdoors (see “Products of ozone-initiated indoor chemistry”).
My aim in this article is to present evidence supporting the hypothesis that indoor exposures to ozone and its oxidation products contribute to ozone’s overall impact on public health. Apportioning ozone’s health impact among indoor and outdoor ozone, as well as indoor and outdoor oxidation products, is more than an academic exercise. If indoor ozone and the products of its chemistry are adversely affecting the public’s health, relatively simple strategies can mitigate these effects.
Indoor Ozone: Exposures and Intakes
Indoor ozone concentrations
Although there are indoor sources of ozone, in most buildings indoor ozone has been transported from outdoors (Weschler 2000). Indoor ozone concentrations track outdoor concentrations with a slight time lag that depends on the air exchange rate. Ozone is removed by indoor surfaces as well as by gas-phase reactions, and hence, indoor concentrations tend to be smaller than co-occurring outdoor levels. Models have been presented that relate indoor ozone concentrations to those outdoors (Nazaroff and Cass 1986; Sabersky et al. 1973; Shair and Heitner 1974). In the absence of indoor sources, the ratio of indoor to outdoor ozone concentrations (I:O) can be estimated using a relatively simple expression (Weschler et al. 1989):
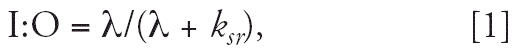 |
where λ is the air exchange rate and ksr is the first-order rate constant for surface removal (both in units of reciprocal time). This equation assumes that the penetration coefficient for ozone is unity, an assumption that remains largely untested, and ignores gas-phase reactions, which tend to be smaller sinks than surface reactions. Numerous investigators have measured surface removal rate constants for ozone, as summarized in several reviews (Grontoft and Raychaudhuri 2004; Nazaroff et al. 1993; Weschler 2000). For example, Lee et al. (1999) found a mean value of 2.8 ± 1.3 hr−1 in 43 Southern California homes. A review of air exchange rates in residences and nonresidences has recently appeared in a draft U.S. EPA report (U.S. EPA 2005). Air exchange rates vary with the region and the season. In general, mean residential values are between 0.6 and 1.7 hr−1, and mean nonresidential values are between 1.5 and 2.0 hr−1. If a value of 3 hr−1 is used for ksr, Equation 1 predicts an I:O of 0.10 at an air exchange rate of 0.33 hr−1, an I:O of 0.33 at 1.5 hr−1 and an I:O of 0.50 at 3 hr−1. These calculated estimates are consistent with measured values, for example, a mean I:O of 0.37 ± 0.25 at 126 Southern California homes (Avol et al. 1998), and a mean I:O of 0.20 ± 0.18 at 145 homes in Mexico City and I:O values between 0.3 and 0.4 at three corresponding schools during class hours (Romieu et al. 1998); see table 2 of Weschler (2000) for a more extensive summary of measured I:Os for ozone.
Table 2.
Personal and outdoor concentrations (μg/m3) of PM2.5 and sulfate for senior citizens in Boston, as well as P:O ratios for each, the difference between these [P:O (PM2.5) − P:O (SO42−)], and the corresponding personal ozone concentrations (pbb).a
| Winter 1 (5 subjects) | Winter 2 (4 subjects) | Winter 3 (5 subjects) | Summer 1 (9 subjects) | Summer 2 (5 subjects) | |
|---|---|---|---|---|---|
| Personal PM2.5 | 10.8 (n = 40) | 15.4 (n = 41) | 16.2 (n = 51) | 17.8 (n = 106) | 20.5 (n = 59) |
| Outdoor PM2.5 | 13.1 (n = 13) | 15.5 (n = 12) | 6.5 (n = 15) | 11.9 (n = 11) | 13.3 (n = 13) |
| P:O (PM2.5) | 0.82 | 0.99 | 2.49 | 1.50 | 1.54 |
| Personal SO42−) | 1.6 (n = 51) | 2.6 (n = 42) | 1.6 (n = 56) | 2.7 (n = 104) | 3.3 (n = 59) |
| Outdoor SO42−) | 3.4 (n = 13) | 4.2 (n = 12) | 1.7 (n = 13) | 3.6 (n = 8) | 4.2 (n = 12) |
| P:O (SO42−) | 0.47 | 0.62 | 0.94 | 0.75 | 0.79 |
| P:O (PM2.5) − P:O (SO42−) | 0.35 | 0.37 | 1.55 | 0.75 | 0.75 |
| Personal O3 | 0.1 (n = 50) | 0.8 (n = 43) | 2.5 (n = 57) | 5.1 (n = 106) | 4.8 (n = 50) |
Data from Sarnat et al. (2000, table 2).
PM2.5, SO42−, and O3 concentrations are based on 24-hr samples. n = total number of measurements for each condition.
Residences with central air conditioning (AC) have I:Os that are typically < 0.10 (Lee et al. 1999, 2004; Stock et al. 1985). This reflects the fact that outdoor air is not deliberately introduced in air-conditioned residences and that the outdoor air exchange due to leakage is typically quite small. Additionally, filters used in air conditioners remove some of the ozone from the air that passes through them (Bekö et al. 2006; Hyttinen et al. 2003, 2006).
Indoor versus outdoor exposures and intakes
“Exposure” to an air contaminant in a given microenvironment has been defined as the concentration of a pollutant in that microenvironment times the amount of time an individual spends there (National Academy of Sciences 1991). Early estimates (Weschler et al. 1989) suggested that ozone exposures occurring indoors are comparable to those occurring outdoors, especially for individuals such as the very young, very old, or chronically ill who spend very little time outdoors. Based on indoor measurements made in six New Jersey homes, Zhang and Lioy (1994) estimated that indoor exposures accounted for more than half the total exposure for the occupants of these homes. In the intervening years, several studies have used passive ozone monitors to measure personal ozone concentrations—ozone concentrations experienced by an individual throughout a 24-hr period—and have made comparisons between these measurements and ozone concentrations measured at outdoor fixed site monitors (Brauer and Brook 1995; Geyh et al. 2000; Lee et al. 2004; Linn et al. 1996; Liu et al. 1993; Sarnat et al. 2005). To a first approximation, the average personal ozone concentration measured in these studies is given by
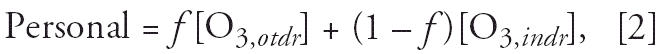 |
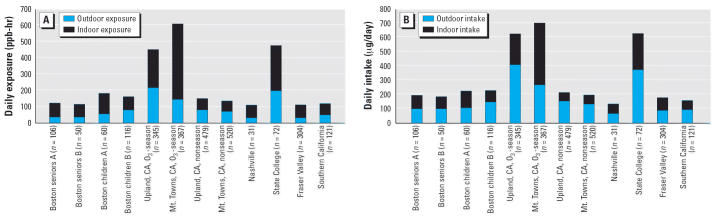
where f is the fraction of time outdoors, [O3,otdr] is the average outdoor ozone concentration while outdoors, 1 − f is the fraction of time indoors, and [O3,indr] is the average indoor ozone concentration while indoors. Figure 1A shows average daily outdoor and indoor ozone exposures, in units of parts per billion per hour, estimated from the studies cited above (each includes sufficient information on the parameters in Equation 2 to enable calculations of these estimates; time in transit has been categorized as time indoors). For the studies shown in Figure 1A, indoor exposures are 43–76% of total daily ozone exposure, with an average of just below 60%.
Figure 1.
(A) Calculated indoor and outdoor ozone exposures. (B) Calculated indoor and outdoor ozone intakes (see text for details). Data from Boston, Massachusetts: Sarnat et al. (2005); Upland and Mt. Towns, California: Geyh et al. (2000); Nashville, Tennessee: Lee et al. (2004); State College, Pennsylvania: Liu et al. (1993); Fraser Valley, British Columbia, Canada: Brauer and Brook (1995); Southern California: Linn et al. (1996).
Figure 1A does not account for the fact that breathing rates for both children and adults vary with activity levels and, on average, tend to be higher outdoors. An individual’s daily ozone inhalation intake (micrograms per day) is estimated by
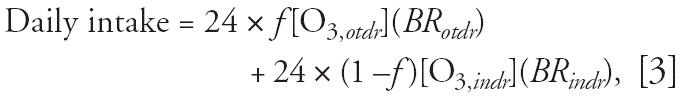 |
where BRotdr and BRindr are the average breathing rate while outdoors and indoors, respectively. Figure 1B shows estimates of daily outdoor and indoor ozone intakes (micrograms per day) calculated using Equation 3. In calculating these intakes, breathing rates corresponding to light exercise (0.95 m3/hr for children, 1.39 m3/hr for adults) were used for BRotdr, and breathing rates corresponding to sedentary activity (0.47 m3/hr for children, 0.54 m3/hr for adults) were used for BRindr (U.S. EPA 1997). Total breathing rates were used rather than fractional rates corresponding only to alveolar ventilation, because most of the inhaled ozone reacts with ascorbic acid, uric acid, glutathione, and unsaturated fatty acids present in the epithelial lining fluid in the conducting airways (Postlethwait and Ultman 2001; Rigas et al. 2000). Although outdoor ozone intakes in Figure 1B tend to be larger, indoor ozone intakes account for between 27 and 60% of total daily ozone intake and average just over 40%. Given that even low levels of ozone have been associated with increased risk of premature mortality (Bell et al. 2006), indoor intakes should not be ignored.
Products of Ozone-Initiated Indoor Chemistry: Exposures and Intakes
Indoor sources of ozone-reactive chemicals
Indoor exposure to ozone is accompanied by exposure to the products of ozone-initiated indoor chemistry (Weschler 2004). In general, these products are a consequence of ozone reacting with many commonly found organic chemicals that contain unsaturated carbon–carbon bonds (e.g., isoprene, styrene, terpenes, sesquiterpenes, squalene, and unsaturated fatty acids and their esters) because such compounds react with ozone much faster than do saturated organic compounds. Table 1 is a summary common indoor sources of ozone-reactive chemicals, including the occupants themselves, soft woods, carpets, linoleum, certain paints, polishes, cleaning products and air fresheners, soiled fabrics, and soiled ventilation filters. These ubiquitous sources result in substantial quantities of indoor chemicals that can react with ozone whenever outdoor concentrations are elevated.
Table 1.
Indoor sources of ozone-reactive chemicals and common stable oxidation products resulting from ozone-initiated reactions with the specified emissions.
| Source | Reactive emissions | Major stable products | References |
|---|---|---|---|
| Occupants (exhaled breath, skin oils, personal care products) | Isoprene, nitric oxide, squalene, unsaturated sterols, oleic acid and other unsaturated fatty acids, unsaturated oxidation products | Methacrolein, methyl vinyl ketone, nitrogen dioxide, acetone, 6MHO, geranyl acetone, 4OPA, formaldehyde, nonanal, decanal, 9-oxo-nonanoic acid, azelaic acid, nonanoic acid | Finlayson-Pitts and Pitts (2000), Fruekilde et al. (1998), Thornberry and Abbatt (2004), Taucher et al. (1997), Wisthaler et al. (2005) |
| Soft woods; wood flooring including cypress, cedar, and silver fir boards; houseplants | Isoprene, limonene, α-pinene, other terpenes and sesquiterpenes | Formaldehyde, 4-AMC, pinonaldehyde, pinic acid, pinonic acid, formic acid, methacrolein, methyl vinyl ketone, SOAs including ultrafine particles | Aoki et al. (2005), Hodgson et al. (2000), Iwashita (2005), Kagi et al. (2005), Atkinson and Arey (2003), SOA references in text |
| Carpets and carpet backing | 4-Phenylcyclohexene, 4-vinylcyclo-hexene, styrene, 2-ethylhexyl acrylate, unsaturated fatty acids and esters | Formaldehyde, acetaldehyde, benzaldehyde, hexanal, nonanal, 2-nonenal | Hodgson et al. (1993), Morrison and Nazaroff (2000, 2002), Weschler et al. (1992a) |
| Linoleum and paints/polishes containing linseed oil | Linoleic acid, linolenic acid | Propanal, hexanal, nonanal, 2-heptenal, 2-nonenal, 2-decenal, 1-pentene-3-one, propionic acid, n-butyric acid | Andersson et al. (1996), Clausen et al. (2005), Wolkoff (1995) |
| Latex paint | Residual monomers | Formaldehyde | Reiss et al. (1995a) |
| Certain cleaning products, polishes, waxes, air fresheners | Limonene, α-pinene, terpinolene, α-terpinene and other terpenes, α-terpineol, linalool, linalyl acetate and other terpenoids, longi-folene and other sesquiterpenes | Formaldehyde, acetaldehyde, glycoaldehyde, formic acid, acetic acid, hydrogen and organic peroxides, acetone, benzaldehyde, 4-hydroxy-4-methyl-5- hexen-1-al, 5-ethenyl-dihydro-5-methyl-2(3H)-furanone, 4-AMC, SOAs including ultrafine particles | Aoki et al. (2005), Destaillats et al. (2006a), Englund et al. (1996), Liu et al. (2004), Nazaroff and Weschler (2004), Shu et al. (1997), Singer et al. (2006), Wolkoff et al. (1998), SOA references in text |
| Natural rubber adhesive | Isoprene, terpenes | Formaldehdye, methacrolein, methyl vinyl ketone | Aoki et al. (2005), Atkinson and Arey (2003) |
| Photocopier toner, printed paper, styrene polymers | Styrene | Formaldehyde, benzaldehyde | Aoki et al. (2005), Wolkoff et al. (1993), Wolkoff (1999) |
| Environmental tobacco smoke | Styrene, acrolein, nicotine | Formaldehyde, benzaldehyde, hexanal, glyoxal, N-methylformamide, nicotinaldehyde, cotinine | Destaillats et al. (2006b), Shaughnessy et al. (2001) |
| Soiled clothing, fabrics, bedding | Squalene, unsaturated sterols, oleic acid and other unsaturated fatty acids | Acetone, geranyl acetone, 6MHO, 4OPA, formaldehyde, nonanal, decanal, 9-oxo-nonanoic acid, azelaic acid, nonanoic acid | Fruekilde et al. (1998), Thornberry and Abbatt (2004), Wisthaler et al. (2005) |
| Soiled particle filters | Unsaturated fatty acids from plant waxes, leaf litter, and other vegetative debris; soot; diesel particles | Formaldehyde, nonanal, and other aldehydes; azelaic acid; nonanoic acid; 9-oxo-nonanoic acid and other oxo-acids; compounds with mixed functional groups (== O, –OH, and –COOH) | Bekö et al. (2006), Hyttinen et al. (2003, 2006), Thornberry and Abbatt (2004) |
| Ventilation ducts and duct liners | Unsaturated fatty acids and esters, unsaturated oils, neoprene | C5 to C10 aldehydes | Morrison et al. (1998) |
| “Urban grime” | Polycyclic aromatic hydrocarbons | Oxidized polycyclic aromatic hydrocarbons | Kahan et al. (2006) |
| Perfumes, colognes, essential oils (e.g. lavender, eucalyptus, tea tree) | Limonene, α-pinene, linalool, linalyl acetate, terpinene-4-ol, γ-terpinene | Formaldehyde, 4-AMC, acetone, 4-hydroxy-4-methyl-5-hexen-1-al, 5-ethenyl-dihydro-5-methyl-2(3H) furanone, SOAs including ultrafine particles | Chao et al. (2005), Karamalegos et al. (2005), Shu et al. (1997), SOA references in text |
| Overall home emissions | Limonene, α-pinene, styrene | Formaldehyde, 4-AMC, pinonaldehdye, acetone, pinic acid, pinonic acid, formic acid, benzaldehyde, SOAs including ultrafine particles | Hodgson et al. (2000), Park and Ikeda (2006), Atkinson and Arey (2003), SOA references in text |
Abbreviations: 4-AMC, 4-acetyl-1-methyl-cyclohexene; 6MHO, 6-methyl-5-heptene-2-one; 4OPA, 4-oxopentanal.
Products of ozone-initiated indoor chemistry
There are several reasons why products of ozone-initiated chemistry tend to have higher concentrations indoors than outdoors. First, there are more ozone-reactive chemicals indoors than outdoors because of the presence of consumer products, architectural coatings, furnishings, and building materials; indeed, some sources occur almost exclusively indoors (e.g., carpets, linoleum, air fresheners). Second, the concentrations of ozone-reactive compounds tend to be higher indoors than outdoors (Brown et al. 1994; Hodgson and Levin 2003; Wolkoff 1995), reflecting more sources and larger emission rates per volumetric flow rate. Third, surface-to-volume ratios (S:V) are roughly two orders of magnitude larger indoors than outdoors based on characteristic mixing heights outdoors (Nazaroff et al. 2003). This is partially counterbalanced by higher deposition velocities, vd, outdoors (Finlayson-Pitts and Pitts 2000) compared with indoors (Weschler 2000). On average, the first-order rate constant that describes surface removal (the product of S:V and vd) is about 30 times larger indoors than outdoors. Indoor surface reactions are major sources of oxidation products (Destaillats et al. 2006b; Fick et al. 2004; Morrison and Nazaroff 2002; Weschler et al. 1992b; Wisthaler et al. 2005). Additionally, unlike gas-phase reactions (Weschler and Shields 2000), surface chemistry can include reactions whose rates are slower than air exchange rates.
Table 1 is a summary of the major stable reaction products anticipated from indoor ozone chemistry. Evidence from field studies suggests that indoor concentrations of some of these stable products (e.g., organic acids and carbonyls) correlate with ozone concentrations (Bako-Biro et al. 2005; Reiss et al. 1995b; Zhang et al. 1994). In addition to stable products, ozone chemistry produces relatively short-lived products. Examples include primary and secondary ozonides, peroxyhemiacetals, α-hydroxy ketones, α-hydroxy hydroperoxides, and peroxyacyl nitrates (Atkinson and Arey 2003; Docherty et al. 2005; Finlayson-Pitts and Pitts 2000; Norgaard et al. 2006; Ziemann 2003). Although short-lived, many of these products exist long enough to be inhaled and transported into the respiratory tract. Indoor ozone also reacts with alkenes to produce hydroxyl radicals (Destaillats et al. 2006a; Fan et al. 2003; Sarwar et al. 2002; Weschler and Shields, 1996, 1997) and with nitrogen dioxide to produce nitrate radicals (Weschler et al. 1992a). These are highly reactive oxidants in their own right. Indeed, at typically anticipated indoor concentrations, ozone-derived nitrate radicals react much faster with alkenes and polycyclic aromatic hydrocarbons (PAHs) than with ozone alone [see table 8 of Nazaroff and Weschler (2004)].
Secondary organic aerosols (SOAs), consisting of primarily fine and ultrafine particles, are an important subgroup of stable products resulting from ozone-initiated chemistry. They are formed from low-vapor pressure–oxidation products that partition between the gas phase and the surface of preexisting particles or nucleate to form new aerosols. The reaction of ozone with various terpenoids in indoor settings has been shown to contribute tens of micrograms per cubic meter to the indoor concentration of submicrometer particles under appropriate conditions (Destaillats et al. 2006a; Fan et al. 2003, 2005; Long et al. 2000; Rohr et al. 2003b; Sarwar et al. 2003, 2004; Wainman et al. 2000; Weschler and Shields 1999).
Studies indicating indoor exposure to SOAs from ozone-initiated chemistry
Particulate organic carbon was analyzed in samples of indoor and outdoor fine particles collected at 173 homes in Houston, Texas; Los Angeles, California; and Elizabeth, New Jersey. At least 40%, but more likely 70–75%, of the particulate organic carbon associated with indoor particles was generated indoors (Polidori et al. 2006). The authors speculated that a portion of this may have been contributions from SOAs generated by indoor ozone chemistry. Table 2 presents data from Sarnat et al. (2005) that support the concept that SOAs generated indoors can make meaningful contributions to personal exposures to particles < 2.5 μm in diameter (PM2.5). The table shows personal (P) and corresponding outdoor (O) concentrations of PM2.5 and fine-mode sulfate (SO42−) for senior citizens living in Boston, Massachusetts, during five monitoring periods. The fine-mode sulfate concentrations are derived from analyses of the PM2.5 filters and serve as markers for personal exposure to PM2.5 of outdoor origin because fine-mode sulfate has few indoor sources (Sarnat et al. 2002). Table 2 also shows personal-to-out-door ratios (P:O) for PM2.5 and SO42−, as well as differences between these ratios [P:O (PM2.5) − P:O (SO42−)]. During winter 1 and winter 2, the difference between P:O (PM2.5) and P:O (SO42−) was approximately 0.35 (Table 2, next to last row). In contrast, during winter 3, summer 1, and summer 2, this difference was much larger, ranging from 0.75 to 1.55. The larger difference indicates that indoor sources were making a larger contribution to personal PM2.5 concentrations during these final three monitoring periods than during the first two monitoring periods. It is unlikely that this was due to recognized (Wallace et al. 2006) indoor sources of PM2.5 such as cooking, cleaning, and personal care because these sources should not be significantly stronger during milder weather. Nor is the larger difference due to more time indoors during the final three periods; the seniors were indoors 97% of the time in the winter and 93% of the time in the summer (time in transit included). Instead, the larger difference may be due to greater amounts of indoor ozone–generated SOAs during winter 3, summer 1, and summer 2 than during winter 1 and winter 2 [personal ozone concentrations (Table 2, last row) were 2.5, 5.1, and 4.8 ppb during the former periods and 0.1 and 0.8 ppb during the latter periods].
Additional data in Sarnat et al. (2005) lend further support to this interpretation. Regression results for measurements made during the summer months indicate a slope of 0.35 (0.22–0.47) for personal sulfate regressed on personal ozone, compared with a slope of 0.72 (0.42–1.01) for personal PM2.5 regressed personal ozone [see table 3 of Sarnat et al. (2005)]. The much larger slope for the latter pairing is consistent with contributions to personal PM2.5 from SOAs generated by ozone-initiated indoor chemistry.
Table 3.
Cities with highest and lowest percent change in daily mortality per 10-ppb increase in daily ozonea, percentage of population growthb, and percentage of housing units with central ACb.
| Change in daily mortality (%) | Population growth, 1990–2000 (%) | Central AC (%) | |
|---|---|---|---|
| Ten cities with highest percent change (of 95 cities) | |||
| New York City | 1.7 | 9.4 | 16 |
| Newark, NY | 1.3 | −0.6 | 47 |
| Philadelphia, PA | 1.3 | −4.3 | 50 |
| Cincinnati, OH | 1.2 | −9.0 | 66 |
| Dallas/Ft. Worth, TX | 1.1 | 18.5 | 91 |
| Shreveport, LA | 1.0 | 0.8 | > 70 |
| Chicago, IL | 0.9 | 4.0 | 62 |
| Syracuse, NY | 0.9 | −10.1 | < 70 |
| Colorado Springs, CO | 0.9 | 28.4 | < 70 |
| Worcester, MA | 0.9 | 1.7 | < 70 |
| Average | 1.1 | 3.9 | — |
| Ten cities with lowest percent change (of 95 cities) | |||
| Orlando, FL | −0.2 | 12.9 | > 70 |
| Denver, CO | 0.0 | 18.6 | 50 |
| San Antonio, TX | 0.1 | 22.3 | 78 |
| Las Vegas, NV | 0.1 | 85.2 | > 70 |
| Little Rock, AR | 0.1 | 4.2 | > 70 |
| Lexington, KY | 0.2 | 15.6 | > 70 |
| Birmingham, AL | 0.2 | −8.7 | 77 |
| San Diego, CA | 0.2 | 10.2 | 34 |
| St. Petersburg, FL | 0.2 | 4.0 | 87 |
| Lafayette, IN | 0.3 | 28.9 | < 70 |
| Average | 0.1 | 20.0 | — |
Health effects of ozone reaction products
Certain ozone reaction products are known to have adverse health effects. For example, formaldehyde has been designated a Group 1 carcinogen in a 2004 International Agency for Research on Cancer evaluation (Cogliano et al. 2005). Acrolein is listed by California as an irritant and carcinogen (California Office of Environmental Health Hazard Assessment 2006). Peroxyactyl nitrate is a known eye irritant (Vyskocil et al. 1998), as are some of the products of ozone/terpene and ozone/isoprene chemistry (Kleno and Wolkoff 2004; Nojgaard et al. 2005). Hydroperoxides formed via the oxidation of terpenes and terpenoids can be potent contact allergens (Gafvert et al. 1994; Karlberg and DoomsGoossens 1997; Matura et al. 2003, 2005; Skold et al. 2002). Leikauf (2002) has listed formaldehyde, acetaldehyde, and acrolein as compounds anticipated to induce or exacerbate asthma. Using a mouse model, Wolkoff and colleagues have demonstrated that ozone/terpene reactions produce strong airway irritants (Clausen et al. 2001; Rohr et al. 2002, 2003a; Wilkins et al. 2001; Wolkoff et al. 1999, 2000). However, an acute exposure study of healthy women exposed for 2 hr to a mixture of volatile organic compounds and ozone (40 ppb) did not result in significant subjective or objective symptoms (Fiedler et al. 2005; Laumbach et al. 2005), suggesting that longer exposures may be necessary to produce a measurable effect.
For some indoor oxidation products, the connection with adverse health effects is more tentative. For example, there is accumulating evidence that outdoor PM2.5 adversely affects morbidity and mortality (Dominici et al. 2006; Pope et al. 2002), and SOAs are major constituents of outdoor PM2.5. However, SOAs from ozone/terpenoid reactions differ in composition from SOAs generated by outdoor photochemical activity. It is not known how the toxicities of these SOAs compare. An additional consideration is the fact that ozone/terpenoid reactions lead to the co-occurrence of peroxides and submicrometer particles (Docherty et al. 2005; Fan et al. 2005; Li et al. 2002), and this may provide a mechanism to transport some of the peroxides deep into the respiratory tract (Friedlander and Yeh 1998). The consequences of inhaling such oxidation products, some of which are known contact allergens (see above), remain to be evaluated.
Hydroxyl and nitrate radicals, derived from ozone reactions, further react to produce still other oxidation products. Toxic products formed in this manner include malaoxon from the OH oxidation of malathion (Brown et al. 1993) and nitrosoamines and nitro-PAHs from reactions involving nitrate radicals (Gupta et al. 1996; Pitts et al. 1985).
Average daily indoor intakes of ozone reaction products
Ignoring gas-phase reactions, the ratio of the indoor concentration of ozone oxidation products to the indoor concentration of ozone, [Prod]:[O3,indr], is roughly estimated by
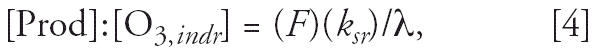 |
where ksr and λ are as defined for Equation 1, and F is the ratio of the molar emission rate of oxidation products to ozone’s surface removal rate (sometimes called the “formation factor”). Wisthaler et al. (2005) found that for every molecule of ozone removed by surfaces in a simulated aircraft cabin, between 0.2 and 0.25 molecules of oxidized products entered the air. For different types of carpets, Morrison and Nazaroff (2002, figure 5) report that for each ozone molecule removed, 0.1–0.7 aldehyde molecules entered the air. For four different types of surfaces in four different homes, Wang and Morrison (2006) report that for each ozone molecule removed, 0.1–0.4 aldehyde molecules entered the air. In the latter two studies, the total number of oxidized molecules that entered the air is presumably larger than that reported for aldehydes alone because common oxidation products such as formic acid, acetic acid, and acetone were not included in the aldehyde numbers. Although more measurements of F are needed in residences and nonresidences, these studies are a beginning. Using a middle estimate of 0.33 for F and combining it with a value of 3 hr−1 for ksr and a range of values from 0.5 to 3 hr−1 for λ, a conservative estimate for [Prod]:[O3,indr] is 0.33–2. This estimate is termed “conservative” because it considers only airborne products derived from surface chemistry; additional oxidation products derived from gas-phase chemistry (e.g., ozone reacting with terpenes) would result in a larger ratio. Hence, it is reasonable to anticipate that ozone oxidation products are present indoors at concentrations that, on a molar basis, are roughly one-third to twice those of ozone alone. This means that average daily indoor intakes of ozone oxidation products are roughly one-third to twice that of ozone (Figure 1B). Because the products of ozone-initiated chemistry tend to have higher concentrations indoors than outdoors (see above) and that greater time spent indoors overwhelms larger breathing rates outdoors, indoor inhalation intakes of oxidation products tend to be much larger than outdoor intakes of oxidation products.
In a region with moderate outdoor ozone levels, persons doing their own day-to-day house cleaning are estimated to inhale an average of 20 μg/day of formaldehyde and 35 μg/day of SOAs (a large fraction of which are ultrafine particles) as a consequence of ozone-initiated reactions with constituents of cleaning agents and air fresheners [table 5.3 of Nazaroff et al. (2006)]. These values are consistent with intakes of oxidation products estimated in the previous paragraph. Such inhalation intakes add to already existing and often significant intakes of formaldehyde and SOAs from other sources. Furthermore, reactions between ozone and constituents of personal-use products (e.g., perfumes, colognes, hair treatments) emit oxidation products in the vicinity of the breathing zone, resulting in inhalation intakes larger than those predicted if the products were evenly distributed throughout a room (Karamalegos et al. 2005).
Connections Between Ill Health and Exposure to Indoor Ozone and Its Oxidation Products
Recent epidemiologic study of mortality in 95 U.S. urban communities
Bell et al. (2004) have used databases from the National Morbidity, Mortality and Air Pollution Study to calculate the average relative rate of mortality associated with short-term ozone concentrations measured at outdoor monitoring stations for 95 U.S. cities between 1987 and 2000. Table 3 presents the 10 cities with the highest percent change in daily mortality per 10-ppb increase in daily ozone and the 10 cities with the lowest percent change. For each of the listed cities, Table 3 also presents the percentage of population growth for the period 1990–2000 and the percentage of residences with central AC. The data were obtained from the U.S. Census Bureau (2006); for selected cities where specific information on central AC was not available, the value is simply listed as being greater than or less than 70% on the basis of comparisons with cities that have similar seasonal dew points and temperatures.
Cities with recent population growth have a larger fraction of new homes and apartments than cities with less growth, and such newer residences tend to have lower air exchange rates (Weisel et al. 2005). Use of central AC is also associated with low air exchange rates (see “Indoor ozone concentrations” above). Conversely, without AC, residents are more likely to open their windows during periods when temperatures are elevated. Hence, compared with older cities that have fewer homes with central AC, newer cities with a higher prevalence of central AC are anticipated to have less outdoor-to-indoor transport and smaller occupant exposures to indoor ozone and the products of ozone-initiated chemistry. Consistent with a connection between such indoor exposures and mortality, 8 of the 10 cities that had the highest percent increase in mortality per 10-ppb increase in ozone had population growth since 1990 < 10% and 8 of the 10 had central AC in < 70% of the structures, whereas 7 of the 10 cities that had the lowest percent increase in mortality per 10-ppb increase in ozone had population growth since 1990 > 10% and 7 of the 10 had central AC in > 70% of its structures (Table 3).
Other suggestive studies
Levy et al. (2005) conducted an empiric Bayes meta regression to examine the relationship between outdoor ozone concentrations and premature mortality based on 48 estimates from 28 time-series studies. The authors deliberately omitted data from the National Morbidity, Mortality and Air Pollution Study (2006) because other investigators were analyzing these data. In other words, their database was independent of data that are the basis for Table 3. One of the conclusions from their meta regression was that AC prevalence was among the strongest predictors of between-study variability. They go on to state that their results suggest “that the ambient ozone-mortality relationship might be lower in cities with greater prevalence of residential central air conditioning (and therefore lower personal exposure to zone).”
Time-series epidemiologic studies often show seasonal differences in the relative risk from ozone (Ito et al. 2005; Levy et al. 2005; Wong et al. 2001; Zhang et al. 2006). Ozone risk estimates are larger for summer than for winter in New York City; Detroit, Michigan; and Cook County, Illinois (Ito et al. 2005). Conversely, ozone risk estimates are larger for winter than for summer in Houston (Ito et al. 2005), Hong Kong (Wong et al. 2001), and Shanghai (Zhang et al. 2006). In New York, Detroit, and Cook County, there is less outdoor-to-indoor transport during the cold winter, when windows tend to be closed, compared with the warmer summer. Indeed, in Boston, a climatically similar urban area, the association between outdoor ozone and personal ozone has been shown to be weaker in winter than in summer (Sarnat et al. 2005). However, in a southern city such as Houston or a subtropical Asian city such as Hong Kong or Shanghai, there is less outdoor-to-indoor transport during the hot, humid summer, when air conditioners are used extensively and buildings tend to be sealed, compared with the cooler winter when buildings tend to be more open.
Conclusions
Indoor ozone and products of ozone-initiated indoor chemistry correlate with ozone measured at fixed outdoor sites. I have cited studies indicating that a) indoor ozone levels are typically 10–50% of outdoor values, b) indoor ozone exposures are typically 45–75% of total exposures, c) indoor ozone inhalation intakes are typically 25–60% of total intakes, d) indoor sources of chemicals that react with ozone are ubiquitous, e) certain oxidation products are known to be toxic and others are anticipated to be toxic, and f ) indoor inhalation intakes of these oxidation products are roughly one-third to twice the indoor intakes of ozone and much greater than outdoor intakes of oxidation products. Smaller indoor intakes of ozone are anticipated for people who spend a large fraction of their indoor time in air-conditioned rooms or in rooms with small air exchange rates during periods when outdoor ozone levels are elevated. Smaller indoor intakes of oxidation products are anticipated for people who live in indoor settings with relatively low concentrations of ozone-reactive chemicals, both in the gas phase and associated with surfaces; smaller indoor intakes of oxidation products also result from higher air exchange rates (Equation 4).
By their nature the cited epidemiologic studies include the indoor exposures discussed in this article. Findings from several epidemiologic studies hint at associations between morbidity and mortality and indoor ozone and its oxidation products. However, these studies were not designed to test this hypothesis. Specific studies can be envisioned to evaluate the contribution of indoor ozone and its oxidation products to ill health (Weschler 2004).
Apportioning risk between outdoor and indoor intakes bears on the strategies used to protect public health. Outdoor ozone is harmful to health; outdoor ozone transported indoors is harmful to health; indoor ozone reacts to form products that are also harmful to health, some perhaps more so than ozone. Contrary to popular wisdom, being indoors does not offer clear protection from ozone-related adverse health effects, but it would if ozone were deliberately removed from ventilation air.
Although it has proven difficult and very costly to reduce outdoor ozone concentrations, relatively simple steps can reduce the concentration of indoor ozone and its oxidation products. For example, charcoal filters (Shair 1981; Shields et al. 1999) or chemically impregnated filters (Kelly and Kinkead 1993) could remove a large fraction of ozone in buildings with mechanical ventilation systems. In naturally ventilated buildings, strategies could be employed that reduce ventilation for the portion of the day when ozone is elevated and increase ventilation when ozone levels are lower. The use of products with ozone-reacting constituents could be limited during periods when indoor ozone levels are elevated. Such steps might be especially valuable interventions in schools, hospitals, and childcare centers in regions that continue to experience elevated outdoor ozone concentrations.
Footnotes
I thank L.B. Weschler, H. Levin, W.W. Nazaroff, L. Wallace, M. Morandi, P.J. Lioy, C.P. Weisel, and R.L. Corsi for their reviews and valuable comments on the draft manuscript, and F. Shair for his pioneering studies.
References
- Andersson K, Andersson B, Nilsson C-A, Sandstrom M. 1996. Naturfarger: Indentifiering av de flyktiga amnen som avges nar aggoljetempera torkar [in Swedish]. Arbetslivsrapport 12. Umea, Sweden.
- Aoki T, Tanabe S, Funaki R, Tanaka H, Nakagawa T, Kihara I. et al. 2005. Generation of submicron particles and secondary emissions from building materials by ozone reactions. In: Indoor Air 2005 (Yang X, Zhao B, Zhao R, eds). Beijing:Tsinghua University Press, 1552–1556.
- Atkinson R, Arey J. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: a review. Atmos Environ. 2003;37:S197–S219. [Google Scholar]
- Avol EL, Navidi WC, Colome SD. Modeling ozone levels in and around Southern California homes. Environ Sci Technol. 1998;32(4):463–468. [Google Scholar]
- Bako-Biro Z, Weschler CJ, Wargocki P, Fanger PO. 2005. Effects of indoor pollution sources and ventilation rate on ozone’s surface removal rate and the occurrence of oxygenated VOCs in an office space. In: Indoor Air 2005 (Yang X, Zhao B, Zhao R, eds). Beijing:Tsinghua University Press, 2320–2324.
- Bekö G, Halas O, Clausen G, Weschler CJ. Initial studies of oxidation processes on filter surfaces and their impact on perceived air quality. Indoor Air. 2006;16(1):56–64. doi: 10.1111/j.1600-0668.2005.00401.x. [DOI] [PubMed] [Google Scholar]
- Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality, and air pollution study. Epidemiology. 2005;16(4):436–445. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987–2000. JAMA. 2004;292(19):2372–2378. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Peng R, Dominici F. The exposure-response curve for ozone and risk of mortality and the adequacy of current ozone regulations. Environ Health Perspect. 2006;114:532–536. doi: 10.1289/ehp.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer M, Brook JR. Personal and fixed-site ozone measurements with a passive sampler. J Air Waste Manage. 1995;45(7):529–537. doi: 10.1080/10473289.1995.10467384. [DOI] [PubMed] [Google Scholar]
- Brown MA, Petreas MX, Okamoto HS, Mischke TM, Stephens RD. Monitoring of malathion and its impurities and environmental transformation products on surfaces and in air following an aerial application. Environ Sci Technol. 1993;27(2):388–397. [Google Scholar]
- Brown S, Sim M, Abramson M, Gray C. Concentrations of volatile organic compounds in indoor air—a review. Indoor Air. 1994;4:123–134. [Google Scholar]
- California Office of Environmental Health Hazard Assessment 2005. Air. Available: http://www.oehha.ca.gov/air.html [accessed 31 May 2006].
- Chao CJ, Wu PC, Chang HY, Su HJ. 2005. The effects of evaporating essential oils on indoor air quality. In: Indoor Air 2005 (Yang X, Zhao B, Zhao R, eds). Beijing:Tsinghua University Press, 2309–2313.
- Clausen PA, Knudsen HN, Larsen K, Kofoed-Sorensen V, Wolkoff P, Wilkins CK. 2005. Use of gas chromatography olfactometry (GC-O) to detect unknown emissions from building products containing linseed oil. In: Indoor Air 2005 (Yang X, Zhao B, Zhao R, eds). Beijing:Tsinghua University Press, 2043–2047.
- Clausen PA, Wilkins CK, Wolkoff P, Nielsen GD. Chemical and biological evaluation of a reaction mixture of R-(+)-limonene/ozone—formation of strong airway irritants. Environ Int. 2001;26(7–8):511–522. doi: 10.1016/s0160-4120(01)00035-6. [DOI] [PubMed] [Google Scholar]
- Cogliano VJ, Grosse Y, Baan RA, Straif K, Secretan MB, El Ghissassi F. Summary of IARC monographs on formaldehyde, 2-butoxyethanol, and 1-tert-butoxy-2-propanol. Environ Health Perspect. 2005;113:1205–1208. doi: 10.1289/ehp.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaillats H, Lunden MM, Singer BC, Coleman BK, Hodgson AT, Weschler CJ, et al. Indoor secondary pollutants from household product emissions in the presence of ozone. A bench-scale chamber study. Environ Sci Technol. 2006a;40(14):4421–4428. doi: 10.1021/es052198z. [DOI] [PubMed] [Google Scholar]
- Destaillats H, Singer BC, Lee SK, Gundel LA. Effect of ozone on nicotine desorption from model surfaces: evidence for heterogeneous chemistry. Environ Sci Technol. 2006b;40(6):1799–1805. doi: 10.1021/es050914r. [DOI] [PubMed] [Google Scholar]
- Docherty KS, Wu W, Lim YB, Ziemann PJ. Contributions of organic peroxides to secondary aerosol formed from reactions of monoterpenes with O3. Environ Sci Technol. 2005;39(11):4049–4059. doi: 10.1021/es050228s. [DOI] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund F, Larsen A, Winther-Funch L, Saarela K, Tirkkonen T. 1996. Emissions of VOC from wooden floors, surface treated with oils and waxes. In: Indoor Air ‘96 (Yoshizawa S, Kimura K, Ikeda K, Tanabe S, Iwata T, eds). Vol 3. Nagoya, Japan: Organizing Committee, Indoor Air ‘96, 9–94.
- Fan ZH, Lioy P, Weschler CJ, Fiedler N, Kipen H, Zhang JF. Ozone-initiated reactions with mixtures of volatile organic compounds under simulated indoor conditions. Environ Sci Technol. 2003;37(9):1811–1821. doi: 10.1021/es026231i. [DOI] [PubMed] [Google Scholar]
- Fan ZH, Weschler CJ, Han IK, Zhang JF. Co-formation of hydroperoxides and ultra-fine particles during the reactions of ozone with a complex VOC mixture under simulated indoor conditions. Atmos Environ. 2005;39(28):5171–5182. [Google Scholar]
- Fick J, Nilsson C, Andersson B. Formation of oxidation products in a ventilation system. Atmos Environ. 2004;38(35):5895–5899. [Google Scholar]
- Fiedler N, Laumbach R, Kelly-McNeil K, Lioy P, Fan ZH, Zhang J, et al. Health effects of a mixture of indoor air volatile organics, their ozone oxidation products, and stress. Environ Health Perspect. 2005;113:1542–1548. doi: 10.1289/ehp.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson-Pitts BJ. Pitts JN Jr. 2000. Chemistry of the Upper and Lower Atmosphere. San Diego:Academic Press.
- Friedlander SK, Yeh EK. The submicron atmospheric aerosol as a carrier of reactive chemical species: case of peroxides. App Occup Environ Hygiene. 1998;13(4):1–5. [Google Scholar]
- Fruekilde P, Hjorth J, Jensen NR, Kotzias D, Larsen B. Ozonolysis at vegetation surfaces: a source of acetone, 4-oxopentanal, 6-methyl-5-hepten-2-one, and geranyl acetone in the troposphere. Atmos Environ. 1998;32(11):1893–1902. [Google Scholar]
- Gafvert E, Shao LP, Karlberg AT, Nilsson U, Nilsson JLG. Contact allergy to resin acid hydroperoxides—hapten binding via free-radicals and epoxides. Chem Res Toxicol. 1994;7(2):260–266. doi: 10.1021/tx00038a020. [DOI] [PubMed] [Google Scholar]
- Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, et al. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. 2003;290(14):1859–1867. doi: 10.1001/jama.290.14.1859. [DOI] [PubMed] [Google Scholar]
- Geyh AS, Xue J, Ozkaynak H, Spengler JD. The Harvard Southern California Chronic Ozone Exposure Study: assessing ozone exposure of grade-school-age children in two Southern California communities. Environ Health Perspect. 2000;108:265–270. doi: 10.1289/ehp.00108265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grontoft T, Raychaudhuri MR. Compilation of tables of surface deposition velocities for O3, NO2 and SO2 to a range of indoor surfaces. Atmos Environ. 2004;38(4):533–544. [Google Scholar]
- Gryparis A, Forsberg B, Katsouyanni K, Analitis A, Touloumi G, Schwartz J, et al. Acute effects of ozone on mortality from the “Air Pollution and Health: A European Approach” project. Am J Respir Crit Care. 2004;170(10):1080–1087. doi: 10.1164/rccm.200403-333OC. [DOI] [PubMed] [Google Scholar]
- Gupta P, Harger WP, Arey J. The contribution of nitro- and methylnitro-naphthalenes to the vapor-phase mutagenicity of ambient air samples. Atmos Environ. 1996;30(18):3157–3166. [Google Scholar]
- Hodgson AT, Levin H. 2003. Volatile Organic Compounds in Indoor Air: A Review of Concentrations Measured in North America since 1990. Report LBNL-51715. Berkeley, CA:Lawrence Berkeley National Laboratory.
- Hodgson AT, Rudd AF, Beal D, Chandra S. Volatile organic compound concentrations and emission rates in new manufactured and site-built houses. Indoor Air. 2000;10(3):178–192. doi: 10.1034/j.1600-0668.2000.010003178.x. [DOI] [PubMed] [Google Scholar]
- Hodgson AT, Wooley JD, Daisey JM. Emissions of volatile organic-compounds from new carpets measured in a large-scale environmental chamber. J Air Waste Manage. 1993;43(3):316–324. doi: 10.1080/1073161x.1993.10467136. [DOI] [PubMed] [Google Scholar]
- Hubbell BJ, Hallberg A, McCubbin DR, Post E. Health-related benefits of attaining the 8-hr ozone standard. Environ Health Perspect. 2005;113:73–82. doi: 10.1289/ehp.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyttinen M, Pasanen P, Kalliokoski P. Removal of ozone on clean, dusty and sooty supply air filters. Atmos Environ. 2006;40(2):315–325. [Google Scholar]
- Hyttinen M, Pasanen P, Salo J, Bjorkroth M, Vartiainen M, Kalliokoski P. Reactions of ozone on ventilation filters. Indoor Built Environ. 2003;12(3):151–158. [Google Scholar]
- Ito K, De Leon SF, Lippmann M. Associations between ozone and daily mortality: analysis and meta-analysis. Epidemiology. 2005;16(4):446–457. doi: 10.1097/01.ede.0000165821.90114.7f. [DOI] [PubMed] [Google Scholar]
- Iwashita G. 2005. Basic study on the chemical reaction between ozone and sesquiterpenes emitted from cedarwood. In: Indoor Air 2005 (Yang X, Zhao B, Zhao R, eds). Beijing: Tsinghua University Press, 3010–3014.
- Kagi N, Fujii S, Tamura H, Nitta J. 2005. Secondary emission from floors by ozone and UV. In: Indoor Air 2005 (Yang X, Zhao B, Zhao R, eds). Beijing:Tsinghua University Press, 1991–1995.
- Kahan TF, Kwamena N-OA, Donaldson DJ. Heterogeneous ozonation kinetics of polycyclic aromatic hydrocarbons on organic films. Atmos Environ. 2006;40(19):3448–3459. [Google Scholar]
- Karamalegos A, Simon H, Zhao P, Morrison G, Siegel J, Corsi RL. 2005. Personal reactive clouds: introducing the concept of near head chemistry. In: Indoor Air 2005 (Yang X, Zhao B, Zhao R, eds). Beijing:Tsinghua University Press, 2356–2360.
- Karlberg AT, DoomsGoossens A. Contact allergy to oxidized d-limonene among dermatitis patients. Contact Dermatitis. 1997;36(4):201–206. doi: 10.1111/j.1600-0536.1997.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Kelly TJ, Kinkead DA. Testing of chemically treated adsorbent air purifiers. ASHRAE J. 1993;35(7):14–23. [Google Scholar]
- Kleno J, Wolkoff P. Changes in eye blink frequency as a measure of trigeminal stimulation by exposure to limonene oxidation products, isoprene oxidation products and nitrate radicals. Int Arch Occup Environ Health. 2004;77(4):235–243. doi: 10.1007/s00420-003-0502-1. [DOI] [PubMed] [Google Scholar]
- Laumbach RJ, Fiedler N, Gardner CR, Laskin DL, Fan ZH, Zhang JF, et al. Nasal effects of a mixture of volatile organic compounds and their ozone oxidation products. J Occup Environ Med. 2005;47(11):1182–1189. doi: 10.1097/01.jom.0000183338.95778.f0. [DOI] [PubMed] [Google Scholar]
- Lee K, Parkhurst WJ, Xue JP, Ozkaynak AH, Neuberg D, Spengler JD. Outdoor/indoor/personal ozone exposures of children in Nashville, Tennessee. J Air Waste Manage. 2004;54(3):352–359. doi: 10.1080/10473289.2004.10470904. [DOI] [PubMed] [Google Scholar]
- Lee K, Vallarino J, Dumyahn T, Ozkaynak H, Spengler JD. Ozone decay rates in residences. J Air Waste Manag Assoc. 1999;49(10):1238–1244. doi: 10.1080/10473289.1999.10463913. [DOI] [PubMed] [Google Scholar]
- Leikauf GD. Hazardous air pollutants and asthma. Environ Health Perspect. 2002;110:505–526. doi: 10.1289/ehp.02110s4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JI, Chemerynski SM, Sarnat JA. Ozone exposure and mortality: an empiric bayes metaregression analysis. Epidemiology. 2005;16(4):458–468. doi: 10.1097/01.ede.0000165820.08301.b3. [DOI] [PubMed] [Google Scholar]
- Li TH, Turpin BJ, Shields HC, Weschler CJ. Indoor hydrogen peroxide derived from ozone/d-limonene reactions. Environ Sci Technol. 2002;36(15):3295–3302. doi: 10.1021/es015842s. [DOI] [PubMed] [Google Scholar]
- Linn WS, Shamoo DA, Anderson KR, Peng RC, Avol EL, Hackney JD, et al. Short-term air pollution exposures and responses in Los Angeles area schoolchildren. J Expo Anal Environ Epidemiol. 1996;6(4):449–472. [PubMed] [Google Scholar]
- Liu LJS, Koutrakis P, Suh HH, Mulik JD, Burton RM. Use of personal measurements for ozone exposure assessment—a pilot-study. Environ Health Perspect. 1993;101:318–324. doi: 10.1289/ehp.93101318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Mason M, Krebs K, Sparks L. Full-scale chamber investigation and simulation of air freshener emissions in the presence of ozone. Environ Sci Technol. 2004;38:2802–2812. doi: 10.1021/es030544b. [DOI] [PubMed] [Google Scholar]
- Long CM, Suh HH, Koutrakis P. Characterization of indoor particle sources using continuous mass and size monitors. J Air Waste Manage. 2000;50(7):1236–1250. doi: 10.1080/10473289.2000.10464154. [DOI] [PubMed] [Google Scholar]
- Matura M, Goossens A, Bordalo O, Garcia-Bravo B, Magnusson K, Wrangsjo K, et al. Patch testing with oxidized R-(+)-limonene and its hydroperoxide fraction. Contact Dermatitis. 2003;49(1):15–21. doi: 10.1111/j.0105-1873.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- Matura M, Skold M, Borje A, Andersen KE, Bruze M, Frosch P, et al. Selected oxidized fragrance terpenes are common contact allergens. Contact Dermatitis. 2005;52(6):320–328. doi: 10.1111/j.0105-1873.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- McDonnell WF, Abbey DE, Nishino N, Lebowitz MD. Long-term ambient ozone concentration and the incidence of asthma in nonsmoking adults: the AHSMOG Study. Environ Res. 1999;80(2 pt 1):110–121. doi: 10.1006/enrs.1998.3894. [DOI] [PubMed] [Google Scholar]
- Morrison GC, Nazaroff WW. The rate of ozone uptake on carpets: experimental studies. Environ Sci Technol. 2000;34(23):4963–4968. [Google Scholar]
- Morrison GC, Nazaroff WW. Ozone interactions with carpet: secondary emissions of aldehydes. Environ Sci Technol. 2002;36(10):2185–2192. doi: 10.1021/es0113089. [DOI] [PubMed] [Google Scholar]
- Morrison GC, Nazaroff WW, Cano-Ruiz JA, Hodgson AT, Modera MP. Indoor air quality impacts of ventilation ducts: ozone removal and emissions of volatile organic compounds. J Air Waste Manage. 1998;48(10):941–952. doi: 10.1080/10473289.1998.10463740. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences 1991. Human Exposure Assessment for Airborne Pollutants. Washington, DC:National Academy Press.
- National Morbidity, Mortality and Air Pollution Study 2006. NMMAPSdata R Package. Baltimore, MD:Johns Hopkins University. Available: http://www.ihapss.jhsph.edu/data/NMMAPS/R/ [accessed 15 August 2006].
- Nazaroff WW, Cass GR. Mathematical modeling of chemically reactive pollutants in indoor air. Environ Sci Technol. 1986;20:924–934. doi: 10.1021/es00151a012. [DOI] [PubMed] [Google Scholar]
- Nazaroff WW, Coleman BK, Destaillats H, Hodgson AT, Liu D-L, Lunden MM. et al. 2006. Indoor Air Chemistry: Cleaning Agents, Ozone and Toxic Air Contaminants. Report 01-336. Sacramento, CA:California Air Resources Board, 145–156.
- Nazaroff WW, Gadgil A, Weschler CJ. 1993. Critique of the use of deposition velocity in modeling indoor air quality. In: Modeling of Indoor Air Quality and Exposure (Nagda N, ed). Philadelphia:American Society for Testing and Materials, 81–104.
- Nazaroff WW, Weschler CJ. Cleaning products and air fresheners: exposure to primary and secondary air pollutants. Atmos Environ. 2004;38(18):2841–2865. [Google Scholar]
- Nazaroff WW, Weschler CJ, Corsi RL. Indoor air chemistry and physics. Atmos Environ. 2003;37(39–40):5451–5453. [Google Scholar]
- Nojgaard JK, Christensen KB, Wolkoff P. The effect on human eye blink frequency of exposure to limonene oxidation products and methacrolein. Toxicol Lett. 2005;156(2):241–251. doi: 10.1016/j.toxlet.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Norgaard A, Nojgaard JK, Larsen K, Sporring S, Wilkins CK, Clausen PA, et al. Secondary limonene endo-ozonide: a major product from gas-phase ozonolysis of R-(+)-limonene at ambient temperature. Atmos Environ. 2006;40(19):3460–3466. [Google Scholar]
- Park JS, Ikeda K. Variations of formaldehyde and VOC levels during 3 years in new and older homes. Indoor Air. 2006;16(2):129–135. doi: 10.1111/j.1600-0668.2005.00408.x. [DOI] [PubMed] [Google Scholar]
- Parodi S, Vercelli M, Garrone E, Fontana V, Izzotti A. Ozone air pollution and daily mortality in Genoa, Italy between 1993 and 1996. Public Health. 2005;119(9):844–850. doi: 10.1016/j.puhe.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Pitts JN, Jr, Sweetman JA, Zielinska B, Atkinson R, Winer AM, Harger WP. Formation of nitroarenes from the reaction of polycyclic aromatic hydrocarbons with dinitrogen pentoxide. Environ Sci Technol. 1985;19(11):1115–1121. doi: 10.1021/es00141a017. [DOI] [PubMed] [Google Scholar]
- Polidori A, Turpin B, Meng QY, Lee JH, Weisel C, Morandi M, et al. Fine organic particulate matter dominates indoor-generated PM2.5 in RIOPA homes. J Expo Sci Environ Epidemiol. 2006;16(4):321–331. doi: 10.1038/sj.jes.7500476. [DOI] [PubMed] [Google Scholar]
- Pope CA, III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwait E, Ultman J. Airspace surface chemistry mediates O3-induced lung injury. Hum Ecol Risk Assess. 2001;7(5):1145–1159. [Google Scholar]
- Reiss R, Ryan PB, Koutrakis P, Tibbetts SJ. Ozone reactive chemistry on interior latex paint. Environ Sci Technol. 1995a;29(8):1906–1912. doi: 10.1021/es00008a007. [DOI] [PubMed] [Google Scholar]
- Reiss R, Ryan PB, Tibbetts SJ, Koutrakis P. Measurement of organic acids, aldehydes, and ketones in residential environments and their relation to ozone. J Air Waste Manag Assoc. 1995b;45(10):811–822. doi: 10.1080/10473289.1995.10467411. [DOI] [PubMed] [Google Scholar]
- Rigas ML, Catlin SN, Ben-Jebria A, Ultman JS. Ozone uptake in the intact human respiratory tract: relationship between inhaled dose and actual dose. J Appl Physiol. 2000;88(6):2015–2022. doi: 10.1152/jappl.2000.88.6.2015. [DOI] [PubMed] [Google Scholar]
- Rohr AC, Shore SA, Spengler JD. Repeated exposure to isoprene oxidation products causes enhanced respiratory tract effects in multiple murine strains. Inhal Toxicol. 2003a;15(12):1191–1207. doi: 10.1080/08958370390229870. [DOI] [PubMed] [Google Scholar]
- Rohr AC, Weschler CJ, Koutrakis P, Spengler JD. Generation and quantification of ultrafine particles through terpene/ozone reaction in a chamber setting. Aerosol Sci Technol. 2003b;37(1):65–78. [Google Scholar]
- Rohr AC, Wilkins CK, Clausen PA, Hammer M, Nielsen GD, Wolkoff P, et al. Upper airway and pulmonary effects of oxidation products of (+)-alpha-pinene, d-limonene, and isoprene in BALB/c mice. Inhal Toxicol. 2002;14(7):663–684. doi: 10.1080/08958370290084575. [DOI] [PubMed] [Google Scholar]
- Romieu I, Lugo MC, Colome S, Garcia AM, Avila MH, Geyh A, et al. Evaluation of indoor ozone concentration and predictors of indoor-outdoor ratio in Mexico City. J Air Waste Manage Assoc. 1998;48(4):327–335. [Google Scholar]
- Sabersky R, Sinema D, Shair F. Concentrations, decay rates, and removal of ozone and their relation to establishing clean indoor air. Environ Sci Technol. 1973;7(4):347–353. [Google Scholar]
- Sarnat JA, Brown KW, Schwartz J, Coull BA, Koutrakis P. Ambient gas concentrations and personal particulate matter exposures: implications for studying the health effects of particles. Epidemiology. 2005;16(3):385–395. doi: 10.1097/01.ede.0000155505.04775.33. [DOI] [PubMed] [Google Scholar]
- Sarnat JA, Long CM, Koutrakis P, Coull BA, Schwartz J, Suh HH. Using sulfur as a tracer of outdoor fine particulate matter. Environ Sci Technol. 2002;36(24):5305–5314. doi: 10.1021/es025796b. [DOI] [PubMed] [Google Scholar]
- Sarwar G, Corsi R, Allen D, Weschler CJ. The significance of secondary organic aerosol formation and growth in buildings: experimental and computational evidence. Atmos Environ. 2003;37(9–10):1365–1381. [Google Scholar]
- Sarwar G, Corsi R, Kimura Y, Allen D, Weschler CJ. Hydroxyl radicals in indoor environments. Atmos Environ. 2002;36(24):3973–3988. [Google Scholar]
- Sarwar G, Olson DA, Corsi RL, Weschler CJ. Indoor fine particles: the role of terpene emissions from consumer products. J Air Waste Manage. 2004;54(3):367–377. doi: 10.1080/10473289.2004.10470910. [DOI] [PubMed] [Google Scholar]
- Shair FH. Relating indoor pollutant concentrations of ozone and sulfur dioxide to those outside: economic reductions of indoor ozone through selective filtration of the make-up air. ASHRAE Trans. 1981;87(1):116–139. [Google Scholar]
- Shair FH, Heitner KL. Theoretical model for relating indoor pollutant concentrations to those outside. Environ Sci Technol. 1974;8(5):444–451. [Google Scholar]
- Shaughnessy RJ, McDaniels TJ, Weschler CJ. Indoor chemistry: ozone and volatile organic compounds found in tobacco smoke. Environ Sci Technol. 2001;35(13):2758–2764. doi: 10.1021/es001896a. [DOI] [PubMed] [Google Scholar]
- Shields H, Weschler CJ, Naik DV. 1999. Ozone removal by charcoal filters after continuous extensive use (5 to 8 years). In: Indoor Air 99, Vol 4 (Raw G, Aizlewood C, Warren P, eds). London:Construction Research Commiunications, 49–54.
- Shu YH, Kwok ESC, Tuazon EC, Atkinson R, Arey J. Products of the gas-phase reactions of linalool with OH radicals, NO3 radicals, and O3. Environ Sci Technol. 1997;31(3):896–904. [Google Scholar]
- Singer BC, Destaillats H, Hodgson AT, Nazaroff WW. Cleaning products and air fresheners: emissions and resulting concentrations of glycol ethers and terpenoids. Indoor Air. 2006;16(3):179–191. doi: 10.1111/j.1600-0668.2005.00414.x. [DOI] [PubMed] [Google Scholar]
- Skold M, Borje A, Matura M, Karlberg AT. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermatitis. 2002;46(5):267–272. doi: 10.1034/j.1600-0536.2002.460504.x. [DOI] [PubMed] [Google Scholar]
- Stock TH, Kotchmar DJ, Contant CF, Buffler PA, Holguin AH, Gehan BM, et al. The estimation of personal exposures to air pollutants for a community-based study of health effects in asthmatics—design and results of air monitoring. J Air Pollut Control Assoc. 1985;35(12):1266–1273. doi: 10.1080/00022470.1985.10466029. [DOI] [PubMed] [Google Scholar]
- Taucher J, Hansel A, Jordan A, Fall R, Futrell JH, Lindinger W. Detection of isoprene in expired air from human subjects using proton-transfer-reaction mass spectrometry. Rapid Commun Mass Spectrom. 1997;11(11):1230–1234. doi: 10.1002/(SICI)1097-0231(199707)11:11<1230::AID-RCM3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Thornberry T, Abbatt JPD. Heterogeneous reaction of ozone with liquid unsaturated fatty acids: detailed kinetics and gas-phase product studies. Phys Chem Chem Phys. 2004;6(1):84–93. [Google Scholar]
- U.S. Census Bureau 2006. American FactFinder and American Housing Surveys. Available: http://factfinder.census.gov/home/saff/main.html?_lang=en [accessed 22 March 2006].
- U.S. EPA 1997. Exposure Factors Handbook. EPA/600/P-95/002Fa. Washington, DC:U.S. Environmental Protection Agency, Office of Research and Development. Available: http://www.epa.gov/ncea/pdfs/efh/ [accessed 22 March 2006].
- U.S. EPA 2005. Ozone Population Exposure Analysis for Selected Urban Areas. Draft Report. Research Triangle Park, NC:U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards. Available: http://www.epa.gov/ttnnaaqs/standards/ozone/data/O3-exposure-draft-TSD.pdf [accessed 30 May 2006].
- U.S. EPA 2006. Air Quality Criteria for Ozone and Related Photochemical Oxidants (Final). EPA/600/R-05/004aF-cF. Washington, DC:U.S. Environmental Protection Agency.
- Vyskocil A, Viau C, Lamy S. Peroxyacetyl nitrate: review of toxicity. Hum Exp Toxicol. 1998;17(4):212–220. doi: 10.1177/096032719801700403. [DOI] [PubMed] [Google Scholar]
- Wainman T, Zhang J, Weschler CJ, Lioy PJ. Ozone and limonene in indoor air: a source of submicron particle exposure. Environ Health Perspect. 2000;108:1139–1145. doi: 10.1289/ehp.001081139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace L, Williams R, Rea A, Croghan C. Continuous week-long measurements of personal exposures and indoor concentrations of fine particles for 37 health-impaired North Carolina residents for up to four seasons. Atmos Environ. 2006;40(3):399–414. [Google Scholar]
- Wang H, Morrison GC. 2006. Ozone initiated secondary emission rates of aldehydes from indoor surfaces in four homes. Environ Sci Technol 10_1021/es060080s [Online 22 July 2006]. [DOI] [PubMed]
- Weisel C, Zhang J, Turpin B, Morandi M, Colome S, Stock T. et al. 2005. Relationships of Indoor, Outdoor, and Personal Air (RIOPA): Part 1. Collection Methods and Descriptive Analyses. Rpt no. 130, Part 1. Boston:Health Effects Institute. [PubMed]
- Weschler CJ. Ozone in indoor environments: concentration and chemistry. Indoor Air. 2000;10(4):269–288. doi: 10.1034/j.1600-0668.2000.010004269.x. [DOI] [PubMed] [Google Scholar]
- Weschler CJ. New directions: ozone-initiated reaction products indoors may be more harmful than ozone itself. Atmos Environ. 2004;38(33):5715–5716. [Google Scholar]
- Weschler CJ, Brauer M, Koutrakis P. Indoor ozone and nitrogen-dioxide—a potential pathway to the generation of nitrate radicals, dinitrogen pentaoxide, and nitric-acid indoors. Environ Sci Technol. 1992a;26(1):179–184. [Google Scholar]
- Weschler CJ, Hodgson AT, Wooley JD. Indoor chemistry —ozone, volatile organic-compounds, and carpets. Environ Sci Technol. 1992b;26(12):2371–2377. [Google Scholar]
- Weschler CJ, Shields HC. Production of the hydroxyl radical in indoor air. Environ Sci Technol. 1996;30(11):3250–3258. [Google Scholar]
- Weschler CJ, Shields HC. Potential reactions among indoor pollutants. Atmos Environ. 1997;31(21):3487–3495. [Google Scholar]
- Weschler CJ, Shields HC. Indoor ozone/terpene reactions as a source of indoor particles. Atmos Environ. 1999;33(15):2301–2312. [Google Scholar]
- Weschler CJ, Shields HC. The influence of ventilation on reactions among indoor pollutants: modeling and experimental observations. Indoor Air. 2000;10(2):92–100. doi: 10.1034/j.1600-0668.2000.010002092.x. [DOI] [PubMed] [Google Scholar]
- Weschler CJ, Shields HC, Naik DV. Indoor ozone exposures. J Air Pollut Control Assoc. 1989;39(12):1562–1568. doi: 10.1080/08940630.1989.10466650. [DOI] [PubMed] [Google Scholar]
- Wilkins CK, Clausen PA, Wolkoff P, Larsen ST, Hammer M, Larsen K, et al. Formation of strong airway irritants in mixtures of isoprene/ozone and isoprene/ozone/nitrogen dioxide. Environ Health Perspect. 2001;109:937–941. doi: 10.1289/ehp.01109937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisthaler A, Tamas G, Wyon DP, Strom-Tejsen P, Space D, Beauchamp J, et al. Products of ozone-initiated chemistry in a simulated aircraft environment. Environ Sci Technol. 2005;39(13):4823–4832. doi: 10.1021/es047992j. [DOI] [PubMed] [Google Scholar]
- Wolkoff P. Volatile organic compounds—sources, measurements, emissions, and the impact on indoor air quality. Indoor Air. 1995;5(suppl 3):9–73. [Google Scholar]
- Wolkoff P. Photocopiers and indoor air pollution. Atmos Environ. 1999;33:2129–2130. [Google Scholar]
- Wolkoff P, Clausen PA, Wilkins CK, Hougaard KS, Nielsen GD. Formation of strong airway irritants in a model mixture of (+)-alpha-pinene/ozone. Atmos Environ. 1999;33(5):693–698. [Google Scholar]
- Wolkoff P, Clausen PA, Wilkins CK, Nielsen GD. Formation of strong airway irritants in terpene/ozone mixtures. Indoor Air. 2000;10(2):82–91. doi: 10.1034/j.1600-0668.2000.010002082.x. [DOI] [PubMed] [Google Scholar]
- Wolkoff P, Schneider T, Kildeso J, Degerth R, Jaroszewski M, Schunk H. Risk in cleaning: chemical and physical exposure. Sci Total Environ. 1998;215(1–2):135–156. doi: 10.1016/s0048-9697(98)00110-7. [DOI] [PubMed] [Google Scholar]
- Wolkoff P, Wilkins CK, Clausen PA, Larsen K. Comparison of volatile organic compounds from processed paper and toners from office copiers and printers: methods, emission rates and modeled concentrations. Indoor Air. 1993;3:113–123. [Google Scholar]
- Wong CM, Ma S, Hedley AJ, Lam TH. Effect of air pollution on daily mortality in Hong Kong. Environ Health Perspect. 2001;109:335–340. doi: 10.1289/ehp.01109335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lioy PJ. Ozone in residential air—concentrations, I/O ratios, indoor chemistry, and exposures. Indoor Air. 1994;4(2):95–105. [Google Scholar]
- Zhang J, Wilson WE, Lioy PJ. Sources of organic acids in indoor air: a field study. J Expo Anal Environ Epidemiol. 1994;4:25–47. [PubMed] [Google Scholar]
- Zhang Y, Huang W, London S, Song G, Chen G, Jiang L, et al. Ozone and daily mortality in Shanghai, China. Environ Health Perspect. 114:1227–1232. doi: 10.1289/ehp.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann PJ. Formation of alkoxyhydroperoxy aldehydes and cyclic peroxyhemiacetals from reactions of cyclic alkenes with O3 in the presence of alcohols. J Phys Chem A. 2003;107(12):2048–2060. [Google Scholar]