Abstract
We have pursued an interdisciplinary research program to develop novel behavioral assessment tools for evaluating specific memory impairments following damage to the medial temporal lobe, including the hippocampus and associated structures that show pathology early in the course of Alzheimer’s disease (AD). Our approach uses computational models to identify the functional consequences of hippocampal-region damage, leading to testable predictions in both rodents and humans. Our modeling argues that hippocampal-region dysfunction may selectively impair the ability to generalize when familiar information is presented in novel recombinations. Previous research has shown that specific reductions in hippocampal volume in non-demented elderly individuals correlate with future development of AD. In two previous studies, we tested non-demented elderly with and without mild hippocampal atrophy (HA) on stimulus-response learning tasks. Individuals with and without HA could learn the initial information, but the HA group was selectively impaired on transfer tests where familiar features and objects were recombined. This suggests that such generalization deficits may be behavioral markers of HA, and an early indicator of risk for subsequent cognitive decline. Converging support for the relevance of these tasks to aging and Alzheimer’s disease comes from our recent fMRI studies of individuals with mild cognitive impairment (MCI). Activity in the hippocampus declines with progressive training on these tasks, suggesting that the hippocampus is important for learning new stimulus representations that support subsequent transfer. Individuals with HA may be able to learn, but in a more hippocampal-independent fashion that does not support later transfer. Ultimately, this line of research could lead to a novel battery of behavioral tests sensitive to very mild hippocampal atrophy and risk for decline to AD, allowing early diagnosis and also allowing researchers to test new Alzheimer’s drugs that target individuals in the earliest stages of the disease – before significant cognitive decline. A new mouse version of one of our tasks shows promise for translating these paradigms into rodents, allowing for future studies of therapeutic interventions in transgenic mouse models of AD.
1. INTRODUCTION
The hippocampal region, including hippocampus, entorhinal cortex, and nearby brain structures, shows pathology very early in the course of Alzheimer’s disease (Fig. 1). Atrophy of these areas is often visible on structural neuroimaging before the onset of behavioral symptoms associated with the disease [1–4] suggesting that hippocampal-region atrophy may represent the prodromal stage of the disease. In fact, for older adults with mild cognitive impairment, HA can predict whether an individual is at short-term risk of decline to dementia [5]. For this reason, behavioral tasks that are sensitive to very mild degrees of hippocampal atrophy could provide a useful early screening tool – and would be relatively simple, quick, and inexpensive to administer. Such early prediction of AD is critical, given that all existing pharmacological interventions for AD work to slow advancement of the disease, rather than reversing or stopping its progress.
Fig. (1).
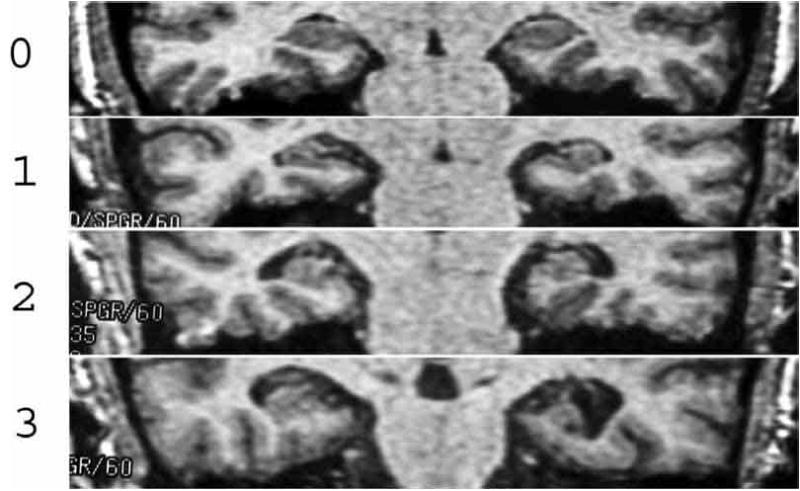
Coronal images of the human brain through the hippocampus in four individuals with increasing degrees of hippocampal atrophy (HA): 0=no atrophy, 1=questionable or mild HA, 2=mild-to-moderate HA, 3=moderate-to-severe HA. Reprinted from [6] Myers et al. (2003) Figure 3.
If hippocampal atrophy is indeed a predictor of AD risk, then it ought to be possible to estimate hippocampal atrophy by behavior alone: if an individual starts to perform poorly on hippocampal-dependent tests, then HA may be occurring and (if this is verified by neuroimaging) aggressive intervention could be considered to prevent or delay onset of AD. Several previous studies with cognitively-normal older adults have shown that HA correlates significantly with performance on memory tests [7–9], although other studies have found that hippocampal volume does not correlate strongly with memory performance [10,11].
One difficulty in trying to assess hippocampal function through general memory tasks (in either animals or humans) is that most memory tasks involve many different brain regions working together, and often allow for multiple alternative methods of solution. If one brain region is disabled, very often other brain regions can compensate for this loss by solving memory problems in a different way than usual. This ability to use multiple alternative strategies (which rely on different brain regions) often allows a person or animal to compensate for hippocampal-region dysfunction. Thus, what is especially important for the study of the hippocampus and assessments of hippocampal function in memory, are tasks or behaviors that are selectively dependent on the hippocampus. To develop such tasks, it is first necessary to determine specifically what role the hippocampus plays in memory.
What Does the Hippocampus Do?
A long history of research in humans and animals with hippocampal-region damage has shown that the hippocampal region is critical for new memory formation e.g. [12]. Humans with hippocampal-region damage are greatly impaired at the ability to form new memories for specific spatio-temporal events (episodic memory) and for general events (semantic memory). These types of memory, often called declarative memory, are characterized by learning after one or a few exposures; such memories are also generally easy to access in forms different from how they were originally acquired [13]. On the other hand, humans with hippocampal-region damage are often spared in their ability to learn new stimulus-response associations and new cognitive and motor skills [14,15]. Given this pattern, it would be expected that recall of new fact or episodic information would be particularly impaired in patients with HA and/or early-stage AD. In fact, there is good evidence that individuals with HA are impaired at tests of declarative memory, such as delayed recall of paragraphs [10,16]. In one study, HA was shown to predict longitudinal decline on tests of delayed paragraph recall [16]. Paragraph delayed recall tasks are also particularly sensitive to hippocampal region damage resulting from other etiologies [17].
Few scientists or clinicians would argue with the strong evidence linking acquisition of new declarative memories to the hippocampal region. A common mistake, however, is to assume that just because the hippocampal region is essential for acquiring new declarative memories, that this is the only function of the hippocampal region. Research over the last decade has demonstrated that the hippocampal region in animals and humans also plays a key role in modulating incremental learning of stimulus-response associations, particularly where such learning requires encoding information about the environmental context or about stimulus-stimulus regularities [18–20]. In other words, although the hippocampal region is critical for learning new declarative information, it is now clear that the hippocampus is involved in many kinds of non-declarative learning too.
Theoretical work by our lab and others has suggested that the hippocampal region is important during initial acquisition of a stimulus-response association to help set up representations of information that allow for subsequent generalization (i.e., transfer) when task demands or contexts change, or when familiar information is presented in new recombinations. Without this hippocampal-mediated flexibility, simple linking of stimulus to response can occur, but such learning will be “hyperspecific” or “inflexible” and will not generalize well [19–21].
To explain our computational models at an intuitive level, it is helpful to draw on a visual metaphor. Recall a famous New Yorker cover, created by Saul Steinberg, that caricatured a typical New Yorker’s mental map of the world. Ninth and Tenth Avenues were drawn in such fine detail that they took up half the map; the remaining cover space was taken up by other areas of New York City. The rest of the country, the area between New Jersey and California, was represented as a small area marked only by a farm silo and a few scattered rocks. This cover painting satirized many New Yorkers’ belief that they are living in the most important place in the world. But the painting also illustrated an important psychological point. Fine distinctions that are meaningful to someone who lives in New York, such as the differences between fashionable street addresses, are emphasized and enlarged in this map. To make room for this expansion, faraway Midwestern States are de-emphasized or compressed and given less space in the map.
To some extent, we all create similar idiosyncratic worldviews with distorted representations; distinctions important to us are enhanced while less relevant ones are deemphasized. Computational modeling in our lab over the last decade has shown how the hippocampal region supports the storage of new memories by emphasizing only those aspects of our experience that are most useful to us. In these computational models, the hippocampus and related brain structures monitor all the information passing through all the senses and use this input to build up an “internal model” of the world (like Steinberg’s map of New York) that is biased to emphasize relevant relationships. Other brain regions that are not damaged in early Alzheimer’s disease may be the final resting place for long-term memory; these other regions act like “clients” of the hippocampus in that they use information from the hippocampus to decide how to encode new memories. Thus, when the hippocampus is damaged in early stages of Alzheimer’s disease, these other brain regions, which are not themselves directly damaged by the disease, are unable to store new hippocampal-dependent information.
This theory has been implemented as a connectionist (neural network) model, and correctly accounts for a range of data in intact and hippocampal-lesioned animals from the domains of classical conditioning [18,22] and instrumental forced-choice discrimination [23], as well as from human category learning [24]. Later elaborations included a role for the neurotransmitter acetylcholine [25–27], suggesting that it operates in a self-regulating feedback loop with the hippocampus, modulating hippocampal dynamics between storage of new (incoming) information and recall of existing (previously-stored) information. AD pathology is, of course, characterized by death of acetylcholine-producing neurons in the basal forebrain, and most existing AD medications are cholinesterase inhibitors, which work to increase brain acetylcholine levels. Thus, this computational modeling approach gives us a starting point to develop behavioral tasks that tap directly into hippocampal-region function and that should be disrupted by HA in prodromal AD. Another advantage of considering stimulus-response learning paradigms, rather than declarative memory tests, is the potential for animal models such as transgenic rodents. Rodents, of course, are non-verbal, but they can easily learn stimulus-response associations, allowing direct cross-species comparison of hippocampal-region function.
2. STRUCTURAL IMAGING IN NON-DEMENTED ELDERLY
The Gluck and Myers computational model of cortico-hippocampal function has led to two published reports of novel behavioral tasks that are more diagnostic than standard neuropsychological tests of mild hippocampal atrophy in non-demented elderly. Each of these tasks takes about 20 minutes, runs automatically on a standard laptop computer, and is relatively fun and engaging (similar to a short video game). Both are related to hippocampal-dependent tasks in rodents.
Concurrent Discrimination with Feature-Irrelevant Transfer
A large literature on animal models has suggested that particular classes of memory task are especially sensitive to hippocampal-region damage. One theme that unifies many of the hippocampal-sensitive tasks is the tradeoff between generalization and specificity. For example, in a study by Howard Eichebaum and colleagues, normal rats trained on a series of odor discriminations ( e.g., A+B−, X+Y−, etc.) would transfer well to novel recombinations of familiar odors (e.g. A+Y−, X+B−) [18]. However, animals with hippocampal-region dysfunction subsequent to fornix transection performed at chance on these novel recombinations. This effect has been interpreted as indicating that hippocampal-lesioned animals overcompress odors: perceiving an AB compound rather than its component odors A and B; presentation of AY therefore represents a novel compound rather than a recombination of familiar components [28]. Humans with hippocampal-region damage are also often characterized as displaying a similar “hyperspecificity”: they are able to retrieve studied information when study and test conditions are identical, but not when test conditions are varied [21, 29]. We therefore hypothesized that individuals with HA might show a similar impairment when challenged to respond to familiar cues in novel recombinations, and that this impairment might be evident before the appearance of more generalized cognitive and memory deficits.
We have designed a concurrent visual discrimination task in which subjects see a pair of objects on each trial, and are asked to learn to choose the correct object from each pair (Fig. 2A). The chosen object is raised and, if the choice was correct, a smiley face icon is revealed underneath. Within each pair, the objects differ in color or shape but not both. Thus, one pair might involve learning to choose a brown mushroom over a brown frame; another pair might involve learning to choose a red cat’s-eye over a yellow cat’s-eye. In the first example, shape is relevant (mushroom vs. frame) but the color is redundant and therefore irrelevant with respect to predicting the location of the smiley face. In the second example, color is relevant (red vs. yellow) but shape is redundant and therefore irrelevant. Training continues until subjects are responding correctly to eight such object pairs, half differing in color and half differing in shape.
Fig. (2).

Concurrent visual discrimination task. (A) On each trial of the initial learning phase, the subject sees a pair of objects on the computer screen (top) and is asked to choose the left or right object. The chosen object is raised and, if the subject’s choice was correct (center) a smiley face is revealed underneath; otherwise (bottom) there is no smiley face. (B) In the transfer phase, the irrelevant feature in each object pair is altered so, in this example, mushroom still beats frame, but the (irrelevant) color has changed. Reprinted from [30] Myers et al. (2002) Figure 2.
There are at least two ways to master this task. The first is to learn simple stimulus-response mappings to each object (so that brown-mushroom beats brown-frame, and so on). The second is to modify stimulus representations to emphasize relevant information and de-emphasize irrelevant information (mushroom beats frame, regardless of color). Note that either strategy is perfectly adequate for solving the task. Our computational model expects that this latter strategy requires processing by the hippocampal-region, so that subjects with hippocampal-region damage will be forced to rely on the simpler object-mapping strategy.
This learning phase is followed by a transfer phase, in which the irrelevant features are changed but the relevant features remained the same (Fig. 2B). For example, the brown frame vs. brown mushroom discrimination might change to green mushroom vs. green frame, with color remaining irrelevant but shape remaining predictive. Now, the strategy used during initial learning becomes critical. Individuals who learned based on relevant features only (mushroom beats frame, regardless of color) should continue to perform very well in the transfer phase. This result is what we expect to see in healthy controls. By contrast, individuals who had learned based on entire objects, treating all features equally, are effectively confronted with novel objects in the transfer phase, and might perform near chance.
We administered this task to a group of 34 non-demented individuals, aged 45–80 years. All were medically healthy, with no clinical or radiographic evidence for structural or metabolic brain abnormalities, no history of alcoholism or psychiatric disorders (including depression) and no medications that might affect cognition. All were also given a structural MRI as part of an ongoing workup, and scans were rated for presence or absence of HA using the four-point scale illustrated in Fig. 1. Of our 34 participants, 18 had at least mild HA (HA>0) and 16 had no visible HA. Consistent with our hypothesis, individuals with and without HA learned the initial discriminations at the same speed (Fig. 3A). However, on transfer, individuals with hippocampal atrophy (HA) made significantly more errors than non-atrophied controls -- and, in fact, their performance on the transfer task was not significantly different than their performance on the initial learning, suggesting that, without hippocampal-region mediation on initial learning, little or no information transfers when task demands change slightly (Fig. 3B).
Fig. (3).
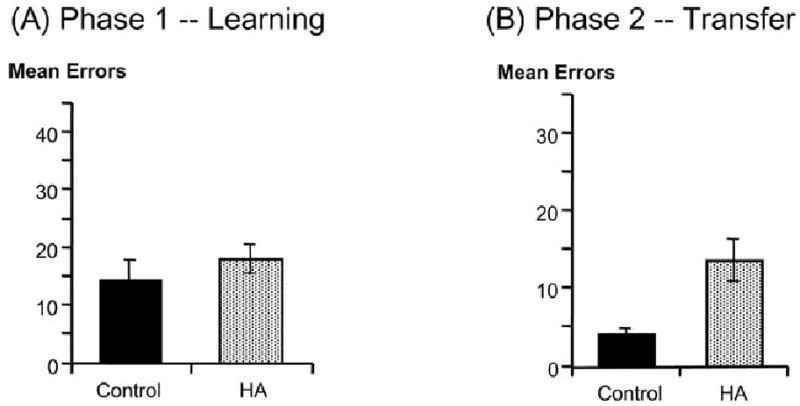
(A) Non-demented elderly individuals with and without HA learned a series of concurrent visual discriminations at the same speed; (B) On the transfer phase, however, the HA group made significantly more errors. Reprinted from [6] Myers et al., 2002, Figure 3A, B.
Importantly, transfer test performance appeared to distinguish between individuals with and without hippocampal atrophy, even though performance on other memory measures, such as the delayed paragraph recall test, did not. This may be because of the relatively mild atrophy in our HA group (only two of our 18 HA subjects had atrophy ratings of 2 or greater bilaterally). This finding suggests that tasks designed to recruit hippocampal-region performance may be especially sensitive to mild hippocampal atrophy, and thus may have some utility as behavioral markers in individuals at risk for future cognitive decline and Alzheimer’s.
Acquired Equivalence
The concurrent discrimination task described above examines subjects’ ability to generalize when the stimulus features are altered. Another aspect of hippocampal-dependent learning in our computational model is the ability to generalize across stimuli that are superficially different but that have the same meaning.
For example, acquired equivalence is a paradigm in which two stimuli having similar histories of association with reinforcement tend to be treated as equivalent. For example, animals may first be trained that stimulus A predicts food and stimulus B also predicts food; if this is followed by training that A now predicts shock, animals tend to generalize and assume that B now also predicts shock e.g. [31,32]. This suggests that the animals have learned an equivalence relationship between the two stimuli. Similar effects have also been obtained in human children [33].
Acquired equivalence involves recognizing that superficially dissimilar stimuli have the same meaning, and so learning about one should generalize to the other. This is one of the functions that our computational model has proposed should depend on hippocampal-region mediation [20]. Accordingly, the model expects that acquired equivalence should be disrupted by hippocampal-region damage: the initial learning should be relatively unimpaired, but transfer should be impaired.
To test this prediction, we have developed a computer-based task based on the logic of an acquired equivalence task. On each trial, subjects see a face and two colored fish, and must learn to choose the fish associated with that face (Fig. 4). Left-right positions of the fish vary from trial to trial. In phase 1, the subject learns four face-fish associations, as shown in Table 1. Faces A and B are each associated with the same fish (X), and so subjects should learn an equivalence relationship between A and B; similarly, C and D are associated with Y, and subjects should learn an equivalence relationship between C and D.
Fig. (4).
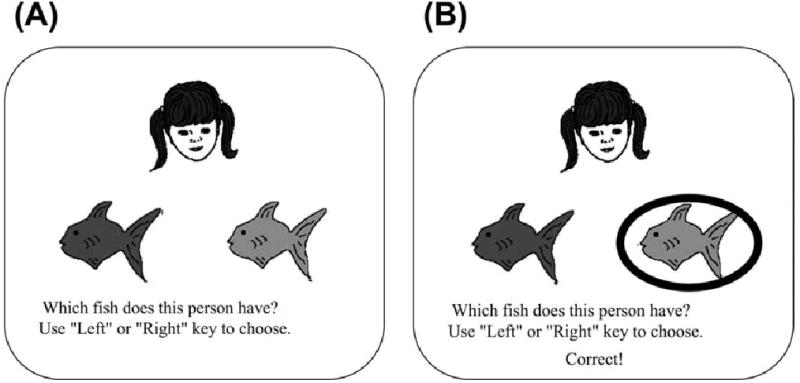
Example screen events during one trial of the acquired equivalence task. (A) On each trial, the subject sees one face and two colored fish. (B) The participant responds by pressing a key to choose the left or right fish, the chosen fish is circled, and feedback is given telling the participant whether this choice was correct or incorrect. Reprinted from [6] Myers et al. (2003) Figure 4.
Table 1.
The Acquired Equivalence Paradigm
| Phase 1: Initial Learning | Phase 2: New Associations Added | Phase 3: Transfer |
|---|---|---|
| Face A -> Choose X over Y | Face A -> Choose W over Z | |
| Face B -> Choose X over Y | Face B -> Choose W or Z? | |
| Face C -> Choose Y over X | Face C -> Choose Z over W | |
| Face D -> Choose Y over X | Face D -> Choose W or Z? |
Next, phase 2 begins without warning to the subject. Continued trials with the learned pairs are interleaved with new discriminations associating faces A and C with new fish W and Z, as shown in Table 1. Finally, phase 3 is a test of learning and transfer; subjects are tested on all six previously-learned associations, as well as the two new discriminations shown in Table 1. No feedback is given in this phase. If subjects had previously learned equivalence relationships in phase 1, then learning in phase 2 should generalize to phase 3: subjects should reliably associate face B with fish W and face D with fish Z. If no equivalence relationship was learned, performance on the new associations should be at chance levels.
We administered this task to a group of non-demented elderly subjects, again divided according to presence or absence of hippocampal atrophy based on structural MRI [6]. As in the prior task, there was no significant difference between groups on learning the initial associations in phases 1 and 2 (Fig. 5A). In phase 3, both groups continued to perform well on the old, previously learned associations. The non-atrophied subjects also tended to transfer their knowledge to new pairs -- associating face B with fish W and face D with fish Z, suggesting that they had formed an acquired equivalence between stimuli associated with common outcomes in phase 1 (Fig. 5B). In contrast, the HA group showed near-chance responding to faces B and D in phase 3, suggesting no acquired equivalence had been learned.
Fig. (5).

(A) Non-demented elderly individuals with and without HA learned at the same speed during the learning phases of our acquired equivalence task. (B) In the testing phase, both groups continued to perform well on previously-learned (old) discriminations. However, the HA group was significantly worse at transferring to novel (new) pairs, indicating they had failed to learn the acquired equivalence during the earlier phases. Reprinted from [6] Myers et al., 2003,
These results are consistent with those from our prior concurrent discrimination learning task. In both cases, it appears that performance on a transfer task, when familiar stimuli are presented with novel features or in novel combinations, is a sensitive measure of hippocampal-region function and of hippocampal atrophy.
4. FUTURE DIRECTIONS
To the extent that hippocampal atrophy predicts risk for future decline to AD, this raises the possibility that such behavioral tasks may be sensitive indicators of AD risk, before deficits begin to show up in “standard” neuropsychological tests of memory or in daily activities. We are currently pursuing longitudinal studies to verify this idea, and to determine whether the same individuals who perform poorly on our transfer tasks are indeed more likely to decline to AD within a few years, compared with individuals who transferred well. Preliminary data from these longitudinal studies is encouraging and a full report will follow in the near future.
Two other directions for current research are, first, the use of functional imaging, and second, the development of mouse versions of these task to facilitate translational research between animal and human studies and the assessment of novel therapeutics in transgenic mouse models of Alzheimer’s disease. These are briefly summarized below.
Functional Imaging in the Mild Cognitively Impaired
The above interpretations of our behavioral tasks assume that HA individuals’ failure to transfer reflect a qualitative difference in initial learning. Specifically, in the presence of hippocampal-region mediation, learning normally includes extra information about context and stimulus regularities, and this extra information supports subsequent transfer. HA impairs this extra learning; HA individuals can still learn the stimulus-response associations needed to master the initial discriminations, but cannot apply this learning flexibly when challenged with a transfer test involving familiar stimuli presented in new ways or new combinations.
One way to test this assumption is by functional neuroimaging (fMRI) of individuals learning these tasks. FMRI is a non-invasive imaging method useful for detecting hemodynamically coupled neurocognitive brain activity during specific cognitive tasks. FMRI offers a method of examining memory-associated brain regions while those regions are functionally engaged in memory tasks. On our tasks, we would expect that individuals who show hippocampal-region activation during initial learning would be more likely to transfer well later, while those individuals who show little hippocampal-region activation during learning might show impaired transfer later.
In initial studies, conducted at the University of Wisconsin Medical School under the direction of Sterling Johnson, we have adapted the concurrent discrimination task shown in (Fig. 2) for event-related fMRI. During the learning phase, subjects were presented with each pair six times over the course of an 8-minute scan. In addition to the object pairs, a control condition was presented in which subjects saw two gray squares side by side; one of the squares was clearly marked as the correct choice, and the subject’s task was merely to choose that square. Feedback was provided for both the object and control trials. Subjects were provided with instructions and practice prior to scanning.
We modeled the cerebral response to the task in two ways. First we examined the main effect of response selection. This produces a robust dorsolateral frontal lobe response in connection with choosing a response among the two response alternatives (irrespective of accuracy or number of repetitions). Next we examined each subject’s time series of images for change in signal associated with increasing response accuracy. In some subjects, but not all, we have seen that the signal in the hippocampus attenuates with increasing response accuracy indicating that the hippocampus is recruited initially during learning, but as the correct response is acquired, the hippocampus becomes progressively less active. A single healthy elderly subject demonstrating this pattern is shown in Fig. 6. These results are consistent with our assumption that, in healthy individuals, the hippocampal region is recruited during the learning phase of this task.
Fig. (6).
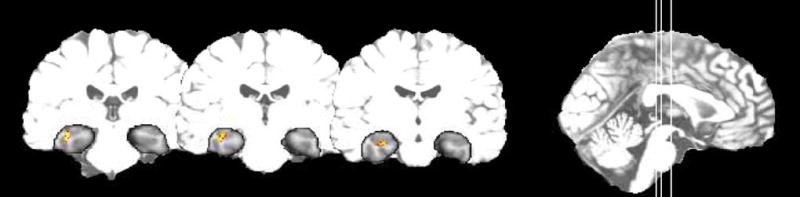
A montage across three slices of the SPM atlas depicting activation from a single 75 year old healthy female subject. The subject exhibited a learning-related adaptation response in the hippocampus. A region of interest analysis was used that focused on the hypothesized location of the mesial temporal lobe (outlined in the Figure).
The next question is whether this pattern of hippocampal-region involvement differs in individuals at risk for Alzheimer’s disease. To investigate this question, we are conducting fMRI studies with our concurrent discrimination task in elderly individuals with amnestic mild cognitive impairment (MCI). Amnestic MCI is a condition involving memory impairment beyond typical age-related declines, and unaccounted for by other medical conditions, and it is a major risk factor for development of AD [34]. F-18 fluoro-deoxy-glucose (FDG) positron emission tomography (PET) studies show reduced cerebral metabolic rate of glucose (CMRgl) in many of the brain areas affected by AD [35], and hippocampal metabolism has been found to correlate with the performance on an encoding task [36].
While CMRgl and structural MRI studies have shown some sensitivity to MCI, the use of fMRI has not been widely applied to this disorder. A small body of functional imaging studies suggest that the hippocampus is responsive to new information in controls more than MCI or early AD [37–40]. Not all studies agree [41,42], but this discrepancy may be due to the fact that MCI subjects in those studies were not required to have deficits in memory function on neuropsychological tests, only memory complaints. Some fMRI studies of mild AD and persons at genetic risk for AD have found greater activation associated with disease presence or risk, perhaps reflecting a compensatory response [43–45]. Further study is needed to resolve the discordant findings.
These prior reports have all more or less relied on novelty detection paradigms that echo the putative role of the hippocampus in forming new memories from previously un-encountered episodic events or stimuli. As reviewed above, models of hippocampal function that incorporate incremental associative learning may provide a fruitful approach to studying hippocampal-region dysfunction in people with MCI, with the goal of identifying those most at risk for conversion to AD.
We have conducted some preliminary fMRI studies in eight volunteers with MCI (mean age 74) and thirteen elderly controls (mean age 73, SD 9). As expected, and per criteria, the MCI subjects exhibited neuropsychological deficits on the encoding trials and delayed recall trials of declarative memory tests including the Rey Auditory Verbal Learning Test and Brief Visuospatial Memory Test-Revised. Other neuropsychological domains were relatively less impaired. The fMRI results for each group were in the direction of our hypotheses, as shown in Fig. 7. The controls on average exhibited attenuation in hippocampal signal over repeated trials associated with performance accuracy, while the MCI subjects did not. Both groups exhibited attenuation in the anterior cingulate over the course of the experiment.
Fig. (7).
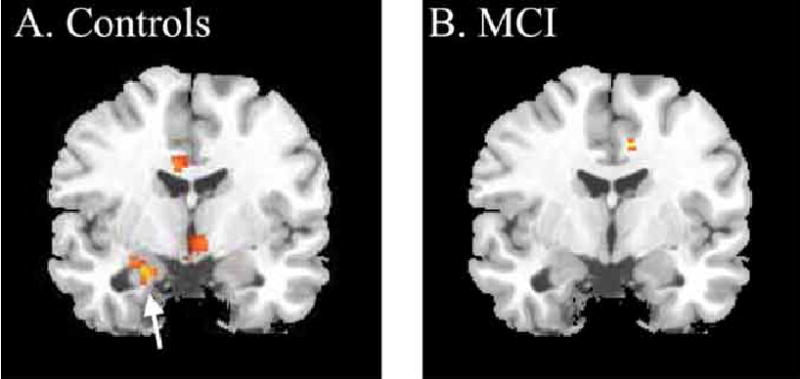
Statistical parametric maps of adaptation during the Choose task in 13 controls and 8 MCI. The activations can be interpreted as regions where the negative slope of change over repeated trials is significantly different from zero. (A) The average slope of adaption in the controls is significant in the anterior medial temporal lobe at the point where the hippocampus and amygdala conjoin (t=4.38, p<.001; x,y,z -22, -4, -22). (B). The MCI patients do not exhibit any significant change over trials in the MTL. The group statistics in A and B are superimposed on the same standard atlas brain at the same slice location. The left side of the brain is on the left side of the image.
These data demonstrate the feasibility of adapting an associative learning task for MCI in the fMRI environment. Future experiments are planned to determine whether the fMRI of associative learning predicts subsequent conversion to AD and whether this adds additional new information beyond behavioral evaluation and existing structural imaging methods.
Translation to Mouse Paradigms
A key value to our associative learning tasks is that –unlike tests of hippocampal-dependent declarative memory (e.g., delayed paragraph recall) – they can be naturally translated into rodent paradigms. In collaboration with Michelle Nicolle at Wake Forest University, we have developed a mouse version of our learning and generalization task (based on the concurrent discrimination task of Fig. 2). The long-term goal is to develop a quick, largely-automated task that can be used for inexpensive, high-throughput drug screening with transgenic mice. This project has recently begun and will proceed as follows. On the human version of the task, subjects begin by learning concurrent discriminations involving pairs of objects that vary in shape and color, with color or shape relevant, and then transfer to new pairs where the relevant features remain the same but the irrelevant features are novel. In the mouse version of this task, we present the animal with discrimination pairs consisting of digging pots that each contain two stimuli, an odor and a digging medium (Fig. 8A). In each pair of pots, either the odor or the medium differs, but not both. Thus, for example, a pair with odor as the relevant stimulus might contain mint-scented sawdust vs. lemon-scented sawdust, while another pair with medium as the relevant stimulus might contain cinnamon-scented confetti vs. cinnamon-scented sand (Fig. 8B). One of the pots is seeded with a chocolate reward at the bottom, and the animal’s task is to learn to dig in the correct pot to obtain the reward. To control for the odor of the food, there is chocolate pellet “dust” distributed in every pot.
Fig. (8).
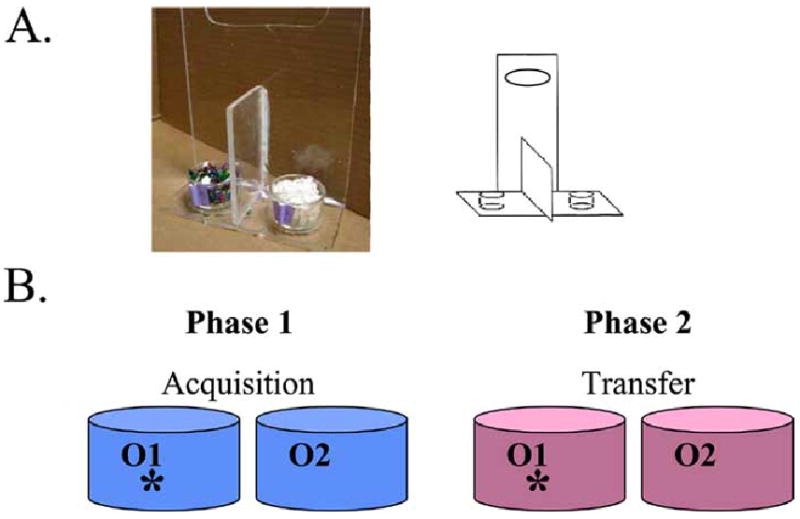
(A) Mouse testing apparatus. (B) Schematic of design of rodent analog of the concurrent discrimination and transfer task.
Our preliminary data from male C57B6 wild-type mice indicates that healthy mice can learn these discriminations easily. The learning phase is followed by a transfer phase analogous to that in the human task: the irrelevant features are changed, but the relevant rules remain the same (so, mint still beats lemon regardless of medium, and confetti still beats sand regardless of odor). Just like the non-atrophied humans, healthy wild-type mice can transfer with relatively few errors, as shown in pilot data in Fig. 9.
Fig. (9).
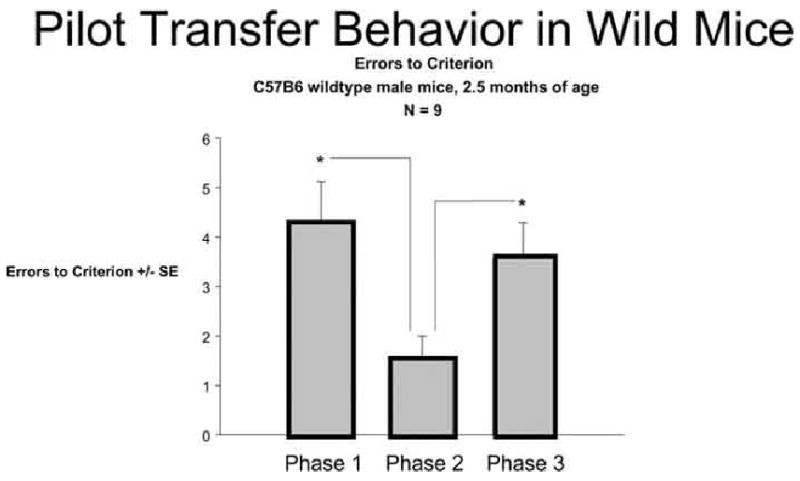
Pilot data from 9 wild-type mice showing enhanced transfer to phase 2 from phase 1 (fewer trials to criterion) when the relevant rule remains the same. A third phase with a novel cue pairing is included as a control for non-specific changes in performance.
The next question is whether we will see the same pattern of spared initial learning but impaired transfer in mouse AD models as we do in humans with HA. The transgenic mouse models of AD will be particularly useful in determining relevance of specific neurobiological and/or pathological changes that may underlie hippocampal-dependent memory dysfunction in AD. If this rodent task is successful, it will be useful in evaluating the effects of new AD drugs on the prevention of AD-related cognitive impairment. New pharmacological agents can be administered to transgenic mice, and their effects on hippocampal-dependent memory assessed by performance on the transfer test. The advantage of using this transfer test over existing rodent paradigms is that it parallels the human task, making direct cross-species comparisons possible and, it would be hoped, speeding evaluation and delivery of new therapeutic agents for Alzheimer’s disease.
CONCLUSIONS
An interdisciplinary research program has lead to the development of novel behavioral assessment tools for evaluating specific memory impairments following damage to the medial temporal lobe, including the hippocampus and associated structures that show pathology early in the course of Alzheimer’s disease (AD). Our approach uses computational models to identify the functional consequences of hippocampal-region damage, leading to testable predictions in both rodents and humans. Our modeling argues that hippocampal-region dysfunction may selectively impair the ability to generalize when familiar information is presented in novel recombinations. In two previous studies, we have shown that generalization deficits may be behavioral markers of HA, and an early indicator of risk for subsequent cognitive decline. Converging support for the relevance of these tasks to aging and Alzheimer’s disease comes from our recent fMRI studies of individuals with mild cognitive impairment (MCI). Ultimately, this line of research could lead to a novel battery of behavioral tests sensitive to very mild hippocampal atrophy and risk for decline to AD, allowing early diagnosis and also allowing researchers to test new Alzheimer’s drugs that target individuals in the earliest stages of the disease – before significant cognitive decline. A new mouse version of one of our tasks shows promise for translating these paradigms into rodents, allowing for future studies of therapeutic interventions in transgenic mouse models of AD.
References
- 1.de Leon M, Golomb J, George A, et al. Hippocampal formation atrophy: Prognostic significance for Alzheimer’s Disease. In: Corain B, Iqbal K, Nicolini M, et al., editors. Alzheimer’s Disease: Advances in Clinical and Brain Research’. New York: John Wiley and Sons; 1993. pp. 35–46. [Google Scholar]
- 2.de Leon M, George A, Golomb J, Tarshish C, Convit A, Kluger A, et al. Frequency of hippocampal formation atrophy in normal aging and Alzheimer’s disease. Neurobiol Aging. 1997;18:1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- 3.de Toledo-Morrell L, Goncharova I, Dickerson B, Wilson R, Bennett D. From healthy aging to early Alzheimer’s disease: In vivo detection of entorhinal cortex atrophy. Ann N Y Acad Sci. 2000;911:240–253. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- 4.Grundman M, Sencakova D, Jack C, et al. Brain MRI hippocampal volume and prediction of clinical status in a mild cognitive impairment trial. J Mol Neurosci. 2002;19(1–2):23–28. doi: 10.1007/s12031-002-0006-6. [DOI] [PubMed] [Google Scholar]
- 5.de Leon M, George A, Stylopoulos L, et al. Early marker for Alzheimer’s disease: The atrophic hippocampus. Lancet. 1989:672–673. doi: 10.1016/s0140-6736(89)90911-2. [DOI] [PubMed] [Google Scholar]
- 6.Myers C, Shohamy D, Gluck M, et al. Dissociating hippocampal versus basal ganglia contributions to learning and transfer. J Cog Neurosci. 2003;15(2):185–193. doi: 10.1162/089892903321208123. [DOI] [PubMed] [Google Scholar]
- 7.Golomb J, de Leon M, Kluger A, et al. Hippocampal atrophy in normal aging: An association with recent memory impairment. Arch Neurol. 1993;50:967–973. doi: 10.1001/archneur.1993.00540090066012. [DOI] [PubMed] [Google Scholar]
- 8.Golomb J, Kluger A, de Leon M, et al. Hippocampal formation size in normal human aging: A correlate of delayed secondary memory performance. Learning and Memory. 1994;1:45–54. [PubMed] [Google Scholar]
- 9.Raz N, Gunning-Dixon F, Head D, et al. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- 10.Soininen H, et al. Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment. Neurology. 1994;44:1660–1668. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan E, et al. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiol Aging. 1995;16(4):591–606. doi: 10.1016/0197-4580(95)00074-o. [DOI] [PubMed] [Google Scholar]
- 12.Squire LR. Memory and Brain. New York: Oxford University Press; 1987. [Google Scholar]
- 13.Squire L, Knowlton B. Memory, hippocampus, and brain systems. In: Gazzaniga M, editor. The Cognitive Neurosciences’. Cambridge, MA: MIT Press; 1995. pp. 825–837. [Google Scholar]
- 14.Gabrieli JDE, McGlinchey-Berroth R, Carrillo MC, et al. Intact delay-eyeblink classical conditioning in amnesia. Behav Neurosci. 1995;109(5):819–827. doi: 10.1037//0735-7044.109.5.819. [DOI] [PubMed] [Google Scholar]
- 15.Gabrieli J. The cognitive neuroscience of human memory. Ann Rev Psych. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- 16.Golomb J, Kluger A, de Leon M, et al. Hippocampal formation size predicts declining memory performance in normal aging. Neurology. 1996;47:810–813. doi: 10.1212/wnl.47.3.810. [DOI] [PubMed] [Google Scholar]
- 17.Squire L, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 18.Eichenbaum H, Mathews P, Cohen NJ. Further studies of hippocampal representation during odor discrimination learning. Behav Neurosci. 1989;103:1207–1216. doi: 10.1037//0735-7044.103.6.1207. [DOI] [PubMed] [Google Scholar]
- 19.Gluck M, Myers C. Hippocampal mediation of stimulus representation: A computational theory. Hippocampus. 1993;3:491–516. doi: 10.1002/hipo.450030410. [DOI] [PubMed] [Google Scholar]
- 20.Gluck MA, Myers CE. Gateway to Memory: An Introduction to Neural Network Models of the Hippocampus and Learning. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- 21.Schacter D. Multiple forms of memory in humans and animals. In: Weinberger N, McGaugh J, Lynch G, editors. Memory Systems of the Brain: Animal and Human Cognitive Processe. New York: Guildford Press; 1985. pp. 351–379. 1985. [Google Scholar]
- 22.Myers C, Gluck M. Context, conditioning and hippocampal re-representation. Behav Neurosci. 1994;108(5):835–847. doi: 10.1037//0735-7044.108.5.835. [DOI] [PubMed] [Google Scholar]
- 23.Myers C, Gluck M. Cortico-hippocampal representations in simultaneous odor discrimination learning: A computational interpretation of Eichenbaum, Mathews and Cohen (1989) Behav Neurosci. 1996;110:685–706. doi: 10.1037//0735-7044.110.4.685. [DOI] [PubMed] [Google Scholar]
- 24.Gluck M, Oliver L, Myers C. Late-training amnesic deficits in probabilistic category learning: A neurocomputational analysis. Learning and Memory. 1996;3:326–340. doi: 10.1101/lm.3.4.326. [DOI] [PubMed] [Google Scholar]
- 25.Myers C, Ermita B, Harris K, et al. A computational model of the effects of septohippocampal disruption on classical eyeblink conditioning. Neurobio Learning Mem. 1996;66:51–66. doi: 10.1006/nlme.1996.0043. [DOI] [PubMed] [Google Scholar]
- 26.Myers C, Ermita B, Hasselmo M, et al. Further implications of a computational model of septohippocampal cholinergic modulation in eyeblink conditioning. Psychobiology. 1998;26(1):1–20. [Google Scholar]
- 27.Rokers B, Mercado E, Allen MT, et al. A connectionist model of septohippocampal dynamics during conditioning: Closing the loop. Behav Neurosci. 2002;116(1):48–62. [PubMed] [Google Scholar]
- 28.Myers C, Gluck M. Cortico-hippocampal representations in simultaneous odor discrimination learning: A computational interpretation of Eichenbaum, Mathews and Cohen (1989) Behav Neurosci. 1996;110(4):685–706. doi: 10.1037//0735-7044.110.4.685. [DOI] [PubMed] [Google Scholar]
- 29.Winocur G, Kinsbourne M. Contextual cueing as an aid to Korsakoff amnesics. Neuropsychologia. 1978;16:671–682. doi: 10.1016/0028-3932(78)90002-7. [DOI] [PubMed] [Google Scholar]
- 30.Myers C, Kluger A, Golomb J, et al. Hippocampal atrophy disrupts transfer generalization in non-demented elderly. Int J Ger Psych Neurol. 2002;15:82–90. doi: 10.1177/089198870201500206. [DOI] [PubMed] [Google Scholar]
- 31.Bonardi C, Rey V, Richmond M, et al. Acquired equivalence of cues in pigeon autoshaping: Effects of training with common consequences and common antecedents. Anim Learn Behav. 1993;21:369–376. [Google Scholar]
- 32.Hall G, Honey R. Contextual effects in conditioning, latent inhibition, and habituation: Associative and retrieval functions of contextual cues. J Exp Psychology: Anim Behav Proc. 1989;15:232–241. [Google Scholar]
- 33.Spiker C. Experiments with children on the hypothesis of acquired and distinctiveness equivalence of cues. Child Dev. 1956;27:253–263. [PubMed] [Google Scholar]
- 34.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda H. Cerebral blood flow and metabolic abnormalities in Alzheimer’s disease. Ann Nucl Med. 2001;15(2):85–92. doi: 10.1007/BF02988596. [DOI] [PubMed] [Google Scholar]
- 36.Chetelat G, Desgranges B, de la Sayette V, et al. Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain. 2003;126(Pt 9):1955–1967. doi: 10.1093/brain/awg196. [DOI] [PubMed] [Google Scholar]
- 37.Johnson S, Baxter L, Susskind-Wilder L, et al. Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia. 2004;42(7):980–989. doi: 10.1016/j.neuropsychologia.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Machulda MM, Ward HA, Borowski B, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61(4):500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Small S, Perera GM, DeLaPaz R. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer’s disease. Ann Neurol. 1999;45(4):466–472. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 40.Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74(1):44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dickerson BC, Salat DH, Bates JF, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56(1):27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dickerson BC, Salat DH, Greve DN, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65(3):404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bondi MW, Houston WS, Eyler LT, et al. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64(3):501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343(7):450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saykin AJ, Flashman LA, Frutiger S, et al. Neuroanatomic substrates of semantic memory impairment in Alzheimer’s disease: Patterns of functional MRI activation. J International Neuropsychol Soc. 1999;5:377–392. doi: 10.1017/s135561779955501x. [DOI] [PMC free article] [PubMed] [Google Scholar]


