Abstract
Hypoxia changes expression of angiogenic genes. Statins were also reported to affect blood vessel formation. However, data on the effects of statins on endothelial cells in hypoxia are limited. Here, effect of hypoxia and atorvastatin was assessed in human microvascular endothelial cells (HMEC-1). Hypoxia (1% O2) up-regulated vascular endothelial growth factor-A (VEGF-A) but, unexpectedly, it decreased interleukin-8 (IL-8) and placenta growth factor (PlGF) expression. Atorvastatin (0.1–1 μM) attenuated PlGF in HMEC-1 in normoxia while it decreased VEGF-A and IL-8 production both in normoxia and hypoxia. Notably, the expression of VEGF-D, macrophage scavenger receptor-1 (MSR1), transforming growth factor β receptor III (TGFβR3) and inhibitor of DNA binding 3 (ID3) was augmented by atorvastatin in cells cultured in normoxia, while in hypoxia the statin attenuated their expression. These data showed that hypoxia influenced in the opposite way the expression of major endothelial genes, augmenting VEGF-A and decreasing IL-8 and PlGF. The influence of atorvastatin on angiogenic gene expression is complex, and final pro- or anti-angiogenic outcome of statin therapy remains to be established for numerous angiogenesis-related diseases.
Keywords: Statins, VEGF-A, IL-8, HIF-1, PlGF
1. Introduction
The main mechanism of statins action is their ability to inhibit cholesterol synthesis via blocking the substrate accessibility to the 3-hydroxy-3-methyl-glutaryl-coenzyme A (3HMG-CoA) reductase, the enzyme that catalyses the rate-limiting step of the cholesterol synthesis pathway in the liver and other tissues (Urbich et al., 2002). By inhibiting L-mevalonic acid synthesis, statins also prevent the intracellular synthesis of other important isoprenoid intermediates of the cholesterol biosynthetic pathway, such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP). Careful analysis of many clinical trials indicate that statins might have effects that are not directly related to their cholesterol-lowering activity (Vaughan et al., 1996). So-called pleiotropic effects of statins, including promotion of angiogenesis, immunomodulatory and anti-inflammatory action (Weitz-Schmidt, 2002) may have beneficial influence on endothelial function, stability of atherosclerotic plaques, reduction of oxidative stress, inflammation and thrombogenic response.
Hypoxia is connected with several vascular pathologies including tumor development, heart and peripheral muscle ischemia or imbalanced wound healing. It increases the expression of many genes, particularly vascular endothelial growth factor-A (VEGF-A), an angiogenic factor essential in the initiation stage of neovascularization. Moreover, interleukin-8 (IL-8), a chemokine with pro-inflammatory and pro-angiogenic activities and placenta growth factor (PlGF), a mitogen for endothelial cells, were also reported to be up-regulated by hypoxic conditions in different cell types (Gleadle et al., 1995; Xu et al., 2004) On the other hand, expression of anti-angiogenic genes like tissue inhibitor of matrix metallo-proteinases (TIMP)-1 and 2 was reported to be diminished in hypoxic conditions (Ben-Yosef et al., 2002).
Surprisingly, although the effect of statins in normoxia have been extensively examined little is known about their influence in hypoxic conditions. Moreover, the pro-angiogenic activity of these drugs has been demonstrated in vitro mostly using macrovascular endothelial cell system such as human umbilical vein endothelial cells (HUVEC), which are, however, not the main endothelial subsets engaged in angiogenic events. Therefore, here we determined the effect of atorvastatin in normoxia and hypoxia on a model of microvascular endothelial cells, the HMEC-1 cell line, as microvascular endothelium is directly involved in angiogenesis. Interestingly, we observed that hypoxia affected in the opposite way the expression of pro-angiogenic VEGF-A, IL-8 and PlGF, enhancing VEGF-A synthesis and decreasing IL-8 and PlGF production. Atorvastatin diminished the expression of both pro-angiogenic VEGF-A, IL-8 and PlGF as well as anti-angiogenic thrombospondin-1 (TSP-1), indicating its complexity of action.
2. Materials and methods
2.1. Reagents
MCDB 131 medium, L-glutamine, epidermal growth factor (EGF) and hydrocortisone were purchased from Sigma (Poznan, Poland). Fetal calf serum (FCS) and chemicals used for reverse transcription (real time RT-PCR) were procured from Invitrogen (Life Technologies, Warszawa, Poland). CytoTox-96 assay, Reverse Transcription System, PCR Core System were obtained from Promega (Madison, WI, USA). ELISA kits for human VEGF-A and IL-8 proteins were from R and D Systems (Abingdon, UK). GEArray expression arrays were purchased from SuperArray Bioscience Corporation. RNA for real-time RT-PCR was isolated using Absolutely RNA Microprep Kit (Stratagene). Chemicals for real-time RT-PCR (Master Mix, primers and probes) were purchased from Applied Biosystem.
2.2. Cell culture and incubation experiments
Human microvascular endothelial cells (HMEC-1) were obtained from Dr. Francis Candal (Centers for Disease Control and Prevention; Atlanta) and cultured in MCDB 131 medium containing 10% FCS, L-glutamine (2 mM), epidermal growth factor (EGF, 10 ng/mL), hydrocortisone (1 μg/mL), penicillin (100 U/mL) and streptomycin (10 μg/mL).
HMEC-1 were routinely cultured at 37 °C in humidified air (normoxia) containing 5% CO2 for 6 or 24 h. For hypoxic stimulation cells were put into a Modular Incubator Chamber (Billups-Rothenberg Inc., Del Mar, CA, USA) which was aired with the gas mixture containing 1% O2, 5% CO2 and 94% N2.
Atorvastatin (Pfizer) was dissolved in DMSO to obtain a stock of 10 mM, and added to the cells at indicated concentrations for the whole incubation period. Mevalonic acid, dissolved in ethanol, was used at the dose 100 μM and was given 1 h before stimulation with atorvastatin. Concentrations of diluents never exceeded 0.1% (v/v) of culture media.
2.3. Detection of gene expression by macroarray hybridization
For the analysis of the differential expression of the set of angiogenesis-associated genes we used gene expression arrays (SuperArray, Inc.). Each GEArray membrane consisted of 96 cDNA fragments of genes involved in angiogenesis as well as positive (housekeeping genes-β-actin, GAPDH, cyclophilin A and ribosomal protein L13a) and negative (pUC18 DNA) controls printed in tetra-spot configuration on specialized nylon membranes. cDNA was prepared from 2 μg RNA by reverse transcription with MMLV reverse transcriptase, labeled with biotin — 16-dUTP (Boehringer Mannhem), then hybridized to membranes overnight with continuous agitation at 60 °C. After washing, the chemiluminescent detection was done and the arrays were exposed to X-ray film. The intensity of each of the gene-specific spots within an individual array was normalized to the expression of housekeeping genes. This allowed comparisons between array experiments. Each experiment was performed twice and only genes which expression has changed more than 1.5 fold up or down have been taken into consideration, according to the approach used in array analysis (Manalo et al., 2005).
2.4. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from the cells by acid guanidinum thiocyanate-phenol-chloroform extraction. Synthesis of cDNA was performed on 2 μg of total RNA with oligo-dT primers for 1 h at 42 °C using MMLV reverse transcriptase, according to vendor’s instruction. Then PCR with Taq polymerase was performed on cDNA for 28–35 cycles using the following protocol: 95 °C — 40 s, 58 °C — 40 s and 72 °C — 50 s. The primers recognizing VEGF-A (5′-CAC CGC CTC/T GGC TTG TCA CAT and 5′-CTG CTG/C TCT TGG GTG CAT/C TG), IL-8 (5′-CTC TCT TGG CAG CCT TCC TGA-3′ and 5′-CCC TCT GCA CCC AGT TTT CCT T-3′), TSP-1 (5′-GCA TTC CAG AGT CTG GCG GAG-3′ and 5′-TCT GTC CGC ACA GCA TCC ACC-3′), and EF2 (5′-GCG GTC AGC ACA ATG GCA TA and 5′-GAC ATC ACC AAG GGT GTG CAG) as a reporter gene have been used. PCR products were analyzed by electrophoresis in 2% agarose gel. The product length for the VEGF-A121 was 431 bp, for VEGF-A165 — 563 bp, for IL-8— 240 bp, for TSP-1— 197 bp and 218 bp for EF2.
2.5. Real-time RT-PCR
Total RNA for real-time RT-PCR was isolated using the Absolutely RNA Microprep Kit according to the manufacturer’s protocol. cDNA synthesis was carried out on 1 μg of total RNA using SuperScript III RNase H−Reverse Transcriptase with random hexamers (Promega). After the RT-reaction cDNA was diluted with distilled water to 10 ng/μl. Exon overlapping primers and Minor Groove Binder (MGB) probes labeled with 6-carboxyfluorescein (FAM) used for real-time RT-PCR were purchased as Assay-on-Demand from Applied Biosystems: IL-8 [Hs00174103_m1], VEGF-A [Hs00173626_m1], PlGF [Hs0018276_m1]. Endothelial cell marker CD31 [Hs00169777] was used as a housekeeping gene as it was stably expressed under the experimental conditions studied. Reactions were performed using 1× TaqMan® Universal PCR master mix, 900 nM of each primer, 250 nM probe and 1 μl cDNA in 10 μl volume. TaqMan real-time PCR was performed in an ABI PRISM 7900HT Sequence Detector (Applied Biosystems) using the following cycling conditions: 2 min 50 °C, 10 min 95 °C, and 45 two-step cycles of 15 s at 95 °C and 60 s at 60 °C. As controls, RNA samples not subjected to reverse transcriptase were analyzed to exclude non-specific signals arising from genomic DNA. Those samples showed no amplification signals. All PCR reactions were carried out in triplicates. Relative quantification of gene expression was calculated based on the comparative CT (threshold cycle value) method (ΔCT = CT gene of interest − CT housekeeping gene). Comparison of gene expression in different samples was performed based on the differences in ΔCT of individual samples (ΔΔCT).
2.6. Transfection with reporter plasmids
A construct containing a full-length human VEGF-A promoter (−2279 to +54) cloned into the luciferase reporter plasmid pGL2 and a pHRE-luc construct containing a fragment of human VEGF-A promoter (−1014 to −903), inserted upstream of the thymidine kinase promoter of pT81luc0 plasmid were kindly provided by Dr. Hideo Kimura (Chiba, Japan). The full-length promoter of the IL-8 gene was cloned upstream of the firefly luciferase coding region in the pGL2-basic vector and was kindly supplied by Dr. Rainer de Martin (Vienna, Austria) (Yeh et al., 2001).
HMEC-1 cells growing to 70% confluence in 24-well plates were transfected with 0.25 μg of DNA and 1.25 μl SuperFect Reagent per well, according to vendor’s protocol. After 24 h cells were treated with indicated stimulants for next 24 h. The activity of reporter genes was determined in cell lysates at 48 h after transfection and normalized to the total protein content as described previously (Grzenkowicz-Wydra et al., 2004).
2.7. ELISA assays
VEGF and IL-8 concentration in cell culture media was determined by a commercially available ELISA kits according to vendor’s protocol (R and D Systems, Abingdon, UK).
2.8. Cell viability assay
Cell viability was assessed by colorimetric measurement of LDH release according to the manufacturer’s instructions (Promega, Madison, WI, USA).
2.9. Statistical analysis
All experiments, if not stated otherwise, were repeated 3–5 times. Data are presented as mean ± SD. Statistical evaluation was done with ANOVA followed by Tukey test. Differences were accepted as statistically significant at p <0.05.
3. Results
3.1. Hypoxia potently enhances VEGF-A and atorvastatin decreases VEGF-A production
VEGF-A expression is strongly induced in hypoxic HMEC-1 both at mRNA (Fig. 1A,B) and protein (Fig. 1C) level. At 1 μM, atorvastatin moderately, but significantly decreased hypoxia-induced VEGF-A protein (Fig. 1C). The changes in VEGF-A protein synthesis were due to atorvastatin effects on mRNA expression, as shown by quantitative real-time RT-PCR (Fig. 1B). Interestingly, VEGF-A protein level after 24 h of hypoxia was increased up to 7 times (Fig. 1C) whereas real-time RT-PCR analysis showed only 2–2.5 fold enhancement of VEGF-A expression both after 6 and 24 h of hypoxia (Fig. 1B), suggesting the significant influence of hypoxia on VEGF-A translation (Stein et al., 1998).
Fig. 1.

Inhibition of hypoxia-induced VEGF-A production in HMEC-1 by atorvastatin. RT-PCR (A) and real-time RT-PCR (B) demonstrating the induction of VEGF-A mRNA expression in hypoxia and moderate down-regulation after 6 h (A) and 24 h (B) treatment with atorvastatin (normoxia — open bars, hypoxia —filled bars). (C) Effect of different concentrations of atorvastatin on VEGF-A synthesis in HMEC-1 after 24 h incubation in normoxia (open bars) and hypoxia (filled bars) measured by ELISA. Representative results from five independent experiments, * p <0.05 vs control.
Hypoxia potently, up to five-fold enhanced the transcription rate of VEGF-A promoter or its hypoxia responsive element (HRE) sequence. Surprisingly, and in contrast to the effect on VEGF protein, activity of both VEGF-A full promoter (Fig. 2A) and HRE sequence (Fig. 2B) was enhanced by atorvastatin in normoxic conditions, as shown by elevated activity of luciferase reporter protein. Addition of mevalonic acid (100 μM), the end product of HMG-CoA reductase, reversed the stimulatory effect on both VEGF-A promoter (Fig. 2A) and HRE (Fig 2B). Again, in hypoxia atorvastatin augmented VEGF-A promoter (Fig. 2C) and HRE activity (Fig. 2D) and both these effects were reversed by mevalonic acid.
Fig. 2.
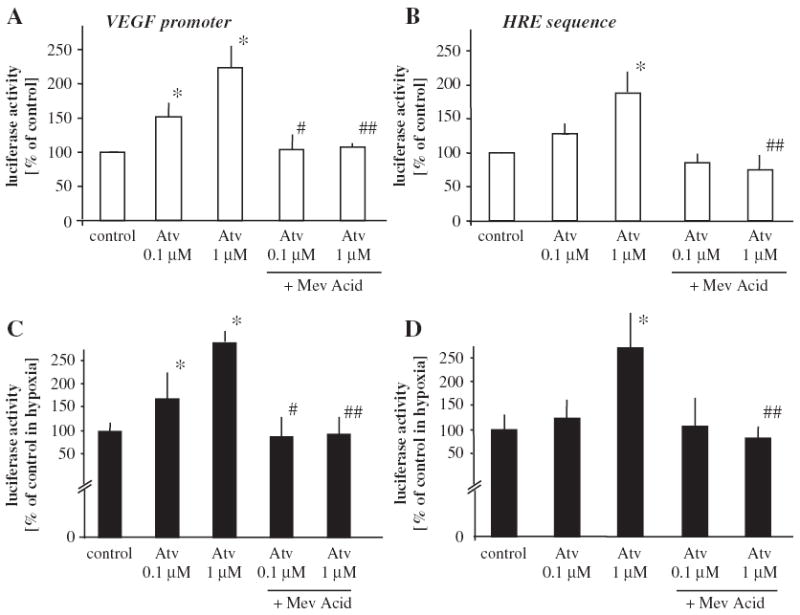
Effect of atorvastatin on VEGF-A promoter and HRE sequence activity in HMEC-1 in normoxic and hypoxic conditions. Luciferase activity demonstrating stimulatory effect of atorvastatin on VEGF-A promoter (A) and HRE sequence (B) in normoxia. Decreased oxygen tension activated VEGF-A promoter by 5.5 ± 0.8 fold and HRE sequence by 9.3 ± 1.1. Additionally, atorvastatin augmented hypoxia-induced VEGF-A promoter (C) and HRE (D) activity. Mevalonic acid (100 μM) completely reversed the stimulatory effect of atorvastatin. Mean of three independent experiments. * p <0.05 vs control, # p <0.05 vs cells treated with 0.1 μM atorvastatin, ## p <0.05 vs cells treated with 1 μM atorvastatin.
To check if the induction of VEGF-A promoter after 24 h could be translated to protein synthesis after longer time, the cells were cultured with 1 μM of atorvastatin for 48 h. However, atorvastatin during such a prolonged incubation became cytotoxic for HMEC-1, what resulted in complete abrogation of VEGF-A production (data not shown).
3.2. Hypoxia and atorvastatin down-regulate IL-8 production in HMEC-1
Basal IL-8 production in HMEC-1 is much higher than the synthesis of VEGF-A and it is at the level of several hundred picograms per 105 cells (Jozkowicz et al., 2001). Interestingly and in contrast to the effect observed in other cells (Galindo et al., 2001; Karakurum et al., 1994), 24 h hypoxia reduced by 60% IL-8 mRNA expression (Fig. 3A,B) and diminished IL-8 protein release by about 25% (Fig. 3C). Atorvastatin at 0.1–1 μM decreased basal expression of IL-8 (Fig. 3B,C) and further aggravated attenuation of IL-8 protein synthesis by hypoxia (Fig. 3C).
Fig. 3.
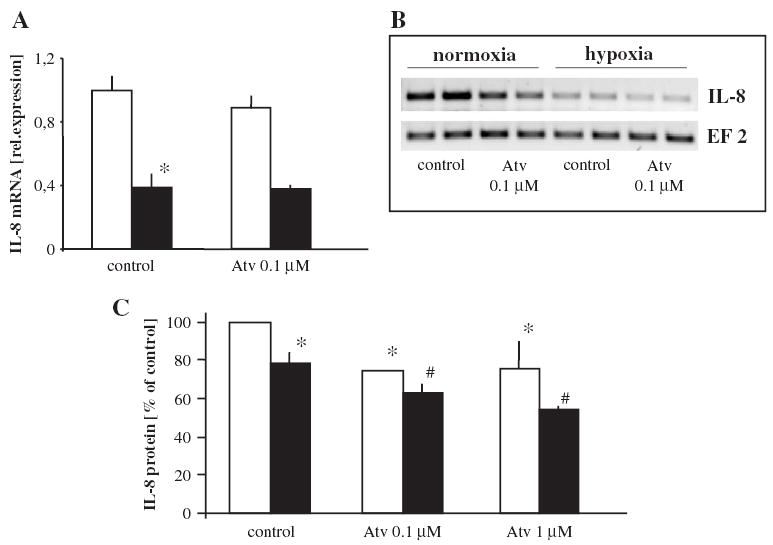
Effect of atorvastatin on IL-8 production in HMEC-1 cultured under different oxygen tension. Down-regulation of IL-8 expression by hypoxia demonstrated by real-time RT-PCR (A), RT-PCR (B) and ELISA (C). Atorvastatin decreased basal synthesis and additionally attenuated IL-8 production in hypoxia (B, C) (normoxia — open bars, hypoxia — filled bars). Mean of two (A) and four (B) experiments, * p <0.05 vs control in normoxia, # p <0.05 vs control in hypoxia.
Surprisingly again, the effect of atorvastatin on IL-8 promoter, when tested in the reporter assay, was different than the effect on IL-8 mRNA expression and IL-8 protein synthesis. Treatment with atorvastatin significantly enhanced reporter luciferase activity both in normoxic (Fig. 4A) and hypoxic (Fig. 4B) conditions. Importantly, the IL-8 promoter was not activated under decreased oxygen tension (100.91 ± 8.82% of normoxia) which relates to the lack of effect of hypoxia on IL-8 mRNA and protein production (Fig. 3).
Fig. 4.
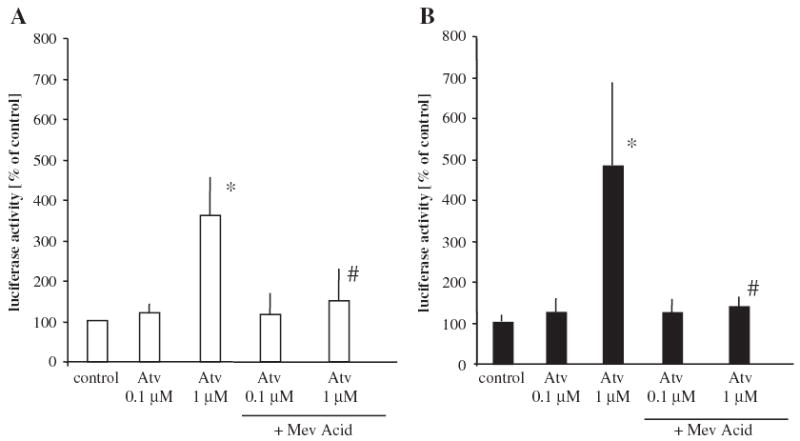
Effect of atorvastatin on IL-8 promoter activity. Atorvastatin enhanced IL-8 promoter activity both in normoxia (A) and in hypoxia (B), as determined by luciferase activity measurement in a reporter gene assay. The effect was reversed by mevalonic acid (100 μM). Mean of two independent experiments, * p <0.05 vs control, # p <0.05 vs cells treated with atorvastatin.
3.3. Macroarray analysis of angiogenic gene expression: effect of hypoxia and atorvastatin
GEArrays contain the cDNA sequences for 96 angiogenic genes and were used to assess the effect of hypoxia and atorvastatin on a larger set of genes. Hybridisation assays showed that hypoxia augmented the expression of some genes represented on the arrays (Table 1). The strongest induction was noted for macrophage scavenger receptor-1 (MSR1) gene, which expression was up-regulated by about 30-fold (Table 1). Expression of VEGF-D, thrombin, VE-cadherin, fibronectin-1 or transforming growth factor beta receptor III (TGFBR3) was also enhanced after hypoxia treatment (Table 1, Fig. 5A). On the other hand, midkine, matrix metalloproteinase-2 (MMP2), osteonectin, TIMP-1 and TIMP-2, as well as PlGF and Tie2 receptor were down-regulated (Table 1). Inhibition of PlGF by hypoxia was confirmed using real-time RT-PCR (Fig. 5B).
Table 1.
Effect of hypoxia on angiogenic genes expression in HMEC-1
| Symbol | Unigene number | Fold of change | |
|---|---|---|---|
| Cadherin 5, type 2 (VE-cadherin) | CDH5 | 76206 | 1.6 |
| Fibronectin 1 | FN1 | 418138 | 1.7 |
| Transforming growth factor, beta receptor III | TGFBR3 | 342874 | 1.8 |
| Coagulation factor II (thrombin) | F2 | 76530 | 2.3 |
| Vascular endothelial growth factor-D | VEGF-D | 11392 | 2.3 |
| Macrophage scavenger receptor-1 | MSR1 | 436887 | 26.5 |
| Midkine | MDK | 82045 | −1.9 |
| Tissue inhibitor of metalloproteinase 2 | TIMP2 | 6441 | −1.9 |
| Matrix metalloproteinase 2 | MMP2 | 367877 | −2.0 |
| Osteonectin | SPARC | 111779 | −2.7 |
| Tissue inhibitor of metalloproteinase 1 | TIMP1 | 522632 | −3.3 |
| Placental growth factor | PlGF | 252820 | −5.2 |
| Tie-2 receptor | Tie-2 | 89640 | −5.6 |
Fig. 5.
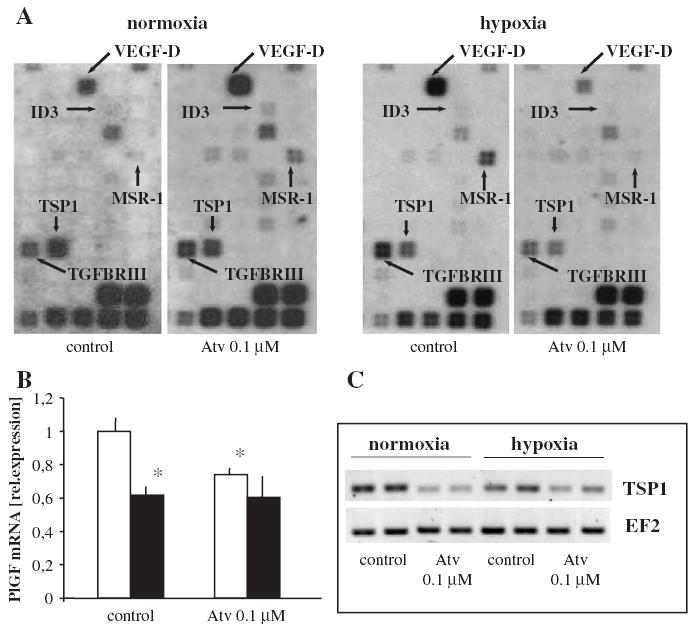
Effect of hypoxia and atorvastatin on angiogenic genes expression in HMEC-1. Macrorray analysis (A) of the expression of 96 angiogenic genes. Note different pattern of dots on each membrane for VEGF-D, ID-3, MSR1 and TGFBR3 demonstrating specific regulation of those genes by atorvastatin in various conditions (stimulation in normoxia, inhibition in hypoxia). (B) Mean of two real-time RT-PCR experiments, showing the inhibition of PlGF expression in hypoxia (filled bars). Note that atorvastatin decreased PlGF expression in normoxia (open bars). * p <0.05 vs control. (C) Representative RT-PCR confirming the inhibitory effect (marked also on the array) of atorvastatin on TSP-1 expression in both normoxic and hypoxic HMEC-1.
Interestingly, atorvastatin exerted dual effect on VEGF-D, MSR1, TGFBR3 and inhibitor of DNA binding 3 (ID3) expression in normoxic and hypoxic conditions (Fig. 5A, Table 2). As shown in Table 2, VEGF-D expression was increased ~3 times in normoxia but in hypoxia it was diminished after atorvastatin treatment. MSR1, TGFBR3 and ID3 expression were regulated in the similar way, as they were strongly inhibited in hypoxic HMEC-1 after 0.1 μM atorvastatin. The strongest changes were noted for MSR1 as under normoxic conditions it was augmented by atorvastatin ~15 times but in hypoxia it became down-regulated ~8 times. (Fig. 5A, Table 2).
Table 2.
Effect of atorvastatin on transcriptome in normoxic and hypoxic HMEC-1
| Symbol | Unigene number | Fold of change | |
|---|---|---|---|
| Normoxia | |||
| Fibroblast growth factor 6 | FGF6 | 166015 | 1.6 |
| Integrin, alpha 5 | ITGA5 | 149609 | 1.6 |
| Tie-2 receptor | Tie-2 | 89640 | 1.7 |
| Matrix metalloproteinase 2 | MMP2 | 367877 | 1.8 |
| Transforming growth factor, beta receptor III | TGFBR3 | 342874 | 1.8 |
| Inhibitor of DNA binding 3 | ID3 | 76884 | 1.9 |
| Midkine | MDK | 82045 | 1.9 |
| Endoglin | ENG | 76753 | 2.2 |
| Restin | RSN | 410113 | 2.6 |
| Transforming growth factor, beta I | TGFB1 | 1103 | 2.6 |
| Vascular endothelial growth factor-D | VEGF-D | 11392 | 2.9 |
| Macrophage scavenger receptor-1 | MSR1 | 436887 | 14.2 |
| Fibronectin 1 | FN1 | 418138 | −1.6 |
| Tumor necrosis factor | TNFA | 241570 | −2.5 |
| Tissue inhibitor of metalloproteinase 1 | TIMP1 | 522632 | −2.7 |
| Hypoxia | |||
| Restin | RSN | 410113 | 1.6 |
| Osteonectin | SPARC | 111779 | 1.7 |
| Tissue inhibitor of metalloproteinase 2 | TIMP2 | 6441 | 1.8 |
| Matrix metalloproteinase 2 | MMP2 | 367877 | 2.4 |
| Tissue inhibitor of metalloproteinase 1 | TIMP1 | 522632 | 2.5 |
| Midkine | MDK | 82045 | 2.9 |
| Chemokine ligand -1 | GRO1 | 789 | 3.2 |
| Tie-2 receptor | Tie-2 | 89640 | 3.3 |
| Thrombospondin 1 | TSP-1 | 164226 | −1.6 |
| Coagulation factor II (thrombin) | F2 | 76530 | −1.9 |
| Transforming growth factor, beta receptor III | TGFBR3 | 342874 | −1.9 |
| Tumor necrosis factor | TNFA | 241570 | −1.9 |
| Inhibitor of DNA binding 3 | ID3 | 76884 | −3.2 |
| Vascular endothelial growth factor-D | VEGF-D | 11392 | −3.2 |
| Cadherin 5, type 2 (VE-cadherin) | CDH5 | 76206 | −3.3 |
| Macrophage scavenger receptor-1 | MSR1 | 436887 | −8.3 |
Atorvastatin enhanced the expression of restin, midkine, Tie-2 receptor and MMP2 both in normoxic and hypoxic HMEC-1 (Table 2). On the other hand, it inhibited the expression of TSP-1 (Table 2, Fig. 5A). This influence was verified using RT-PCR (Fig. 5C). Moreover, atorvastatin diminished PlGF expression in normoxic but not in hypoxic HMEC-1, as determined by real-time RT-PCR (Fig. 5B).
4. Discussion
The salient finding of the present study is that in human microvascular endothelial cells hypoxia influenced, in the opposite way, the expression of two crucial pro-angiogenic genes, augmenting VEGF-A and decreasing IL-8. Surprisingly hypoxia also down-regulated the expression of PlGF. Next, the complexity of the effect of atorvastatin on angiogenic gene expression in HMEC-1 was revealed. Thus, both in normoxia and hypoxia, at pharmacologically relevant concentrations the drug moderately, but significantly inhibited the production of pro-angiogenic VEGF-A and IL-8. Moreover, it decreased PlGF expression in normoxic HMEC-1. On the other hand, the expression of VEGF-D, MSR1, TGFBR3 and ID3 was up-regulated by atorvastatin in normoxia but it was diminished in hypoxia.
Statins have been reported to affect angiogenesis in the biphasic way, exerting stimulatory effect at low, nanomolar concentrations, while inhibiting the blood vessel formation at high, micromolar concentrations (Frick et al., 2003; Urbich et al., 2002; Weis et al., 2002). Importantly, the effect of different statins appears to be similar (Frick et al., 2003; Urbich et al., 2002; Weis et al., 2002). Pro-angiogenic activities of statins are due to their effects on both mature endothelial cells and endothelial progenitor cells, which are protected from senescence and apoptosis (Assmus et al., 2003). At the molecular level those protective activities of statins are mostly ascribed to the stimulation of the PI3/Akt kinase pathway, resulting in the phosphorylation of eNOS, a critical mediator of angiogenic and anti-apoptotic activity of endothelial cells (Urbich et al., 2002). On the other hand, higher, micromolar concentrations of statins may exert weak or no effect on Akt kinase phosphorylation, while they enhance eNOS expression (Urbich et al., 2002).
VEGF-A is strongly up-regulated by hypoxia, an effect mediated by hypoxia inducible factor-1 (HIF-1) binding to HRE of VEGF promoter. Additionally, hypoxia strongly augments translation of VEGF-A (Stein et al., 1998). Low oxygen tension stabilizes HIF-1α subunit which together with HIF-1β forms heterodimer and binds to HRE present in the regulatory regions of many genes including VEGF-A, erythropoietin or glucose transporters (for a review see (Zagorska and Dulak, 2004). HIF-1 binding activity increases only two-fold between 20% and 6% O2 but ten-fold between 6% and 0.5% O2 (Jiang et al., 1996).
Previous studies demonstrated the stimulatory effect of low nanomolar concentrations of statins on VEGF-A production in microvascular endothelial cells (Weis et al., 2002). However, in the present study we observed that this is not the case when therapeutically relevant concentrations of atorvastatin were applied. Importantly, we observed for the first time that 0.1–1 μM amounts of atorvastatin down-regulated hypoxia-induced VEGF-A synthesis, and affected IL-8 and TSP-1 production in such conditions.
Surprisingly, atorvastatin at micromolar concentrations both in normoxic and hypoxic conditions enhanced significantly the transcriptional activity of VEGF-A promoter, including its HRE part, as demonstrated in the reporter gene assay. However, those influences are reflected neither in the VEGF-A mRNA nor protein, which decreased under the same conditions and were not enhanced even under prolonged treatment with statins. Similarly, atorvastatin decreased IL-8 protein synthesis but it enhanced IL-8 promoter activity. Interestingly, similar opposite effects of statins on protein synthesis (inhibitory) and mRNA expression (stimulatory) have been noted in the case of metalloproteinases (Ikeda et al., 2000). Also in the present study we noted an increase in MMP-2 gene expression by atorvastatin both in normoxia and hypoxia.
Promoter activation is determined by the measurement of luciferase activity, therefore one may presume that the effect of atorvastatin on luciferase protein is different than on either VEGF-A or IL-8 mRNA or protein, which may be destabilized by statins in contrast to luciferase.
Another pro-angiogenic factor which we studied extensively was IL-8. It is a member of the chemokine family and has been shown to play an important role in tumor growth, angiogenesis, and metastasis (Yuan et al., 2005). Moreover, it is a survival factor for endothelial cells as it enhanced anti-apoptotic Bcl-xL :Bcl-xS and Bcl-2:Bax ratios (Li et al., 2003). IL-8 has pro-inflammatory activities and its elevated level is detected in hypercholesterolemic patients (Porreca et al., 1999). Moreover, it has been implicated in modulation of atherogenesis (Boisvert, 2004), as up-regulation of this chemokine was observed in atherosclerotic plaques (Wang et al., 1996). Our results showing a decrease in IL-8 synthesis after statins treatment are in agreement with recent data demonstrating such an effect both in vitro and in vivo. Down-regulation of this chemokine in response to statins has been observed in patients with coronary artery disease (CAD) (Waehre et al., 2003), in hypercholesterolemic patients (Rezaie-Majd et al., 2002) as well as in in vitro studies on monocytes/macrophages (Terkeltaub et al., 1994), vascular smooth muscle cells (Ito et al., 2002) and endothelial cells (Morikawa et al., 2002). In our experiments only ~30% reduction in IL-8 mRNA occurs after atorvastatin treatment while in a study by Morikawa a 3-fold inhibition of IL-8 mRNA after 8 and 24 h incubation has been noted (Morikawa et al., 2002). However, the strong suppression could be associated with the drug concentration used in this study (6.6 μM). In our experiments we applied up to 1 μM of atorvastatin, which is in the range of pharmacological concentrations in patients receiving this drug for treatment of hypercholesterolemia. Stronger decrease in IL-8 protein synthesis by atorvastatin despite moderate effect on IL-8 mRNA suggests that similarly to the effect on VEGF atorvastatin affects IL-8 translation or protein stability.
Interestingly, apart from the regulation of IL-8 expression by atorvastatin we have found that hypoxia significantly diminished the level of this cytokine in HMEC-1. Such result is in contrast to other studies where increase in IL-8 mRNA and protein level has been observed in endothelial cells (Karakurum et al., 1994), human ovarian carcinoma cells (Yoshino et al., 2003) or fibroblasts (Galindo et al., 2001). We confirmed the inhibitory effect of hypoxia using different methods, and both RT-PCR and quantitative real-time RT-PCR as well as ELISA demonstrated down-regulation of IL-8 in HMEC-1. Importantly, IL-8 production is diminished similarly to hypoxia in primary microvascular endothelial cells treated with desferoxamine, an iron chelator activating hypoxia-inducible factor (HIF-1) (Koo et al., 2003). Also, treatment with cobalt chloride, another HIF-1 activator, does not induce IL-8 expression in HMEC-1 (Loboda et al., 2005). Moreover, recently published study clearly demonstrated that hypoxia does not enhance IL-8 expression and HIF-1 induction may even impede the synthesis of IL-8 in many cancer cells (Mizukami et al., 2005). Thus, IL-8 appears to be differentially affected by hypoxia or its mimics in microvascular endothelial cells in contrast to other cell types and the detailed mechanism remains to be elucidated.
The regulation of PlGF, another important pro-angiogenic gene could be also cell type specific. Our results demonstrate its down-regulation in hypoxia, which is in contrast to data reported in other studies (Gleadle et al., 1995; Green et al., 2001). Similarly to the effect on IL-8, atorvastatin seems to decrease its expression in normoxic conditions.
Thrombospondin-1 (TSP-1), is a glycoprotein with major roles in cellular adhesion and vascular smooth muscle proliferation and migration. It has been shown both to stimulate and inhibit angiogenesis by influencing endothelial cell adhesion, migration or apoptosis (Esemuede et al., 2004). We have found that expression of TSP-1 was significantly decreased after 24 h incubation with atorvastatin in hypoxic HMEC-1. Our data are in line with observations reported by Riessen et al. (1999), who studied statins effects on human vascular smooth muscle cells. They noticed that both cerivastatin and lovastatin had an inhibitory effect on TSP-1 expression in these cells. As elevated level of TSP-1 was also observed in cholesterol-induced atherosclerosis and intimal hyperplasia (Roth et al., 1998), this action of statins could be beneficial for treatment of both cancer and atherosclerosis.
Interestingly, MSR1, encoding macrophage scavenger receptor A was the most strongly regulated gene in macroarray studies. The significance of this receptor for angiogenic processes is, however, not known, and remains to be elucidated.
In conclusion, down-regulation of IL-8 and VEGF-A expression after atorvastatin treatment, the effect observed also in hypoxia, could be related to the anti-angiogenic and anti-inflammatory activities of statins. Such an inhibitory effect could be additionally potentiated by inhibition of the expression of other pro-angiogenic mediators, like VEGF-D, ID3 or VE-cadherin in hypoxia. However, the effects exerted by pharmacological concentrations of atorvastatin are small and although one may not exclude accumulating effect due to influence of statins on many mediators, the relevance of those observations for many angiogenesis-dependent conditions, including cancers, growth of atherosclerotic plaques, psoriasis and rheumatoid arthritis remains to be further investigated.
Acknowledgments
The study has been supported by research grants from Pfizer (Poland) and PBZ-107/P04/2004 grant from the Polish Ministry of Scientific Research and Information Technology awarded to J. Dulak. Dr Jozkowicz is an International Senior Research Fellow of Wellcome Trust.
References
- Assmus B, Urbich C, Aicher A, Hofmann WK, Haendeler J, Rossig L, Spyridopoulos I, Zeiher AM, Dimmeler S. HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ Res. 2003;92:1049–1055. doi: 10.1161/01.RES.0000070067.64040.7C. [DOI] [PubMed] [Google Scholar]
- Ben-Yosef Y, Lahat N, Shapiro S, Bitterman H, Miller A. Regulation of endothelial matrix metalloproteinase-2 by hypoxia/reoxygenation. Circ Res. 2002;90:784–791. doi: 10.1161/01.res.0000015588.70132.dc. [DOI] [PubMed] [Google Scholar]
- Boisvert WA. Modulation of atherogenesis by chemokines. Trends Cardiovasc Med. 2004;14:161–165. doi: 10.1016/j.tcm.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Esemuede N, Lee T, Pierre-Paul D, Sumpio BE, Gahtan V. The role of thrombospondin-1 in human disease. J Surg Res. 2004;122:135–142. doi: 10.1016/j.jss.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Frick M, Dulak J, Cisowski J, Jozkowicz A, Zwick R, Alber H, Dichtl W, Schwarzacher SP, Pachinger O, Weidinger F. Statins differentially regulate vascular endothelial growth factor synthesis in endothelial and vascular smooth muscle cells. Atherosclerosis. 2003;170:229–236. doi: 10.1016/s0021-9150(03)00299-5. [DOI] [PubMed] [Google Scholar]
- Galindo M, Santiago B, Alcami J, Rivero M, Martin-Serrano J, Pablos JL. Hypoxia induces expression of the chemokines monocyte chemoattractant protein-1 (MCP-1) and IL-8 in human dermal fibroblasts. Clin Exp Immunol. 2001;123:36–41. doi: 10.1046/j.1365-2249.2001.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleadle JM, Ebert BL, Firth JD, Ratcliffe PJ. Regulation of angiogenic growth factor expression by hypoxia, transition metals, and chelating agents. Am J Physiol. 1995;268:C1362–C1368. doi: 10.1152/ajpcell.1995.268.6.C1362. [DOI] [PubMed] [Google Scholar]
- Green CJ, Lichtlen P, Huynh NT, Yanovsky M, Laderoute KR, Schaffner W, Murphy BJ. Placenta growth factor gene expression is induced by hypoxia in fibroblasts: a central role for metal transcription factor-1. Cancer Res. 2001;61:2696–2703. [PubMed] [Google Scholar]
- Grzenkowicz-Wydra J, Cisowski J, Nakonieczna J, Zarebski A, Udilova N, Nohl H, Jozkowicz A, Podhajska A, Dulak J. Gene transfer of CuZn superoxide dismutase enhances the synthesis of vascular endothelial growth factor. Mol Cell Biochem. 2004;264:169–181. doi: 10.1023/b:mcbi.0000044386.45054.70. [DOI] [PubMed] [Google Scholar]
- Ikeda U, Shimpo M, Ohki R, Inaba H, Takahashi M, Yamamoto K, Shimada K. Fluvastatin inhibits matrix metalloproteinase-1 expression in human vascular endothelial cells. Hypertension. 2000;36:325–329. doi: 10.1161/01.hyp.36.3.325. [DOI] [PubMed] [Google Scholar]
- Ito T, Ikeda U, Yamamoto K, Shimada K. Regulation of interleukin-8 expression by HMG-CoA reductase inhibitors in human vascular smooth muscle cells. Atherosclerosis. 2002;165:51–55. doi: 10.1016/s0021-9150(02)00194-6. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- Jozkowicz A, Dulak J, Prager M, Nanobashvili J, Nigisch A, Winter B, Weigel G, Huk I. Prostaglandin-J2 induces synthesis of interleukin-8 by endothelial cells in a PPAR-gamma-independent manner. Prostaglandins Other Lipid Mediat. 2001;66:165–177. doi: 10.1016/s0090-6980(01)00155-1. [DOI] [PubMed] [Google Scholar]
- Karakurum M, Shreeniwas R, Chen J, Pinsky D, Yan SD, Anderson M, Sunouchi K, Major J, Hamilton T, Kuwabara K, et al. Hypoxic induction of interleukin-8 gene expression in human endothelial cells. J Clin Invest. 1994;93:1564–1570. doi: 10.1172/JCI117135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SW, Casper KA, Otto KB, Gira AK, Swerlick RA. Iron chelators inhibit VCAM-1 expression in human dermal microvascular endothelial cells. J Invest Dermatol. 2003;120:871–879. doi: 10.1046/j.1523-1747.2003.12144.x. [DOI] [PubMed] [Google Scholar]
- Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- Loboda A, Jazwa A, Wegiel B, Jozkowicz A, Dulak J. Heme oxygenase-1-dependent and -independent regulation of angiogenic gene expression: effect of cobalt protoporphyrin and cobalt chloride on VEGF and IL-8 synthesis in human microvascular endothelial cells. Cell Mol Biol. 2005;51:347–355. [PMC free article] [PubMed] [Google Scholar]
- Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, Zimmer MA, Iliopoulos O, Zukerberg LR, Kohgo Y, Lynch MP, Rueda BR, Chung DC. Induction of interleukin-8 preserves the angiogenic response in HIF-1 alpha-deficient colon cancer cells. Nat Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Takabe W, Mataki C, Kanke T, Itoh T, Wada Y, Izumi A, Saito Y, Hamakubo T, Kodama T. The effect of statins on mRNA levels of genes related to inflammation, coagulation, and vascular constriction in HUVEC. Human umbilical vein endothelial cells. J Atheroscler Thromb. 2002;9:178–183. doi: 10.5551/jat.9.178. [DOI] [PubMed] [Google Scholar]
- Porreca E, Sergi R, Baccante G, Reale M, Orsini L, Febbo CD, Caselli G, Cuccurullo F, Bertini R. Peripheral blood mononuclear cell production of interleukin-8 and IL-8-dependent neutrophil function in hypercholesterolemic patients. Atherosclerosis. 1999;146:345–350. doi: 10.1016/s0021-9150(99)00160-4. [DOI] [PubMed] [Google Scholar]
- Rezaie-Majd A, Maca T, Bucek RA, Valent P, Muller MR, Husslein P, Kashanipour A, Minar E, Baghestanian M. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2002;22:1194–1199. doi: 10.1161/01.atv.0000022694.16328.cc. [DOI] [PubMed] [Google Scholar]
- Riessen R, Axel DI, Fenchel M, Herzog UU, Rossmann H, Karsch KR. Effect of HMG-CoA reductase inhibitors on extracellular matrix expression in human vascular smooth muscle cells. Basic Res Cardiol. 1999;94:322–332. doi: 10.1007/s003950050158. [DOI] [PubMed] [Google Scholar]
- Roth JJ, Gahtan V, Brown JL, Gerhard C, Swami VK, Rothman VL, Tulenko TN, Tuszynski GP. Thrombospondin-1 is elevated with both intimal hyperplasia and hypercholesterolemia. J Surg Res. 1998;74:11–16. doi: 10.1006/jsre.1997.5209. [DOI] [PubMed] [Google Scholar]
- Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol Cell Biol. 1998;18:3112–3119. doi: 10.1128/mcb.18.6.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkeltaub R, Solan J, Barry M, Jr, Santoro D, Bokoch GM. Role of the mevalonate pathway of isoprenoid synthesis in IL-8 generation by activated monocytic cells. J Leukoc Biol. 1994;55:749–755. doi: 10.1002/jlb.55.6.749. [DOI] [PubMed] [Google Scholar]
- Urbich C, Dernbach E, Zeiher AM, Dimmeler S. Double-edged role of statins in angiogenesis signaling. Circ Res. 2002;90:737–744. doi: 10.1161/01.res.0000014081.30867.f8. [DOI] [PubMed] [Google Scholar]
- Vaughan CJ, Murphy MB, Buckley BM. Statins do more than just lower cholesterol. Lancet. 1996;348:1079–1082. doi: 10.1016/S0140-6736(96)05190-2. [DOI] [PubMed] [Google Scholar]
- Waehre T, Damas JK, Gullestad L, Holm AM, Pedersen TR, Arnesen KE, Torsvik H, Froland SS, Semb AG, Aukrust P. Hydroxymethylglutaryl coenzyme a reductase inhibitors down-regulate chemokines and chemokine receptors in patients with coronary artery disease. J Am Coll Cardiol. 2003;41:1460–1467. doi: 10.1016/s0735-1097(03)00263-8. [DOI] [PubMed] [Google Scholar]
- Wang N, Tabas I, Winchester R, Ravalli S, Rabbani LE, Tall A. Interleukin 8 is induced by cholesterol loading of macrophages and expressed by macrophage foam cells in human atheroma. J Biol Chem. 1996;271:8837–8842. doi: 10.1074/jbc.271.15.8837. [DOI] [PubMed] [Google Scholar]
- Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- Weitz-Schmidt G. Statins as anti-inflammatory agents. Trends Pharmacol Sci. 2002;23:482–486. doi: 10.1016/s0165-6147(02)02077-1. [DOI] [PubMed] [Google Scholar]
- Xu L, Pathak PS, Fukumura D. Hypoxia-induced activation of p38 mitogen-activated protein kinase and phosphatidylinositol 3′-kinase signaling pathways contributes to expression of interleukin 8 in human ovarian carcinoma cells. Clin Cancer Res. 2004;10:701–707. doi: 10.1158/1078-0432.ccr-0953-03. [DOI] [PubMed] [Google Scholar]
- Yeh M, Leitinger N, de Martin R, Onai N, Matsushima K, Vora DK, Berliner JA, Reddy ST. Increased transcription of IL-8 in endothelial cells is differentially regulated by TNF-alpha and oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2001;21:1585–1591. doi: 10.1161/hq1001.097027. [DOI] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Koga K, Hirota Y, Yano T, Tsutsumi O, Fujimoto A, Kugu K, Momoeda M, Fujiwara T, Taketani Y. Upregulation of interleukin-8 by hypoxia in human ovaries. Am J Reprod Immunol. 2003;50:286–290. doi: 10.1034/j.1600-0897.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- Yuan A, Chen JJ, Yao PL, Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853–865. doi: 10.2741/1579. [DOI] [PubMed] [Google Scholar]
- Zagorska A, Dulak J. HIF-1: the knowns and unknowns of hypoxia sensing. Acta Biochim Pol. 2004;51:563–585. [PubMed] [Google Scholar]


