Abstract
In the current study, we extended our previous works on natural endogenous reverse transcription (NERT) and further examined its potential as a virucide molecular target in sexual transmission of primate lentiviruses. HIV-1 and SIV virions were pretreated with select nucleoside (NRTIs) and nonnucleoside RT inhibitors (NNRTIs), either alone or in combination with NERT-stimulating substances. The effects of these antiretrovirals on virion inactivation were analyzed in human T cell lines and primary cell cultures. Pretreatment of HIV-1 virions with physiologic NERT-stimulants and 3′-azido-3′-deoxythymidine 5′-triphosphate (AZT-TP) or nevirapine potently inactivated cell-free HIV-1 virions and resulted in strong inhibition of the viral infectivity. Pretreatment of chimeric SHIV-RT virions with NERT-stimulating cocktail and select antiretrovirals also resulted in virion inactivation and inhibition of viral infectivity in T cell lines. Our findings demonstrate the potential clinical utility of approaches based on inhibiting NERT in sexual transmission of HIV-1, through the development of effective anti-HIV-1 microbicides, such as NRTIs and NNRTIs.
Keywords: HIV-1, SIV, NERT, NRTI, NNRTI, RT, Virucide
Introduction
Reverse transcriptase (RT) represents a virally encoded protein with three enzymatic activities, RNA- and DNA-dependent DNA polymerase and RNase H (Baltimore, 1970; Coffin, 1990; Temin and Mizutani, 1970). Previous studies have shown that these activities can be measured either within virions made permeable by low levels of detergent or amphipathic peptides, such as melittin, to allow access of deoxyribonucleoside triphosphates (dNTPs) (Boone and Skalka, 1981a, 1981b; Garapin et al., 1970; Gilboa et al., 1979) or by complete disruption of the virions with high concentrations of nonionic detergents (Poiesz et al., 1980), representing endogenous and exogenous RT activity, respectively. Endogenous RT (ERT) reactions have been a key in the evaluation of the kinetics and molecular intermediates of reverse transcription (Boone and Skalka, 1981a, 1981b; Borroto-Esoda and Boone, 1991; Gilboa et al., 1979). Early studies have demonstrated that nonionic detergents are helpful in permeabilizing retroviral virions to allow near full-length viral DNA synthesis (Gilboa, 1979; Yong et al., 1990). Nevertheless, endogenous RT activity was also noted in nonpermeabilized HIV-1 virions, by several groups, including ours (Yong et al., 1990; Borroto-Esoda and Boone, 1991; Debyser et al., 1992; Zhang et al., 1993; Zhang et al., 1996b, 1996c). We and others have demonstrated that in a detergent-free system, addition of dNTPs to isolated HIV-1 virions could stimulate RT activity, leading to higher levels of virions carrying strong stop negative strand moieties and more complete negative strand intermediates (Borroto-Esoda and Boone, 1991; Zhang et al., 1996b). The intravirion reverse transcripts could be detected in HIV-1 virions, not just in vitro but also in vivo (Zhang et al., 1994, 1996a). The efficiency of intravirion reverse transcription in HIV-1 virions is augmented by certain physiologic substances such as polyamines (e.g., spermine and spermidine). It is notable that polyamines reach very high concentrations in seminal fluids. HIV-1 virions in seminal plasma were found to harbor dramatically higher levels of full-length or nearly full-length reverse transcripts, as compared with virions isolated from the peripheral blood of HIV-1-seropositive men (Zhang et al., 1996b). HIV-1 virions, which have processed a certain amount of ERT, would have a kinetic advantage to infect initially resting CD4 T cells and macrophages (Zhang et al., 1996b; Dornadula et al., 1999). As intravirion reverse transcription is a biochemically active process that can take place without nonphysiological virion permeabilization, it has been entitled “natural endogenous reverse transcription (NERT)” (Zhang et al., 1996b, 1998).
We have further demonstrated that the amphipathic domains at the C-terminus of HIV-1 gp41 are able to make the viral envelope naturally permeable to dNTPs, which underlines the molecular mechanism of NERT phenomenon (Zhang et al., 1996c). Deletion of these amphipathic domains can eliminate the exogenous dNTP-driven intravirion reverse transcription (Zhang et al., 1996c). It has been demonstrated that the peptides derived from the amphipathic domains at the C-terminus of gp41 [named as lentiviral lytic peptide (LLP)] are able to lyse either prokaryotic or eukaryotic cells (Miller et al., 1991; Miller et al., 1993). These peptides can also form a pore in planar phospholipid bilayer membrane (Chernomordik et al., 1994). We have found that the synthesized LLP, like melittin, can allow dNTPs to pass through viral envelope which lacks the LLP domain (Zhang et al., 1996c).
At this stage in AIDS pandemic, prevention of sexual transmission of HIV-1 is of highest priority. Many investigators have attempted to develop various virucides to inactivate the cell-free virions and therefore prevent the sexual transmission of HIV-1 (Balzarini et al., 1998; Fauci, 1993; Fichorova et al., 2005; van Damme and Rosenberg, 1999). As the viral envelope is naturally permeable to triphosphates of nucleosides and polyamines in human seminal plasma can significantly enhance the NERT activity such that a large amount of reverse transcripts can be found within cell-free HIV-1 virions in seminal fluids, we propose that intravirion reverse transcription is an ideal target for inactivating the virions in seminal fluids. We have now extended our previous studies (Dornadula et al., 1997, 1999; Zhang et al., 1993, 1994, 1996a, 1996b, 1996c, 1998) and herein present recent findings on inhibiting NERT by means of select agents and its potential usage to prevent sexual transmission of human and nonhuman primate lentiviruses.
Results
As HIV-1 virions are naturally permeable to triphosphates of nucleosides (e.g., dNTPs), we hypothesized that triphosphates of chain terminators could also pass through the viral envelope. We first conducted experiments to test the effect of the NRTI [e.g., 3′-azido-3′-deoxythymidine 5′-triphosphate (AZT-TP)] or NNRTI (e.g., nevirapine) on inhibiting NERT. As shown in Fig. 1, pretreatment of virions (200 ng of HIV-1 p24 equivalent) with 4 μM of the NNRTI, nevirapine, led to decreased NERT, as compared to NERT-stimulated controls (virions pretreated with polyamines and dNTPs at physiological concentration in seminal fluids) (Zhang et al., 1996b) by analysis of intravirion DNA, but somewhat less than AZT-TP (approximately 90% versus 95% decrease in gag copy numbers by phosphor-imager analyses). As expected, pretreatment of virions with AZT, which was used as a negative control in our experiments, did not affect NERT.
Fig. 1.
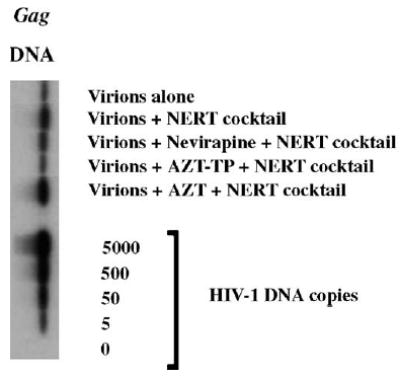
Inhibitory effects of select antiretroviral agents on NERT. Intravirion HIV-1 reverse transcripts were assayed by DNA-PCR. Virions were pretreated with nevirapine, AZT-TP and/or AZT at 4 μM, with or without NERT-stimulating cocktail. After 4 h, the DNA was extracted and subjected to semi-quantitative PCR and Southern blotting analysis. The figure is representative of two independent studies.
We have also analyzed the effects of nevirapine and AZT-TP on inactivating virions in initially quiescent human PBL cultures, which were infected with antiretroviral agent (nevirapine, AZT-TP and/or AZT at 4 μM) pretreated or untreated virions. HIV-1NL4-3 (X4-tropic) virions were pretreated with different concentrations of antiviral reagents, with or without NERT-stimulating cocktail at physiological concentrations in seminal fluids and then used to infect initially quiescent human peripheral blood lymphocytes (PBLs). Both nevirapine and AZT-TP demonstrated a potent inhibitory effect (approximately a 25-fold decrease) on viral infectious titer (Fig. 2a), indicating that these NERT inhibitors indeed function as virucides and effectively inactivate the virions. We have also tested various concentrations of NERT inhibitors upon viral infectivity. As shown in Fig. 2b, both AZT-TP and nevirapine again exerted a dramatic inhibitory effect on viral growth in PBL cultures 12 days post-infection, but only in the presence of the NERT-stimulating cocktail. AZT-TP, at a concentration of 1 μM and in the presence of polyamines/dNTPs, was found to be a more potent inhibitor of NERT, as compared to nevirapine at the same concentration. Both drugs at a concentration of 10 μM virtually ablated viral replication. Our findings demonstrate that polyamines and dNTPs are essential not only for the inhibitory effects of the NRTI, AZT-TP, but also for the NNRTI, nevirapine. Similar results were obtained in testing the inhibitory effects of nevirapine and AZT-TP on NERT in macrophage cultures. HIV-1ADA virions (R5-tropic) were pretreated with different concentrations of drugs in the presence or absence of physiologic NERT stimulators and used to infect macrophages. As illustrated in Fig. 2c, both drugs at a concentration of 10 μM in the presence of a NERT stimulatory cocktail, reduced HIV-1ADA growth by at least 50%, while NERT inhibition was less profound in the absence of polyamines and dNTPs. This finding indicates that R-tropic viruses are also sensitive to NERT inhibitors and is of great significance, as R-tropic viruses play a leading role for sexual transmission (Zhu et al., 1993).
Fig. 2.
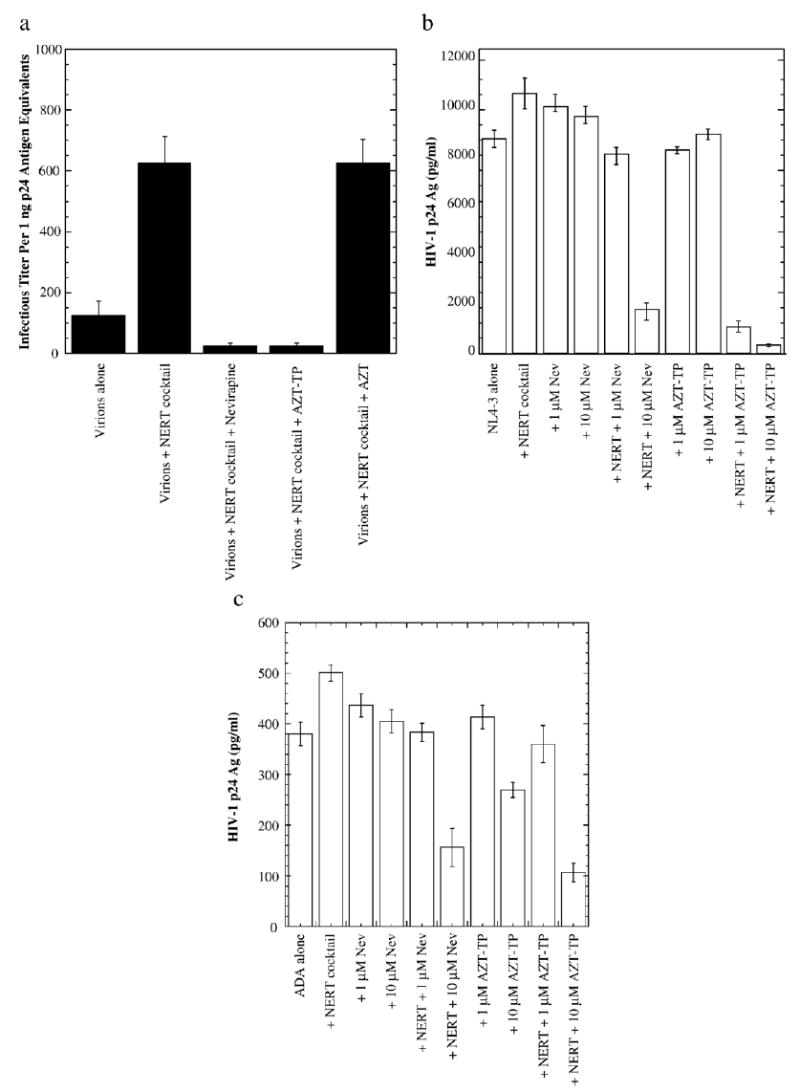
Effects of select antiretroviral agents on NERT and HIV-1 infectivity in human primary cells. (a) Infectivity of HIV-1 virions pretreated with select antiretroviral agents. One nanogram of HIV-1NL4-3 p24 antigen was serially diluted from pretreated virion preparations and used to infect initially quiescent PBLs. Viral infectious titer was assessed via HIV-1 p24 antigen expression (ELISA) at day 28 post-infection. The figure is representative of at least two independent studies. (b) Infectivity of HIV-1 virions pretreated with select antiretroviral agents with or without the NERT-stimulating cocktail. Ten ng of HIV-1NL4-3 p24 antigen equivalents from virion preparations, pretreated (or nontreated) with various concentrations of select antiretroviral agents in the presence or absence of the NERT-stimulating cocktail, were used to infect human PBL cultures. Viral production was assessed via HIV-1 p24 antigen expression (ELISA) by day 12 post-infection. The figure is representative of two independent studies. NL4-3: cells infected with untreated viral preparation only; Nev: nevirapine; NERT: NERT-stimulating cocktail (100 nM dNTPs, 3 mM spermine and 0.1 mM spermidine). (c) Ten nanograms of HIV-1ADA p24 antigen equivalents from virion preparations, pretreated (or nontreated) with various concentrations of select antiretroviral agents in the presence or absence of the NERT-stimulating cocktail, were used to infect human monocyte/macrophage cultures. Viral production was assessed via HIV-1 p24 antigen expression (ELISA) by day 5 post-infection. The figure is representative of two independent studies. ADA: cells infected with untreated HIV-1 only; Nev: nevirapine, NERT: NERT-stimulating cocktail (100 nM dNTPs, 3 mM spermine and 0.1 mM spermidine).
To examine whether dNTPs and/or polyamines (NERT-stimulating cocktail simulating physiological concentration in seminal fluids) might actually stabilize virions in the incubation period during induction of ERT, time-course experiments were conducted. Changes in stability of HIV-1 virions might confound analysis of NERT-altering virion infectivity. To explore this issue, certain HIV-1NL4-3 virion preparations were not treated with dNTPs and/or polyamines but incubated for different time periods (0 to 16 h) at 37 °C in serum-negative, RPMI-1640 media (i.e., same conditions as during stimulation of NERT). No decreases in infectivity of virion preparations were demonstrated with up to 16 h of incubation, which is the limit used in NERT stimulation (after 16 h the virion infectivity began to slowly deteriorate). A modest increase in virion infectivity at 4 to 8 h of incubation may be secondary to low levels of baseline NERT occurring during incubation or maturation of HIV-1 virions (Fig. 3a). These data suggest that decreasing virion stability during NERT stimulation conditions, which could be altered by polyamines (spermine and spermidine) and/or dNTPs at physiological concentration in seminal fluids, does not take place. In addition, it was theoretically possible that super-physiological stabilization of virions might be possibly induced by polyamines and/or dNTPs at 37 °C. As such, time-courses (i.e., different incubation times; 0 to 16 h) of HIV-1NL4-3 virions treated with physiological concentrations (as above) of polyamines and dNTPs at 37 °C were also conducted. There was no difference in infectivity in treated and untreated virion preparations at the 0–h time point (i.e., prompt isolation of virions after treatment), and only minor differences at 1 h (Fig. 3a). Only at 4 h post-incubation, the activity of NERT-cocktail-pretreated virions was significantly increased. These data argue against super-physiologically increased stabilization of virions by polyamines/dNTPs, as compared to the “normal” stability of HIV-1 virions. As well, these data also point against at least gross binding to virion surfaces and carry-over of RT-stimulating reagents into target cells.
Fig. 3.
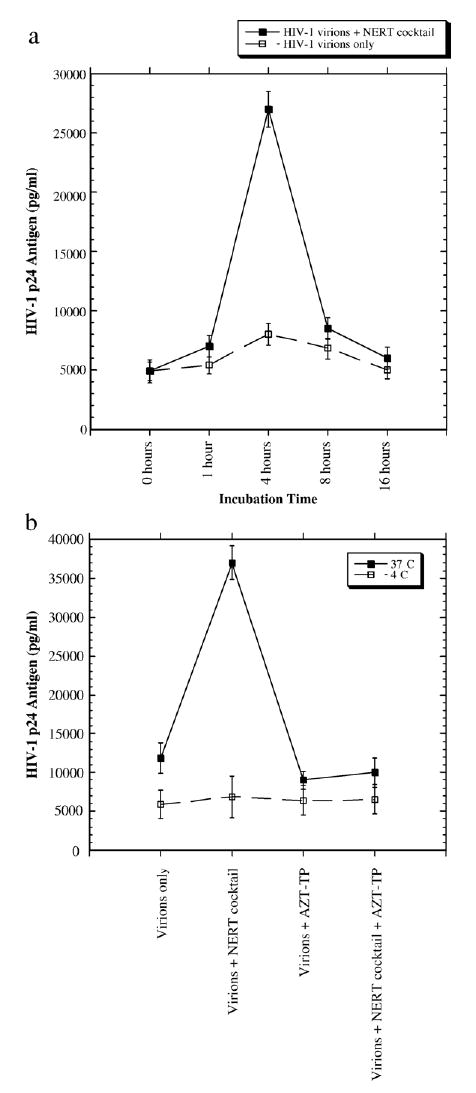
Time-course analysis of the effects of a NERT-stimulating cocktail on HIV-1 stability and NERT-altering agents on HIV-1 infectivity at different temperature conditions. (a) HIV-1NL4-3 virion preparations (1 ng of HIV-1 p24 antigen equivalents) were either pretreated with the NERT-stimulating cocktail, or left untreated. Subsequently, the virion preparations were incubated in RPMI-1640 medium at 37 °C for different time periods. Infectivity was assessed on initially quiescent PBLs, via HIV-1 p24 antigen expression on day 10 post-infection. The 0 h represents prompt re-isolation of virions after mixing with media containing, or not containing the NERT-stimulating cocktail. (b) HIV-1NL4-3 virion preparations (4 ng of HIV-1 p24 antigen equivalents) were pretreated with 4 μM of AZT-TP in the presence or absence of the NERT-stimulating cocktail (100 nM dNTPs, 3 mM spermine and 0.1 mM spermidine) for 15 h at different temperature conditions (37 °C and 4 °C). After re-isolation by ultracentrifugation, the virion preparations were used to infect initially quiescent human PBLs. Viral production was assessed via HIV-1 p24 antigen expression (ELISA) by day 10 post-infection.
To further evaluate whether dNTPs and/or polyamines might be “carried-over” into target cells, either intravirion or bound to virions, during induction of ERT and stimulate intracellular RT, accounting for increased virion infectivity, functional temperature experiments were performed. Treatment of HIV-1 virions with dNTPs/polyamines was conducted at 4 °C and 37 °C. As RT does not process at 4 °C, any change in infectivity on target cells would be due to intracellular RT induction from “carry-over” of dNTPs/polyamines. In these studies, HIV-1NL4-3 virions were treated with a NERT-stimulating cocktail and inhibiting agents (AZT-TP at 4 μM), and maintained at 4 °C and 37 °C for 15 h of incubation. As shown in Fig. 3b, there was no change in virion infectivity on initially quiescent human PBMCs as target cells, when virions were treated and incubated at 4 °C.
As the simian immunodeficiency virus (SIV) RT is not inhibited by most NNRTIs, yet these may be critical moieties as molecular virucides against HIV-1, we also utilized an SHIV-RT construct (Uberla et al., 1995), in initial in vitro studies. SHIV-RT is a key construct in use within many macaque model systems of lentiviral transmission and pathogenesis (Balzarini et al., 1997; Mori et al., 2000; Rosenwirth et al., 1999; Uberla et al., 1995). By utilizing SIV mac251 and SHIV-RT mac239env on CEM-174 target cells to determine the viral infectious titer, we conducted similar studies to those performed with HIV-1 virions (Fig. 2a). SIV mac251 virions treated with 4 μM of nevirapine showed no dramatic decrease in viral infectivity, unlike that observed with 4 μM of AZT-TP with dNTPs/polyamines (Table 1). This might be anticipated as NNRTIs have little effect on the SIV RT. As expected, no inhibition was observed on virions pretreated with AZT, and/or AZT with NERT-stimulating cocktail, which served as negative controls. Next, the SHIV-RT virions were evaluated. As hypothesized, by utilizing a SHIV-RT construct (i.e., chimeric with an HIV-1 RT gene), the virions’ infectivity was now sensitive to treatment with nevirapine (at least a five-fold dilution decrease) (Table 2). No differences were observed on virions pretreated with 4 μM of nevirapine, as compared to virions treated with 4 μM of nevirapine in the presence of NERT stimulators. Once again, no inhibition was observed with virions pretreated with AZT, and/or AZT with the NERT-stimulating cocktail.
Table 1.
Effects of NERT-altering agents on SIV: viral infectivity assay upon CEM-174 cells—SIVMAC251 (shown: Day 28 post-infection)
| SIVMAC251 viral input—dilutions (25 ng of SIV-1 p27 antigen)
|
||||||
|---|---|---|---|---|---|---|
| Treatment | 1:1 | 1:5 | 1:25 | 1:125 | 1:625 | 1:3125 |
| Virions only | ++++ | ++++ | +++ | − | − | − |
| Virions+NERT cocktail | ++++ | ++++ | ++++ | − | − | − |
| Virions+nevirapine | ++++ | ++++ | + | − | − | − |
| Virions+NERT cocktail+nevirapine | ++++ | ++++ | + | − | − | − |
| Virions+AZT-TP | ++++ | ++++ | ++ | − | − | − |
| Virions+NERT cocktail+AZT-TP | ++++ | +++ | − | − | − | − |
| Virions+AZT | ++++ | ++++ | + | − | − | − |
| Virions+NERT cocktail+AZT | ++++ | ++++ | +++ | − | − | − |
−: <125 pg/ml; +: 125 to 250 pg/ml; ++: 250 to 500 pg/ml; +++: 500 to 1000 pg/ml; ++++: >1 ng/ml p27 antigen units.
Four μM concentrations of nevirapine, AZT-TP and AZT were used.
NERT-stimulating cocktail: 100 nM of dNTPs+3 mM spermine+0.1 mM spermidine.
Table 2.
Effects of NERT-altering agents on SHIV-RT: viral infectivity assay upon CEM-174 cells—SHIV-RT (shown: Day 28 post-infection)
| SHIV-RT/SIV-mac239env viral input—dilutions (25 ng of SIV-1 p27 antigen)
|
||||||
|---|---|---|---|---|---|---|
| Treatment | 1:1 | 1:5 | 1:25 | 1:125 | 1:625 | 1:3125 |
| Virions only | ++++ | ++++ | +++ | + | − | − |
| Virions+NERT cocktail | ++++ | ++++ | +++ | +++ | − | − |
| Virions+nevirapine | ++++ | ++++ | + | − | − | − |
| Virions+NERT cocktail+nevirapine | ++++ | ++++ | + | − | − | − |
| Virions+AZT-TP | ++++ | ++++ | +++ | + | − | − |
| Virions+NERT cocktail+AZT-TP | ++++ | ++++ | + | − | − | − |
| Virions+AZT | ++++ | ++++ | +++ | + | − | − |
| Virions+NERT cocktail+ZT | ++++ | ++++ | +++ | ++ | − | − |
−: <125 pg/ml; +: 125 to 250 pg/ml; ++: 250 to 500 pg/ml; +++: 500 to 1000 pg/ml; ++++: >1 ng/ml p27 antigen units.
Four μM concentrations of nevirapine, AZT-TP and AZT were used.
NERT cocktail: 100 nM of dNTPs+3 mM spermine+0.1 mM spermidine.
Discussion
The molecular mechanisms that facilitate HIV-1 sexual transmission have been incompletely characterized. As such, analyses of the viral life-cycle in seminal and cervical/vaginal secretions are of critical importance. One stage of the lentiviral life-cycle, which may be altered by specific extracellular milieus, is intravirion reverse transcription (Zhang et al., 1993, 1996b). Determining the effects of NERT of primate lentiviruses on viral transmission promises to assist in the rational design of lentivirucides. In this report, we have further extended our studies, and herein, we analyzed in vitro potential molecular virucide agents towards primate lentiviruses by targeting ERT. We have examined HIV-1, SIV, and SHIV-RT with respect to NERT inhibition as well as inhibiting viral infectivity by select antiretroviral “virucidal” agents on human primary cells or CEM-174 cell lines. Potential lentivirucides included the triphosphorylated derivative NRTI, AZT-TP, as well as the NNRTI, nevirapine. We have also examined the effects of NERT stimulators on NERT and NERT inhibition by AZT-TP and nevirapine. It is not surprising that AZT-TP has stronger inhibitory effect upon viral infectivity when the NERT is initiated. As AZT-TP functions as a chain terminator, it would have higher chance to incorporate into the extending nascent DNA chain during NERT process. In the presence of NERT stimulators, the RT enzyme will start the DNA polymerization in a very short time. The extending DNA chain, no matter how short it is, will be the target for the chain terminator AZT-TP. It is interesting that nevirapine also has stronger inhibitory effect upon viral infectivity after NERT is initiated. This phenomenon could be due to that NERT process requires an active form of RT enzyme, which could facilitate a tighter interaction between nevirapine and RT enzyme. We would like to emphasize that, the NERT stimulators, which contain dNTPs at 100 nM or polyamines (spermine at 3 mM, and spermidine at 0.1 mM), have naturally existed in seminal fluids of HIV-1-seronegative men (Zhang et al., 1996b). If these virucides identified in this report are used in clinic, NERT cocktail used in this study is not necessary to be simultaneously administrated, as seminal fluids have naturally harbored these reagents. Conversely, since most NNRTIs cannot be utilized against SIV NERT, as they rather specifically inhibit HIV-1 RT, we employed a SHIV-RT construct that was used to infect CEM-174 cells (Balzarini et al., 1997; Uberla et al., 1995). Our findings demonstrate that both NRTIs and NNRTIs have strong potentials to function as virucides, in developing future molecular approaches to inhibit NERT and its potential role in sexual transmission. The design and development of novel RT inhibitors and studying their effects on intravirion reverse transcription are thus of importance for future clinical approaches.
We have also conducted time-course experiments to analyze whether NERT stimulants at physiological concentration in seminal fluids might actually stabilize HIV-1 virions in the incubation period during induction of ERT, since changes in virion stability might confound analysis of NERT-altering virion infectivity. As shown in Fig. 3, we did not observe any decrease in infectivity of virion preparations for up to 16 h of incubation. A modest increase observed in virion infectivity at 4 to 8 h of incubation, may be secondary to low levels of baseline NERT occurring during incubation. Therefore, our findings do not support decreasing virion stability during NERT stimulation conditions, which could be altered by polyamines and dNTPs. In addition, our findings point against super-physiologically increased stabilization of virions by polyamines and dNTPs, as compared to the “normal” stability of HIV-1 virions. Furthermore, these data also complement our “temperature-dependent” studies and strongly suggest against gross binding to HIV-1 envelope and carryover of NERT stimulants into target cells. Thus, throughout our past and present studies on intravirion reverse transcription, we have continuously attempted to evaluate alternate potential hypotheses, which would mitigate against NERT stimulation or inhibition leading directly to altered virion infectivity (Dornadula et al., 1997, 1999; Zhang et al., 1996a, 1996b, 1996c, 1998).
We have already shown that purified SIV virions also contain virus-specific DNA, which is a result of partial reverse transcription. Further, it was demonstrated that viral DNA synthesis can be initiated in SIV virions in the presence of polyamines and dNTPs at physiological concentrations (Dornadula et al., 1997). The viral infectivity upon initially quiescent cells was significantly increased, when levels of intravirion reverse transcripts were modulated, suggesting that NERT of SIV may play an important role for SIV pathogenesis and transmission. This is of importance since, in contrast to HIV-1, these phenomena may be further directly investigated by in vivo model systems (Miller et al., 1994, 1998). In our current in vitro studies, we utilized a SHIV-RT construct, which represents a key approach for use within certain macaque model systems. Similarly to our HIV-1 studies, we analyzed the effects NERT-altering agents in SIVmac251 and SHIV-RT on CEM-174 target cells. Consistently, we found that AZT-TP with NERT stimulant pretreatment of SIVmac251 virions results in dramatic decreases of viral infectivity, while utilization of the chimeric SHIV-RT lead to inhibition of SIV infectivity upon pretreatment with the NNRTI, nevirapine.
In summary, our studies demonstrate that intravirion reverse transcription may alter molecular mechanisms underlying efficient transmission of human and nonhuman primate lentiviruses. Inhibiting intravirion reverse transcription represents an important virucidal approach, as virions are made “nonviable”, and significantly less infectious after treatment. Our studies demonstrate the strong potential clinical utility of approaches based on inhibiting sexual transmission of HIV-1, on a molecular level with virion-specific agents. As such, these may lead to the development of prophylactic therapeutics to alter both heterosexual and homosexual transmission of HIV-1 and possibly in altering vertical transmission of HIV-1 from mother to infant perinatally. The development of effective antilentiviral virucides may be one of the most important potential development tasks, prior to a vaccine, for stemming the HIV-1 pandemic in the developing world.
Materials and methods
Reagents
AZT was obtained from Sigma, AZT-TP from Moravek Biochemicals (CA), and nevirapine was obtained from the NIH AIDS Research and Reference Reagent program.
Human peripheral blood samples
All blood samples were obtained from healthy HIV-1-seronegative normal donors from the Hospital Blood Bank at Thomas Jefferson University, via approved protocols.
Viral stocks
X4-tropic HIV-1 strain NL4-3, and the R5-tropic strain ADA were produced by transfection of 293T cells with the respective proviral DNA, as described previously (Argyris et al., 2003; Zhang et al., 2000) The SIVmac251 virus was kindly provided by Dr. C. J. Miller at the Primate Research Center, University of California at Davis (Miller et al., 1994, 1998) and the SHIV-RT (genomic backbone SIVmac239 with the RT gene of HIV-1) was obtained from Dr. Joseph Sodroski (Dana-Farber Cancer Institute) (Balzarini et al., 1997; Letvin et al., 1995; Uberla et al., 1995).
Quantitation of intravirion HIV-1 DNA by PCR
Intravirion HIV-1 reverse transcripts were quantitated by DNA-PCR, as described previously (Zhang et al., 1996a, 1996b, 1996c; Dornadula et al., 1997, 1999). Briefly, HIV-1NL4-3 virions (200 ng of HIV-1 p24 antigen equivalents) were pretreated with select antiretroviral agents at concentrations of 4 μM, with or without a NERT-stimulating cocktail consisting of 100 nM dNTPs and polyamines (3 mM spermine and 0.1 mM spermidine). After incubating at 37 °C for 4 h, DNA was extracted with a “quick lysis” methodology and quantitative PCR was performed.
Viral infectivity assays
Viral infectivity assays were performed to determine the influence of physiological substances upon intravirion HIV-1 and SIV reverse transcription and lentiviral replication, as well as the effects of select NRTIs and NNRTIs on NERT (Zhang et al., 1996a, 1996b, 1996c). Infectivity was measured in CEM-174 cell cultures, peripheral blood mononuclear cells (PBMCs), or monocyte/macrophages respectively.
CEM-174 cell cultures
SIVmac251 and SHIV-RT (genomic backbone SIVmac239) were pretreated with select antiretroviral agents at concentrations of 4 μM, with or without NERT-stimulating cocktail as described previously (Dornadula et al., 1997) and further purified via ultracentrifugation. The infectivity of each viral preparation was analyzed utilizing CEM-174 cells, via increasing dilutions of a set viral input (25 ng of p27 antigen). After 28 days, p27 antigen expression was quantitated via ELISA, as described previously (Dornadula et al., 1997).
PBLs
HIV-1NL4-3 virions in the fresh supernatants of virus-producing cells (CEM cells) were pretreated with various reagents and purified with ultracentrifugation to remove the reagents, as described previously (Zhang et al., 1996a, 1996b, 1996c). The virions (10 ng of HIV-1 p24 antigen equivalents) were then resuspended in RPMI-1640 medium and allowed to infect 4×106 quiescent PBL (and/or PBLs) from an HIV-1-seronegative person at 37 °C for 4 h. The unbound viruses were washed off, via three vigorous washes with PBS. The infected cells (2×06) were then cultured, in duplicate, in 2 ml of RPMI-1640 medium plus 10% fetal calf serum (FCS). After overnight incubation, the supernatants (0.5 ml) were collected for HIV-1 p24 antigen detection on day 0. Forty-eight hours post-infection, PHA (5 μg/ml; Sigma) and natural human IL-2 (50 U/ml; BRL-GIBCO) were added to the cultures. On the fifth day post-infection, PHA and IL-2 were washed off by pelleting the infected cells via centrifugation. The cells were then cultured in 2 ml of RPMI-1640 medium plus 20% FCS with IL-2 (10 U/ml). Samples (0.5 ml) of the supernatants were collected at specified time points. The HIV-1 p24 antigen levels were quantitated by ELISA (kits from Dupont).
Monocyte/Macrophages
Isolation of monocytes/macrophages was performed by a previously described protocol (Collman et al., 1989; Zhang et al., 1996b). Briefly, Ficoll discontinuous gradient-purified peripheral blood mononuclear cells were placed onto 2% gelatin-coated flasks to allow attachment of monocyte/macrophages. Nonadherent cells were vigorously washed off. The monocyte/macrophages (4×105 cells per well) were then cultured in a mixture of 10% FCS, 10% horse serum, 0.1 mM nonessential amino acids (NEAA) (GIBCO), 10 U of granulocyte–macrophage colony-stimulating factor, GM-CSF, per ml (Sigma), and 10 U of macrophage colony-stimulating factor, M-CSF per ml (Sigma), for 7 days. HIV-1ADA virions were preincubated with various reagents and purified as above. The virions (10 ng of HIV-1 p24 antigen equivalents) were resuspended in Dulbecco’s modified Eagle’ medium (DMEM) and allowed to infect 4×105 monocyte/macrophages at 37 °C for 16 h. The unbound viruses were washed off, via three vigorous washes with DMEM. The infected cells were then cultured, in duplicate, in 2 ml of DMEM plus 10% FCS, 10% horse serum, and 0.1 mM NEAA. After overnight incubation, the supernatants (0.5 ml) were collected for HIV-1 p24 antigen detection on day 0. The cells were then cultured in 2 ml of DMEM plus 10% FCS, 10% horse serum, and 0.1 mM NEAA. Samples (0.5 ml each) of the supernatants were collected at specified time points. The HIV-1 p24 antigen levels were quantitated by ELISA.
Statistical analyses
Paired Student’s t tests were utilized to compare the differences between samples treated with different viral preparations. All tests were two-tailed. A P value of 0.05 or less was considered statistically significant.
Acknowledgments
We thank Dr. C. J. Miller at the Primate Research Center, University of California at Davis, for supplying SIVmac251 viruses and Dr. Joseph Sodroski at Dana-Farber Cancer Institute for supplying SHIV-RT. This work was supported in part by US PHS grant AI52732 to H.Z.
References
- Argyris EG, Acheampong E, Nunnari G, Mukhtar M, Williams KJ, Pomerantz RJ. Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts. J Virol. 2003;77:12140–12151. doi: 10.1128/JVI.77.22.12140-12151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Viral RNA-dependent DNA polymerase. Nature. 1970;226:1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Balzarini J, DeClercq E, Uberla K. SIV/HIV-1 hybrid virus expressing the reverse transcriptase gene of HIV-1 remains sensitive to HIV-1-specific reverse transcriptase inhibitors after passage in rhesus macaques. J AIDS. 1997;15:1–4. doi: 10.1097/00042560-199705010-00001. [DOI] [PubMed] [Google Scholar]
- Balzarini J, Naesens L, Verbeken E, Laga M, Van Damme L, Parniak M, Van Mellaert L, Anne J, De Clercq E. Preclinical studies on thiocarboxanilide UC-781 as a virucidal agent. AIDS. 1998;12:1129–1138. doi: 10.1097/00002030-199810000-00004. [DOI] [PubMed] [Google Scholar]
- Boone LR, Skalka AM. Viral DNA synthesized in vitro by avian retrovirus particles permeabilized with mellittin. J Virol. 1981a;37:109–116. doi: 10.1128/jvi.37.1.109-116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone LR, Skalka AM. Viral DNA synthesized in vitro by avian retrovirus particles permeabilized with melittin. J Virol. 1981b;37:117–126. doi: 10.1128/jvi.37.1.117-126.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroto-Esoda K, Boone LR. Equine infectious anemia virus and HIV DNA synthesis in vitro: characterization of the endogenous reverse transcriptase reaction. J Virol. 1991;65:1952–1959. doi: 10.1128/jvi.65.4.1952-1959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L, Chanturiya AN, Suss-Toby E, Nora E, Zimmerberg J. An amphipathic peptide from the C-terminal region of the human immunodeficiency virus envelope glycoprotein causes pore formation in membranes. J Virol. 1994;68:7115–7123. doi: 10.1128/jvi.68.11.7115-7123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin JM. Retroviridae and their replication. In: Fields BN, editor. Virology. Raven Press Ltd; New York: 1990. pp. 1437–1499. [Google Scholar]
- Collman R, Hassan NF, Walker R, Godfrey B, Cutilli J, Hastings JC, Friedman H, Douglas SD, Nathanson N. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med. 1989;170:1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debyser Z, Vandamme AM, Pauwels R, Baba M, Desmyter J, DeClercq E. Kinetics of inhibition of endogenous human immunodeficiency virus type 1 reverse transcription by 2,3-dideoxynucleoside 5-triphosphate, tetrahydroimidazo-[4,5,1-jk][1,4]-benzodiazepin-2(1H)-thione, and 1-[(2-hydroxyethoxy) methyl]-6-(phenylthio) thymine derivatives. J Biol Chem. 1992;267:11769–11776. [PubMed] [Google Scholar]
- Dornadula G, Zhang H, Bagasra O, Pomerantz RJ. Natural endogenous reverse transcription of simian immunodeficiency virus. Virology. 1997;227:260–267. doi: 10.1006/viro.1996.8317. [DOI] [PubMed] [Google Scholar]
- Dornadula G, Zhang H, Shetty S, Pomerantz RJ. HIV-1 virions produced from replicating peripheral blood lymphocytes are more infectious than those from nonproliferating macrophages due to higher levels of intravirion reverse transcripts: Implications for pathogenesis and transmission. Virology. 1999;253:10–16. doi: 10.1006/viro.1998.9465. [DOI] [PubMed] [Google Scholar]
- Fauci AS. Multifactorial nature of HIV disease: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Zhou F, Ratnam V, Atanassova V, Jiang S, Strick N, Neurath AR. Anti-human immunodeficiency virus type 1 microbicide cellulose acetate 1,2-benzenedicarboxylate in a human in vitro model of vaginal inflammation. Antimicrob Agents Chemother. 2005;49:323–335. doi: 10.1128/AAC.49.1.323-335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapin A, McDonnell JP, Levinson W, Quintrell N, Fanshier L, Bishop JM. Deoxyribonucleic acid polymerases associated with Rous sarcoma virus and avian myeloblastosis: properties of the enzyme and its product. J Virol. 1970;6:589–598. doi: 10.1128/jvi.6.5.589-598.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E, Mitra SW, Goff S, Baltimore D. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979;18:93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Letvin NL, Li J, Halloran M, Cranage MP, Rud EW, Sodroski J. Prior infection with a nonpathogenic chimeric simian–human immunodeficiency virus does not efficiently protect macaques against challenge with simian immunodeficiency virus. J Virol. 1995;69:4569–4571. doi: 10.1128/jvi.69.7.4569-4571.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Garry RF, Jaynes JM, Montelaro RC. A structural correlation between lentivirus transmembrane proteins and natural cytolytic peptides. AIDS Res Hum Retroviruses. 1991;7:511–519. doi: 10.1089/aid.1991.7.511. [DOI] [PubMed] [Google Scholar]
- Miller MA, Cloyd MW, Liebmann J, Rinaldo CR, Jr, Islam KR, Wang SZ, Mietzner TA, Montelaro RC. Alterations in cell membrane permeability by the lentivirus lytic peptide (LLP-1) of HIV-1 transmembrane protein. Virology. 1993;196:89–100. doi: 10.1006/viro.1993.1457. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Marthas M, Torten J, Alexander NJ, Moore JP, Doncel GF, Hendrickx AG. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J Virol. 1994;68:6391–6400. doi: 10.1128/jvi.68.10.6391-6400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Marthas M, Greenier J, Lu D, Dailey PJ, Lu Y. In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J Virol. 1998;72:3248–3258. doi: 10.1128/jvi.72.4.3248-3258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Yasutomi Y, Sawada S, Villinger F, Sugama K, Rosenwith B, Heeney JL, Uberla K, Yamazaki S, Ansari AA, Rubsamen-Waigmann H. Suppression of acute viremia by short-term post exposure prophylaxis of simian/human immunodeficiency virus SHIV/RT-infected monkeys with a novel reverse tanscriptase inhibitor ( GW420867) allows for development of potent antiviral immune responses resulting in efficient containment of infection. J Virol. 2000;74:5747–5753. doi: 10.1128/jvi.74.13.5747-5753.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwirth B, Bogers WM, Nieuwenhuis IG, Haaft PT, Niphuis H, Kuhn EM, Bischofberger N, Erfle V, Sutter G, Berglund P, Liljestrom P, Uberla K, Heeney JL. An anti-HIV strategy combining chemotherapy and therapeutic vaccination. J Med Primatol. 1999;28:195–205. doi: 10.1111/j.1600-0684.1999.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Temin HM, Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970;226:1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Uberla K, Stahl-Hennig C, Bottiger D, Matz-Rensing K, Kaup FJ, Li J, Haseltine WA, Fleckenstein B, Hunsmann G, Oberg B, et al. Animal model for the therapy of acquired immunodeficiency syndrome with reverse transcriptase inhibitors. Proc Natl Acad Sci U S A. 1995;92:8210–8214. doi: 10.1073/pnas.92.18.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Damme L, Rosenberg ZF. Microbicides and barrier methods in HIV prevention. AIDS. 1999;13:S85–S92. [PubMed] [Google Scholar]
- Yong WH, Wyman S, Levy JA. Optimal conditions for synthesizing complementary DNA in the HIV-1 endogenous reverse transcriptase reaction. AIDS. 1990;4:199–206. doi: 10.1097/00002030-199003000-00004. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang Y, Spicer TP, Abbott LZ, Abbott M, Poiesz BJ. Reverse transcription takes place within extracellular HIV-1 virions: potential biological significance. AIDS Res Hum Retroviruses. 1993;9:1287–1296. doi: 10.1089/aid.1993.9.1287. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bagasra O, Niikura M, Poiesz BJ, Pomerantz RJ. Intravirion reverse transcripts in the peripheral blood plasma of human immunodeficiency virus type 1 infected individuals. J Virol. 1994;68:7591–7597. doi: 10.1128/jvi.68.11.7591-7597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Dornadula G, Wu Y, Havlir D, Richman DD, Pomerantz RJ. Kinetic analysis of intravirion reverse transcription in the blood plasma of human immunodeficiency virus type 1-infected individuals: Direct assessment of resistance to reverse transcriptase inhibitors in vivo. J Virol. 1996a;70:628–634. doi: 10.1128/jvi.70.1.628-634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Dornadula G, Pomerantz RJ. Endogenous reverse transcription of human immunodeficiency virus type 1 in physiological microenvironinents: an important stage for viral infection of non-dividing cells. J Virol. 1996b;70:2809–2824. doi: 10.1128/jvi.70.5.2809-2824.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Dornadula G, Alur P, Laughlin MA, Pomerantz RJ. Amphipathic domains in the C terminus of the transmembrane protein (gp4l) permeabilize HIV-1 virions: a molecular mechanism underlying natural endogenous reverse transcription. Proc Natl Acad Sci U S A. 1996c;93:12519–12524. doi: 10.1073/pnas.93.22.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Dornadula G, Pomerantz RJ. Natural endogenous reverse transcription of HIV type 1. AIDS Res Hum Retroviruses, Suppl. 1998;1:93–95. [PubMed] [Google Scholar]
- Zhang H, Pomerantz RJ, Dornadula G, Sun Y. HIV-1 Vif protein is an integral component of an mRNP complex of viral RNA and could be involved in the viral RNA folding and packaging process. J Virol. 2000;74:8252–8261. doi: 10.1128/jvi.74.18.8252-8261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Mo H, Wang N, Nam DS, Cao Y, Koup RA, Ho DD. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]


