Abstract
In recent years, the concept of self-organization has been used to understand collective behaviour of animals. The central tenet of self-organization is that simple repeated interactions between individuals can produce complex adaptive patterns at the level of the group. Inspiration comes from patterns seen in physical systems, such as spiralling chemical waves, which arise without complexity at the level of the individual units of which the system is composed. The suggestion is that biological structures such as termite mounds, ant trail networks and even human crowds can be explained in terms of repeated interactions between the animals and their environment, without invoking individual complexity. Here, I review cases in which the self-organization approach has been successful in explaining collective behaviour of animal groups and societies. Ant pheromone trail networks, aggregation of cockroaches, the applause of opera audiences and the migration of fish schools have all been accurately described in terms of individuals following simple sets of rules. Unlike the simple units composing physical systems, however, animals are themselves complex entities, and other examples of collective behaviour, such as honey bee foraging with its myriad of dance signals and behavioural cues, cannot be fully understood in terms of simple individuals alone. I argue that the key to understanding collective behaviour lies in identifying the principles of the behavioural algorithms followed by individual animals and of how information flows between the animals. These principles, such as positive feedback, response thresholds and individual integrity, are repeatedly observed in very different animal societies. The future of collective behaviour research lies in classifying these principles, establishing the properties they produce at a group level and asking why they have evolved in so many different and distinct natural systems. Ultimately, this research could inform not only our understanding of animal societies, but also the principles by which we organize our own society.
Keywords: self-organization, animal groups, societies, collective behaviour
1. Introduction
I am sitting at my office window watching the students come out of the lecture theatre. They queue to buy coffee. When served, they leave in groups of three or four and edge towards the café's tables, looking nervously at each others faces to ascertain some weak preference for a seat in the sun or shade. Once the unspoken decision is made they sit down quickly, sip their coffee and begin to relax, the morning's lectures behind them. Faces light up and their conversation turns in directions I cannot possibly imagine.
For those five or so minutes after the lecture the students' behaviour is almost entirely predictable. The picture I have painted is not simply something I see every morning, but is familiar to university campuses all over the world. Different personal histories and drink preference aside, the pattern of waiting our turn, making joint, unspoken decisions about where to sit and relaxing together after a period of concentration is familiar to us all. Every day we see examples of groups of autonomous individuals behaving collectively in a manner that can be described in a few simple words.
Although familiar to us all, a few students standing in a queue probably qualifies as one of the least spectacular examples of collective behaviour of human or animal groups. It is difficult to know where to start when choosing the most spectacular. A flock of birds twisting in the evening light; a fish school wincing at the thought of a predator; the cram to leave an underground station; ants marching in an endless line; the stop and start traffic jams; the quiet hum of a honey bee hive; the pulsating roar of a football crowd; a swarm of locusts flying across the desert; or even the bureaucracy of the European Union (figure 1). In all these examples, the individual is submerged as the group takes on a life of its own. The individual units do not have a complete picture of their position in the overall structure and the structure they create has a form that extends well beyond that of the individual units.
Figure 1.
Examples of collective animal behaviour. (a) Fish milling (reproduced with permission from Philip Colla, oceanlight.com). (b) The entrance crater to a nest of the ant Messor barbarus (from Theraulaz et al. 2003). (c) Traffic flow in Paris (reproduced with permission from Anthony Atkielski). (d) A bifurcation in a Pharaoh's ant trail (reproduced with permission from Duncan Jackson). (e) A Mexican wave at an American football game (taken from Farkas 2002). (f) A band of marching locusts (reproduced with permission from Iain Couzin).
There is a sense in which all these collective patterns are regular and even predictable. In the hustle and bustle of a busy street we can forget the complex reasons each of us had for shopping on this particular Saturday morning. Brought together to search for a roost, the migrating birds do not reflect on the long day's flight behind them. The submergence of the individual brings a new order to the group. Nevertheless, understanding how coordinated patterns emerge from a mass of interactions between individuals poses a difficult problem. The regularity of collective animal behaviour leaves us feeling that there must be some unifying laws which govern these different phenomena. But, while the line of commuting cars might remind us of a trail of ants, are there deep similarities which connect them? If so, can we determine a set of principles that allow us to classify and understand collective animal behaviour?
It was on the basis that ‘experimental evidence, as well as daily observation, show that systems involving a large number of interacting subunits can present, under certain conditions, a marked coherent behaviour extending well beyond the scale of the individual subunit’ that Nicolis & Prigogine (1977) wrote ‘Self-organization in Nonequilibrium Systems’. Their desire was to go beyond simple analogies and pin down a rigorous theory of these ‘self-organized’ systems. Such a theory should explain how complex structures arise from repeated interactions between the individual units. It should show why certain structures are created and persist and explain the similarities between systems at very different scales and levels of biological organization. For example, could the flow of traffic be described by the mathematics of fluid flow? And if this was the case could we make general statements about the flow of any type of matter, be it swarms of locusts, crowds leaving football grounds or water running down the drain.
Nicolis and Prigogine were partially successful in their enterprise. They showed that many of the mathematical equations used for describing chemical reactions could equally well be considered as models of, for example, predator–prey interactions or the building behaviour of termites. By applying the same mathematical models to very different systems they argued for a formal correspondence, above that of mere analogy, between the modelled systems.
Nicolis and Prigogine were not alone in their belief that many aspects of collective behaviour could be modelled mathematically, and thus direct and useful comparisons drawn between diverse systems. The preceding decades had seen books by Ashby (1947), Wiener (1948) and von Bertalanffy (1968) all of which aimed at providing a framework for the study of collective behaviour. Von Bertalanffy argued for the existence of general growth laws of social entities as diverse as manufacturing companies, urbanization and Napoleon's empire. His approach was ‘empirico-intuitive’, in that he used observations on the growth of organisms and applied the principles to other systems. However, neither von Bertalanffy, Nicolis and Prigogine nor any of the other early pioneers of self-organization experimentally validated the models they proposed for collective phenomena in humans or animals.
There was good reason for the lack of experimental testing of the theories of self-organization. For most collective phenomena involving humans it is simply impossible to perform experiments. How do you do an experiment to test a hypothesized model for the growth of Napoleonic empires? Even for the collective behaviour of animals, such as flocking birds, the collection of field data requires a large number of cameras and sophisticated computer tracking software. However, around the time of Nicolis & Prigogines' book another member of their Brussels group, Jean–Louis Deneubourg, began to develop and test a theory of self-organization in social insect societies. Based on the assumption that insect societies follow simple behavioural rules, Deneubourg's approach was to write down mathematical models of how colonies of ants forage and termites build mounds (Deneubourg 1977; Deneubourg & Goss 1989). By conducting laboratory experiments where the insects were constrained to relatively simple environments, Deneubourg and his colleagues were able to test and, in some cases, validate these models (Camazine et al. 2001).
Similar approaches, in many cases developed independently of the Brussels's school, have been applied to understanding the collective behaviour of fish, cockroaches, humans and other animals. Nicolis and Prigogine's basic observation, that individuals following simple behavioural rules can produce complex behavioural patterns, has not only proved a useful idiom for describing a whole range of collective phenomena; even more importantly, the fact that these simple rules can be encapsulated in mathematical models has meant that clear, testable predictions about the collective behaviour of animal groups and societies can be made. Making and testing these predictions has become the field of ‘self-organization’.
2. Examples of self-organization
(a) Ant trails
The canonical example of a self-organized animal behaviour is ant pheromone trails. Many species of ants deposit chemicals, known as pheromones, to mark the route from food to nest (Wilson 1971). After finding a food source and feeding, an ant returns to the nest, pausing at regular intervals on its way to leave small amounts of pheromone. The ant then makes repeated trips from nest to food source, often leaving more pheromone to reinforce its trail. Other ants, which were previously unaware of the food source and encounter the trail, will follow the trail and find the food source. Once they have collected food, these follower ants also leave pheromone on their return journey. Through this positive reinforcement, the pheromone trail builds up and after a short time we see a steady trail of ants walking between food and nest. Pheromone trails are formed purely on the basis of local information. They are started by a single individual or a small group of ants responding to the presence of food and they are reinforced by ants that encounter and follow the trail.
Despite their simplicity, pheromone trails can be used to solve the problem of directing the majority of ants on the shortest route from food to nest. For example, Beckers et al. (1992) presented starved colonies of the ant Lasius niger with two alternative bridges between food and nest, then measured the number of ants using the two bridges 30 min after the first ant had found food. When one of the bridges was only 40% longer than the other, over 80% of the ants took the shorter bridge in 16 out of the 20 experimental trials. Individual ants make little or no comparison of the two bridges, instead the slightly longer trip time means that pheromone is laid less rapidly on the longer bridge. Thus, when trail following ants make the choice between two bridges they detect a higher concentration of pheromone on one of the bridges, the shorter one (Beckers et al. 1993). The shorter bridge is thus chosen with a higher probability by the follower ants and when these ants return home they further reinforce the shortest path. Since pheromone continually evaporates on both paths but is more strongly reinforced only on the shortest path, the ants rapidly concentrate their trail on the shorter path.
While lab experiments are usually on the scale of tens of centimetres, in nature some species of ants build trail networks on the scale of kilometres or even hundreds of kilometres (Hölldobler & Wilson 1990). Using computer simulations, Deneubourg et al. (1989) showed that the complex trail networks created by army ants during a raid could be reproduced by the simple rules for pheromone laying and following found in the double bridge experiments. By manipulating the food distribution, Franks et al. (1991) showed that the model accurately predicted network structure. It is in this sense we call ant foraging self-organized: ants follow only local rules regarding the laying and following of pheromone, but the resulting trail structure is built on a scale well beyond that of a single ant.
To be more specific, we can say that ant trails result from positive feedback. The one ant which first finds the food starts a feedback loop as more and more ants are recruited to the food, and as more ants are recruited the rate of recruitment increases further. Similar positive feedback loops are also seen in the recruitment dance of the honey bee (Seeley et al. 1991; Seeley 1995), the construction of termite mounds (Bonabeau et al. 1998) and cemetery building by Messor sancta ants (Theraulaz et al. 2002). In each of these cases, the mechanism for recruitment is different but the same pattern of rapid amplification of some initial event is seen. Positive feedback is not limited to social insects, where there are high levels of genetic relatedness and thus co-operation between the colony members, but is also observed within groups of unrelated animals. For example, cockroaches rest for longer periods under shelters with more cockroaches leading to aggregation (Ame et al. 2004) and solitarious locusts are excited through multiple contacts with other solitarious locusts leading to gregarization and collective migration (see figure 1e; Collett et al. 1998; Simpson et al. 2001).
(b) Flocks, schools and crowds
Some of the most mesmerizing examples of collective behaviour of animal groups can be seen overhead every day. V-shaped formations of migrating geese, starlings dancing in the evening sky and hungry seagulls swarming over a fish market, are just some of the wide variety of shapes formed by bird flocks. Fish schools also come in many different shapes and sizes: stationary swarms; predator avoiding vacuoles and flash expansions; hourglasses and vortices; highly aligned cruising parabolas, herds and balls (Partridge 1982; Partridge et al. 1983; Parrish et al. 2002). These shapes are often seen on a scale that far exceeds the size or even the range of interaction of individual fish. Herring schools can consist of more than 5000 individuals spread over 700 m2 (Mackinson 1999).
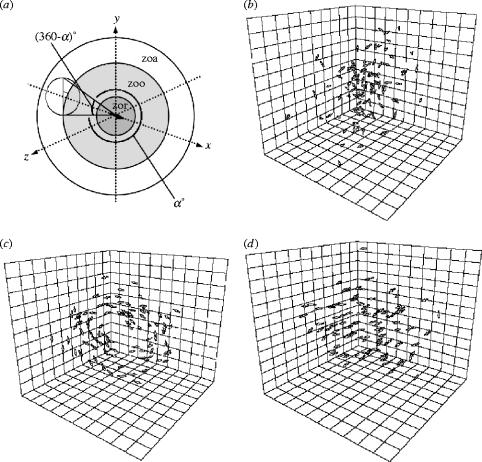
Despite the variety of shapes and motions of animal groups it is possible that many of the different collective patterns are generated by small variations in the rules followed by individual group members. Several authors have developed computer simulation models, known as self-propelled particle (SPP) models, that attempt to capture the collective behaviour of animal groups in terms of the interactions between group members (Okubo 1986; Reynolds 1987; Gueron et al. 1996; Czirok & Vicsek 2000). For example, Couzin et al. (2002) proposed a model in which individual animals follow three simple rules of thumb: (i) move away from very nearby neighbours; (ii) adopt the same direction as those that are close by and (iii) avoid becoming isolated. Each individual thus has three zones—repulsion, alignment and attraction—which increase in size, so that individuals are attracted to neighbours over a larger range than they align, but decrease in priority, so that an individual would always move away from neighbours in the repulsion zone (figure 2a). Keeping the repulsion and attraction radii constant, Couzin et al. (2002) found that as the alignment radius increased, individuals would go from a loosely packed stationary swarm (figure 2b), to a torus where individuals circle round their centre of mass (figures 1a and 2c) and, finally, to a parallel group moving in a common direction (figure 2d). Far from requiring a distinct set of behaviours, these three very different collective patterns self-organize in response to a small adjustments to one factor: the radius over which individuals align with each other (Couzin & Krause 2003). Hoare et al. (2004) later used a similar model to explain the group size distribution they observed in laboratory experiments.
Figure 2.
Couzin et al. (2002) model of fish dynamics. (a) Illustration of the rules governing an individual in the fish model. The individual is centred at the origin: zor, zone of repulsion; zoo, zone of orientation; zoa, zone of attraction. The possible ‘blind volume’ behind an individual is also shown, α, field of perception. Collective behaviours exhibited by the model: (b) swarm, (c) torus and (d) dynamic parallel group.
If the complex patterns produced by animal groups can be explained in terms of a few simple rules, is it possible to use simulation models to predict and even control the behaviour of human crowds? Helbing et al. (2000) proposed a simulation model for pedestrians attempting to escape life-threatening situations through a limited number of exits. They assumed that, in such a situation pedestrians' behaviour is limited to following a few simple rules: try to minimize travel time, avoid collisions with walls and move in the direction of other people. Tragically, these natural behaviours are also those which lead to increased blockages and decreased probability of escape. Helbing et al. (2000) were also able to show that widening corridors could actually lead to slower collective motion, because pedestrians at the end of the widening slow down traffic by trying to squeeze back in to the main flow. Helbing's modelling approach has been usefully applied in understanding: why pedestrians walking on a busy street form bands moving in alternative directions (Milgram & Toch 1969; Helbing & Molnar 1995), the speed of Mexican waves in football stadiums (see figure 1e; Farkas et al. 2002, 2003); as well as the formation of traffic jams (Helbing & Huberman 1998; Helbing & Treiber 1998). These models, which treat humans as particles that interact according to a set of ‘social forces’, have been remarkably successful in predicting the shape and dynamics of crowds.
(c) Audience applause
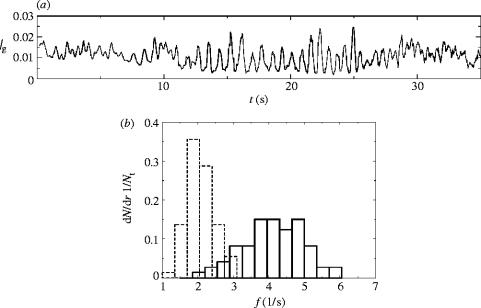
After any good concert performance, audiences express their appreciation by a loud round of applause. In Eastern Europe and Scandinavia this applause is often rhythmical, with the entire audience clapping simultaneously and periodically. Neda et al. (2000a,b) recorded and analysed the clapping of theatre and opera audiences in Romania and Hungary and found a common pattern: first an initial phase of incoherent but loud clapping, followed by a relatively sudden jump into synchronized clapping that, after about half a minute, was again rapidly replaced by unsynchronized applause (figure 3a). A surprising observation was that the average volume of the synchronized clapping is lower than that of unsynchronized applause, both before and after the synchronized bouts. While an audience presumably wants to maximize their volume and thus their appreciation of the performance, they are unable to combine louder volumes with synchronized clapping.
Figure 3.
The emergence of synchronized clapping (from Neda et al. 2000a,b). (a) The average noise intensity of a crowd through time. The first 10 s shows unsynchronized fast clapping, followed by a change to regular slower clapping until around 27 s, followed by synchronized clapping again. (b) A normalized histogram of clapping frequencies for 73 high school students (isolated from each other) for Mode I (solid) and Mode II (dashed) clapping.
Neda et al. (2000a,b) went on to record small local groups in the audience and asked individuals, isolated in a room, to clap as if (I) ‘at the end of a good performance’ or (II) ‘during rhythmic applause’. Both modes of clapping were rhythmical at the individual level, with individuals clapping in mode I twice as fast as those clapping in mode II (figure 3b). The important difference was in the between individual variation for the two modes. When asked to clap rhythmically, isolated individuals chose similar, though not precisely identical, clapping frequencies, while when given the freedom to applaud spontaneously the chosen frequencies spread over a much wider range.
To interpret this observation, Neda et al. (2000a,b) used a classical mathematical result about coupled oscillators. Kuramoto (1975) showed that if a large number of oscillators, for example pendulums hanging on a wall, each with its own frequency, are coupled together so that they continually adjust their frequency to be nearer that of the average frequency, then provided the oscillators' initial frequencies are not too different they will eventually adopt the same frequency and oscillate synchronously (Kuramoto 1984). This is what happens to audiences clapping according to mode II. Their initial independent clapping frequencies are close together, and by listening to the clapping of others, they synchronize their clapping. Audiences clapping in mode I, however, have initial clapping frequencies which are less similar to each other. Thus, even if they try to adjust their clapping in reaction to the sound around them, the Kuramoto model predicts that they will never arrive at a state of synchronized clapping. This is exactly what happened in the audiences that were recorded: faster clapping, with greater inter-individual variation never synchronized. Theatre audiences are thus forced to choose between two different manners of showing their appreciation: loud, frequent, unsynchronized or quieter, less frequent, synchronized clapping.
Synchronized rhythmic activity is seen in many different animal groups and across all of biology (Strogatz 2003). For example, Leptothorax ants synchronize their bouts of activity (Franks et al. 1990; Cole 1991; Boi et al. 1999), human females' menstrual cycles become synchronized when living or working closely together (Stern & McClintock 1998) and, probably the best studied example, fireflies synchronize their flashing (Buck & Buck 1976). The importance of Kuramoto's model is that it shows that individuals with slightly different frequencies can synchronize, each by moving their frequency slightly towards the average. This observation is by no means obvious, and the prediction that above some critical amount of between-individual variation synchronization cannot occur has wide ranging implications. For fireflies, the prediction is that species that do not synchronize their flashing will have higher between-individual variation, and possibly more rapid flashing.
3. Properties of self-organization
Beyond the fact that individuals produce collective patterns, is there anything more specific we can say about these phenomena we have labelled self-organized? The answer to this question lies in the relationship between similarities in the rules governing and the patterns generated by very different systems. Opera audiences and groups of fireflies are different in shape and size, method of communication and their objectives, but both are restricted by inter-individual differences when trying to synchronize their actions. Birds fly, fish swim and pedestrians walk, but they all exhibit the tendency to move away from those that are too close while avoiding being left completely out on their own. These simple rules result in similar patterns of aggregation and directed movement, independent of the details of the animals involved.
If we are to build a useful theory of self-organization of animal groups it is not enough to say that certain things ‘look’ similar. In the above examples, the aspect that links different systems together is similarity in the mathematical models we used to describe their behaviour. For pheromone trails, cockroach shelter choice and ant cemeteries, mechanisms of positive and negative feedback produced aggregations and collective decisions; for oscillating fireflies and hand clapping, the Kuramoto model of coupled oscillators provided understanding of the role of variation in synchronization; and the dynamics of fish schools and human crowds were all captured by SPP models. If the same mathematical model captures the behaviour of different systems then we can talk about similarities between systems that go further than simple analogy. We can use our mathematical models to make predictions that apply to many different systems. Furthermore, if two systems obey the same mathematical laws, we can perform experiments on one system and infer how another system might behave under similar conditions.
(a) More than the sum of its parts
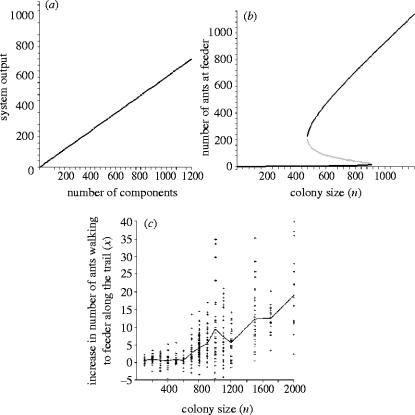
A concrete example of a mathematical prediction that spans all systems subject to positive feedback is that these systems are ‘more than the sum of their parts’ (Aristotle, Metaphysica, 10f–1045a). That is to say that when we plot system ‘output’ against number of system components we do not get a straight line (figure 4). For example, if an ant in a small colony finds a food source a long way from the nest, then by the time another ant passes over the place she left a pheromone trail, the pheromone will probably have evaporated. In this case, the trail does not help other ants find the food. For large colonies of ants, however, it is more likely that an ant will cross the pheromone trail before it evaporates and reinforce it. The reinforcement leads to the familiar positive feedback loop and a well-established trail between nest and food. Thus, the output of the system, i.e. number of ants visiting the feeder and hence food collected, is low for small ant colonies but rapidly increases as the colony becomes larger.
Figure 4.
How different systems increase output as a function of number of system components. (a) A linear increase in system output with number of components. (b) Model prediction of how the number of foragers visiting a feeder changes with number of ants in the colony. The black line is the predicted stable equilibrium for number of foragers visiting the feeder. The grey line is the unstable equilibrium. If fewer ants than the unstable equilibrium initially discover the source, then the total increase in foragers will be determined by the lower stable equilibrium. However, if the initial discovery is by a larger group than the unstable equilibrium, then the increase will be to the upper equilibrium. The model for the rate of change of ants going to the feeder, x, is The figure shows the equilibrium solutions for parameter values: α=0.004 5, β=0.000 15, s=10 and n, the total number of ants, varied between 0 and 65 (see Beekman et al. 2001 for details). (c) Colony size versus the maximum increase in the number of ants walking to a feeder within 40 min of its introduction to an arena containing a starved ant colony (see Beekman et al. 2001 for details). The solid line connects the means of all trials at a given colony size, while crosses represent single trials.
Beekman et al. (2001) formalized this verbal argument in a mathematical model of trail laying. The model predicted that (a) as the number of ants in the colony increased the number of ants visiting the feeder would increase nonlinearly and (b) provided the rate at which ants found the food without following a trail was small, then at a critical colony size there would be a sudden switch from few ants visiting the feeder to a large proportion of the ants visiting the feeder (figure 4b). Both these predictions were confirmed experimentally (figure 4c). Small colonies were unable to establish an effective pheromone trail, while above a critical size, in this case 700 ants, trails were formed between nest and food. In small groups the ant colony was merely a sum of its parts, the amount of food collected being a total of that collected by ants that discovered food independently. But together, in large numbers, the positive feedback of pheromone communication meant a jump in efficiency that made the colony more than the sum of independently working ants.
We can also think of bird flocks and other animal herds as being more than the sum of their parts. Using a simple SPP model, Czirok et al. (1999) showed that a group of individuals that align their direction with that of their neighbours undergo a rapid transition from random motion at low densities, where each individual moves largely independently of the others, to directed motion at high densities where all individuals move in a common direction. We can think of the output of the flock to be a common direction, e.g. a shared migration route, and as the flock's density increases there is a continuous, but rapid nonlinear change to having a shared direction. More recently, it has been shown that under some conditions the transition from random to a common direction can be discontinuous, as it was for ant foraging (Gregoire et al. 2003; Gregoire & Chate 2004).
(b) The central limit theorem
Not all of the collective patterns produced by animal groups are more than the sum of their parts. Symmetrical structures, such as the domes built by wood ants or the craters built by M. barbarus ants (Chretien 1996; Theraulaz et al. 2003), can result from the independent actions of the colony's ants (figure 1b). For example, Chretien (1996) showed that when an individual M. barbarus ant leaves the nest hole with a sand pellet, she moves in a straight line away from the hole in a random direction. Once the ant is, on average, 4.8 cm from the hole she drops the pellet. The fact that the direction chosen by the ant is independent of the direction taken by the other ants in the colony produces a symmetrical crater. The height of this crater, which here can be considered the output of the colony, is proportional to the number of building ants (as in figure 4a). It is purely the sum of the parts that created it.
The fact that the crater wall is equally high on all sides of the ants' nest entrance is a consequence of a remarkable mathematical theorem that applies to all systems consisting of large numbers of independent individuals: the central limit theorem. The theorem states that if each of a large number of independent individuals contributes a small randomly distributed quantity to some total output, then that total output is distributed according to a Normal distribution. Moreover, the standard deviation of total output increases in proportion to the square root of the number of individuals. Since the height of the ants' wall increases in proportion to the number of individuals and, by the central limit theorem the standard deviation around the wall increases as its square root, once the wall is reasonably high the variation in its height will be small relative to its average height. Thus, despite, and indeed because of, the ants working independently an even outer wall is constructed.
The central limit theorem is the most basic statement about and the cornerstone for understanding all collective phenomena. It proves that systems consisting of independent parts are ‘usually’ no more than a square root of the number of parts more or less than the sum of their parts. The Normal distribution is universal, in the sense that all systems consisting of independent parts are subject to it. It thus provides a null hypothesis against which all data taken from systems purporting to exhibit some kind of feedback or self-organization can be compared. Indeed, in this sense all self-organized systems can be explained in terms of the manner in which their output deviate from a Normal distribution (Sornette 2004).
(c) Sensitivity to initial conditions
M. barbarus' crater is not particularly sensitive to the distribution of sand before building begins. If a small pile of sand was put on one side of the nest hole, this pile would grow at the same rate as all other sides of the crater. Once the walls of the crater had grown, the initial difference may remain but look small relative to the total height of the surrounding walls. Such insensitivity to initial distribution is not the case for ant trails. For example, Beckers et al. (1992) repeated their bridge experiments described earlier, this time starting with only the longer bridge (28 cm between nest and food) available. Once the ants established a trail on this bridge a shorter bridge (14 cm) was introduced. However, the established feedback on the longer bridge was so strong, that in 16 out of 20 trials the ants did not switch to the shorter bridge. Strong positive feedback had locked the ant in a suboptimal path choice.
A standard test for whether a particular collective animal behaviour is sensitive to initial conditions is the identical binary choice experiment. In the absence of positive feedback we would expect a 50 : 50 split between two equal alternatives. This does not occur for systems subject to positive feedback. When Beckers et al. (1992) offered Lasius niger ants two identical bridges between food and nest, after 30 min the majority of the ants took only one of the two bridges. Sumpter & Beekman (2003) reported similar results for Pharaoh's ants when they offered the ants two identical feeders in opposite directions from the nest. Instead of a 50 : 50 split between feeders, the split was closer to 70 : 30 or 30 : 70, giving a u-shaped distribution of number of ants at one of the feeders over all the trials (figure 5a). The ‘winning’ feeder was the one that had the most ants nearby when it was initially placed in the foraging arena.
Figure 5.
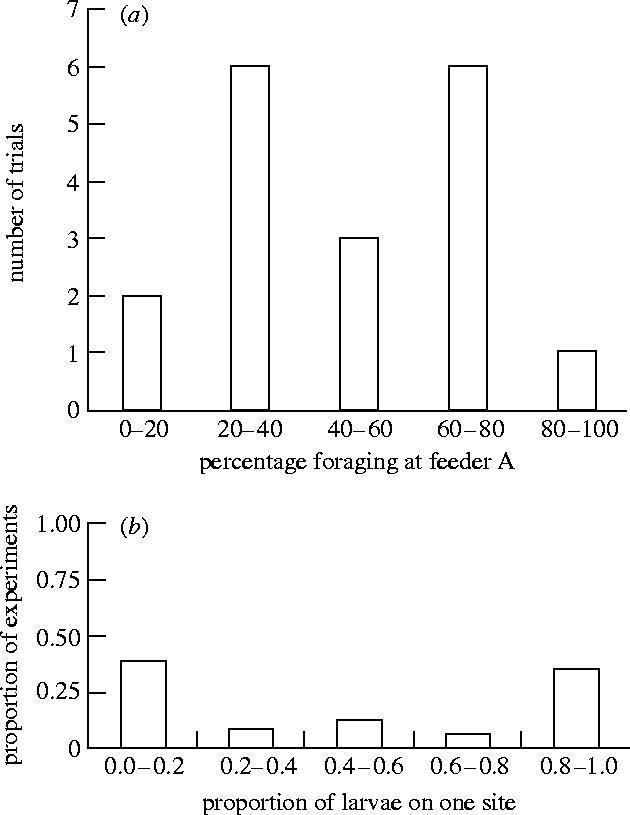
Distribution of the proportion of individuals choosing one of two identical options in binary choice experiments where (a) Pharoah's ant are offered two identical (1.0 M sugar solution) food sources (reproduced from Sumpter & Beekman 2003) and (b) cockroaches are offered two identical shelters (reproduced from Ame et al. 2004).
Similar results have been observed from cockroaches to consumers. For the cockroach, Blattella germanica, the positive feedback mechanism for aggregation is resting time: cockroaches rest for longer at sites containing more cockroaches (Ame et al. 2004; Jeanson et al. 2005). The result is the same as for the ants, a u-shaped distribution of shelter choice (figure 5b). This same resting time feedback operates in spiders (Jeanson et al. 2004) as well as chain formation by weaver ants (Deneubourg et al. 2002). It has been argued that similar patterns are seen in consumer choice (Ormerod 1998). When two similar products are released on the market, such as Betamax and VHS video recorders in the 1980s, after a short period of both products being used, one of them often out-competes the other. Fashion and group pressure could provide a powerful positive feedback mechanism whereby small differences in products are amplified as the herd of consumers follow the initial choices of a few individuals.
4. Individual versus group complexity
The examples of self-organization I have discussed so far illustrate how, for various types of interaction between individuals, we can use mathematical models to predict complex group level patterns of behaviour. Similarities and differences in the mathematical models applied to these systems were helpful in drawing useful comparisons between the systems themselves. I have even outlined some useful and general predictions about these systems: that if the system consists of independent units its output will be normally distributed, that if the units are subject to positive feedback the systems output will be ‘more than the sum of its parts’ and strongly subject to its initial configuration. It may appear now that, through this wonderful theory of self-organization and mathematical modelling, we are well on our way to fully understanding and determining relationships between everything from the foraging of ant colonies to consumer behaviour in our own society.
Unfortunately, such understanding is all too far away. Unlike the simple units composing physical systems, animals are complex entities. While the assumption that humans follow a simple set of rules may hold in certain limited social situations—such as after a concert, in a panic or when queuing for coffee—in a wider social context, each individual's behaviour is a combination of countless genetic and environmental factors. The same is true for other mammals, birds, fish and even insects. For example, individual honey bees are known to use at least 17 different communication signals, the most famous of which is the waggle dance, and adjust their behaviour in response to at least 34 different cues (reviewed in Seeley 1998). While foraging for food, honey bees exhibit at least seven different behavioural states, e.g. scout, recruit, inspector etc. (see figure 6b; reviewed in Biesmeijer & de Vries 2001), and exhibit a range of signals about the location and availability of food (Seeley 1995).
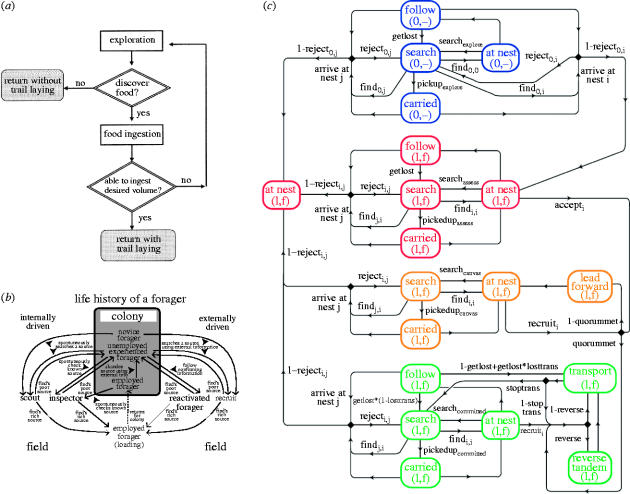
Figure 6.
Algorithms for social insect behaviour. (a) The ‘simple rule of thumb’ used by Lasius niger ants when exploiting liquid food sources (Detrain & Deneubourg 2002; from results of Mailleux et al. 2000). (b) Behaviour control structure of a honey bee forager. Depicted are the seven behavioural categories of foragers. The left part of the diagram represents internally driven categories, the right part, the externally driven categories. Upon locating a rich source, a searching forager becomes employed, whereas upon locating a poor source (including no source), a searching forager returns to the hive and becomes unemployed (for details see Biesmeijer & de Vries 2001). (c) Model of the behaviour of Temnothorax ants when emigrating. Boxes represent behavioural states and arrows transitions between them, labels indicate measured probabilities (Pratt et al. 2005). Colours indicate the four major levels of an ant's commitment to a candidate nest site: exploration (blue), assessment (red), canvassing (amber) and commitment (green).
While studies of self-organization have shown that simple interacting units can become more than the sum of their parts and self-organize (Bonabeau et al. 1997; Camazine et al. 2001) and that they sometimes follow ‘simple rules of thumb’ (figure 6a; see also Detrain & Deneubourg 1997; Detrain et al. 1999; Detrain & Deneubourg 2002), this does not necessarily imply that individual social insects are simple units (Seeley 2002). There is evidence that only ant species with large colonies use pheromone trails for communicating the presence of food (Beckers et al. 1989), but there is little evidence from between species comparison that individual complexity decreases with increased colony size (Anderson & McShea 2001). Honey bees are just one example of species with both large colony sizes and individuals that exhibit a complicated array of communication signals and behavioural states (figure 6b). Even the humble Pharoah's ant, with its exclusive reliance on pheromones for recruiting to food, and the subject of Beekman et al. (2001) colony size study, has been shown to use at least three different pheromone signals to organize traffic on its trails (Jackson et al. 2004; Jackson et al. in press; Duncan Jackson & Elva Robinson personal communication). Thus, self-organization gives us, through mathematical models, a means of predicting the consequences of certain interactions between individuals, but it is by no means an exclusive principle by which insect societies are organized.
One example of a collective behaviour that has been extensively studied from the viewpoint of both individual and group level complexity is the emigration of ants of the genus Temnothorax (Möglich 1978; Mallon et al. 2001; Pratt 2005, in press; Pratt et al. 2002, 2005). These ants, whose colonies consist of between 50 and 500 individuals, live in small preformed cavities. In the laboratory, a colony whose nest has been damaged moves to a new site within a few hours, reliably choosing the best site from as many as five alternatives, discriminating among sites according to cavity area and height, entrance size, and light level (Pratt & Pierce 2001; Franks et al. 2003a,b). Around 30% of the ants actively partake in the process of choosing a new nest site. These active ants undergo four phases of graded commitment to a particular nest site (figure 6c). Each ant begins in an exploration phase during which she searches for nest sites. Once she finds a site she enters an assessment phase, carrying out an independent evaluation of the site, the length of the evaluation being inversely proportional to the quality of the site (Mallon et al. 2001). Once she has accepted the site she enters a canvassing phase, whereby she leads tandem runs, in which a single follower is slowly led from the old nest to the new site. These recruited ants then in turn make their own independent assessments of the nest. Once the nest population has reached a quorum (a threshold population), the ant enters a committed phase, rapidly transporting passive adults and brood items.
Like the self-organized systems discussed earlier, the collective ability to choose the best of several new nest sites does not rely on one ant possessing information on more than a small part of the colony's task. Tandem run recruitment also has elements of the simple positive feedback seen in the pheromone trails of Lasius ants and the aggregation of cockroaches. However, the four phase decision-making process, the use of a quorum threshold to decide whether to perform a tandem runs or a transport (Pratt 2005, in press; Pratt et al. 2002), and the fact that some ants find both nests and choose the superior one (Mallon et al. 2001), makes the behaviour of individual migrating ants more complicated than ‘simple rules of thumb’. Temnothorax ants combine elements of self-organization, whereby a global solution to the problem of finding a new nest emerges from the interactions of the multiple ants, with a sophisticated behavioural algorithm, whereby individual ants continually monitor the progress of the emigration and change their behaviour accordingly.
The detailed experimental understanding of Temnothorax migration has made it possible to determine the behavioural algorithm followed by, and the communication pathways between the ants. Pratt et al. (2002, 2005) have, as new experimental data have become available, systematically refined this behavioural algorithm in order to capture everything that is known about ant migration in a model (figure 6c). Their approach follows that used in modelling gene regulatory (von Dassow et al. 2000) and other complex networks (Kitano 2002). Once the behavioural algorithm is developed, the role of its various components can be tested. For example, Pratt et al. (2002) showed that the requirement that the nest site population reaches quorum before transport commences leads to a reduction in incidence of colony splitting. Franks et al. (2003a,b) went on to show experimentally that the ants reduce the size of their quorum in situations where migration speed takes priority over the avoidance of splitting. Detailed behavioural algorithm models are thus a tool for reconciling individual and group level complexity, allowing for meaningful analysis of how each part of the algorithm contributes to overall system function (Fewell 2003).
The construction of detailed models of the algorithms followed by individuals also allows for between species comparison. For example, Camazine et al. (1999), Seeley & Buhrman (2001), Seeley (2003) and Seeley & Visscher (2003) have studied the migration of honey bees and found that they use a similar combination of positive feedback (dancing instead of tandem running) and quorum thresholds (Seeley & Visscher 2004) as Temnothorax. When algorithms are similar across very different species we can ask why they have evolved in so many different cases. What is it about the combination of positive feedback and response thresholds that produces effective collective decision making? The power of the behavioural algorithm approach is that the algorithm can be analysed and compared across systems. Indeed, rather than simply simulating algorithms in order to reproduce experiments, the algorithms can be studied to find out the principles that underlie them.
Before leaving the subject of individual complexity it is worth pointing out that, when viewed at certain spatial and temporal scales, very complex individuals can produce very simple group level dynamics, provided they exhibit a reasonable degree of independence. For example, though a highly complex algorithm may have brought each individual to town in the first place, the number of people passing by a point on a quiet shopping street during a 5 min interval is likely to be randomly distributed. This prediction is based on the ‘law of small numbers’, that independent low frequency events in a large population follow a Poisson distribution (Bortkiewicz 1898). While on very small time scales there are socially enforced gaps between people and on very large time scales there are patterns determined by shops opening and closing, on the time scale of an hour on a Monday morning passers-by do so more or less at random. Once a large number of factors begin to influence behaviour, the complex begins to seem simple again.
5. Principles of collective behaviour
As far as understanding collective animal behaviour I have come full circle. First, I marvelled at the wonderful patterns produced by animal groups, then showed that some of these patterns could be explained by simple mathematical models, but found myself back at the beginning when we discovered the true complexity of the individuals and the need for detailed behavioural algorithms. These algorithms appear no easier to understand than the phenomena they purport to model and lack the elegance provided by self-organization. There were, however, points on this journey where it felt like progress was made: when completely different systems seemed to be logically equivalent and were given a new explanation by the application of a mathematical model.
Similarities between the logical structure underlying different systems have led researchers to dream of a unifying theory for the study of complex and self-organized systems. The dream is to develop some sort of ultracalculus, a way of seeing clearly the consequences of a myriad of complex interactions (Strogatz 2003). Currently, the only universal theorem of this type is the central limit theorem, but it is precisely those systems consisting of non-independent individuals that our ultracalculus should apply to. For Nicolis and Prigogine the unifying theory was the thermodynamics of open systems; for Bak (1996) it was self-organized criticality and sand piles; for Sornette (2004) it is the generalization of the normal distribution to power law distributions; for Kauffman (1993) it is Boolean networks; and for Wolfram (2002) it is cellular automata. Elegant as these mathematical ideas are, none of them have proved truly universal, and more importantly, nor have they been shown to apply to large numbers of biological systems. Experimental tests of these theories are few and far between.
My personal view is more pragmatic than the development of a universal theory or an ultracalculus of self-organization. Studying systems of collective animal behaviour should proceed on a case-by-case basis. For each particular system, we classify how individuals interact with each other and build behavioural algorithms based on these observations. The behavioural algorithms are then the basis for mathematical models. If a similar mathematical model has been previously applied to another system, this helps us understand the behavioural algorithm and thus the system, but the equivalence of mathematical models alone should not guide the way in which we study our chosen system. Through this approach, mathematical models become a tool for investigating natural systems and the fact that the same model is applicable to many different systems is a happy co-incidence rather than a proof of universal laws.
The pragmatic approach does not deny that there are strong similarities between very different systems. On the contrary, it is essential to such an approach that systems are classified in terms of their logical or mathematical similarities and differences. The approach I take now is to list some of the principles for the behavioural algorithms that produce collective animal behaviour. This approach builds on the idea of self-organization that many of an animal group's activities can be described in terms of three principles: positive feedback, negative feedback and the amplification of random fluctuations (Bonabeau 1997; Camazine et al. 2001). Here, I add to this list the principles of individual integrity; response thresholds; leadership; inhibition; redundancy; synchronization and selfishness. My current list is not intended as exhaustive, but it does include many commonly observed features of collective animal behaviour. The ultimate aim is to find a set of principles on which the behavioural algorithms followed by individuals are built. These principles should be both empirical, i.e. describe behaviour that really does occur, and sufficiently abstract that mathematical models based on these principles can be developed and studied in a way that leads to insight into a number of different systems.
Bearing in mind that individuals do not follow only simple rules, it is important that we do not think of these principles in isolation. Indeed, we should consider how these different principles interact with each other to produce collective patterns. For example, how does individual integrity mix with positive feedback in allowing information about food sources to flow quickly through a honey bee colony without the colony losing the ability to discover new sources? Or, how can synchronization of mating fireflys occur when it is to the advantage of one firefly to cheat by flashing faster than the others? Many of the big questions for the future of collective animal behaviour concern how these principles fit together to generate complex collective patterns.
(a) Integrity and variability
Each of the animals in a group is different, in terms of their genes and/or their previous experience. For example, if every bee always collected the same type of food from the same place then their colony's nutritional needs could not be met. Worse still, when the uniquely selected food source is depleted, flow of food into the colony stops until the next food source is found. Such a situation is avoided in honey bee colonies by a high degree of individual variation. From only one week old, before they have left the hive for the first time, honey bees have different levels of response to sucrose, which later in life determines their propensity to collect water, nectar and pollen (Pankiw & Page 2000). Honey bees and other social insects are highly variable in the direction, intensity and focus of food collection and other tasks (Jeanne 1988). Sometimes this variability alone is sufficient to produce collective patterns. For example, the circular structure of M. barbarus craters was due to each individual choosing a random direction by which to leave the nest. Even when not all activities are carried out independently, individual variability is of central importance to ensuring that different solutions to a problem are explored.
The importance of individual variability in human society is highlighted by an observation by Galton (1907). He examined 800 entries into a ‘guess the weight of the ox competition’, where a crowd of fairgoers each paid a small amount to guess how much a large ox would weigh after slaughter, with the most accurate guess winning a prize. Although the guesses had a wide variation, differing significantly from a Normal distribution, the average guess was only 1 pound (450 g) less than the 1197 pounds (544.5 kg) that the ox weighed. Acting independently, the crowd ‘knew’ the weight of the ox. There are many such examples of collective accuracy: ask the audience on ‘Who wants to be a millionaire’; the accurate prediction of American presidential elections by betting; and the Google search engine using links to a webpage to measure its popularity are just some (Surowiecki 2004). In all animal groups, high inter-individual variation can provide a continual supply of new solutions to the problems the group aims to solve.
(b) Positive feedback
Positive feedback is the amplification of events through recruitment or reinforcement (Bonabeau et al. 1997; Camazine et al. 2001). A cockroach stops in a shelter, another one pauses nearby. An ant finds a food source, another one follows her trail there. A fish turns to the left, its neighbour follows soon after. A few people recommend a particular brand of video recorder, others go out and buy it. As such imitation or recruitment behaviour continues the number performing an activity explodes exponentially. An isolated behaviour is quickly subsumed by a mass of similar behaviours. Positive feedback is the best studied component of collective animal behaviour and we have already discussed many of the collective patterns it can produce.
While individual integrity generates new group level solutions, positive feedback spreads this information quickly through the group. For example, an ant finding a food source relies on the particular search path of that ant, but the trail it leaves on the way home is the start of a positive feedback loop that homogenizes the behaviour of those following the trail. Followers submit their own integrity to the discoveries of others. Too much positive feedback can lead to suboptimal solutions when conditions change, such as when ants stick to an established a trail even when a shortcut is introduced (Beckers et al. 1992). Successful problem solving by collective behaviour often involves striking the correct balance between individual variation and positive feedback. Information should spread but also be kept up to date by new discoveries.
(c) Negative feedback
If positive feedback builds up a collective pattern then it is negative feedback that stabilizes it. For example, once lots of ants are collecting from a food source, new arrivals will find it hard to collect food and search elsewhere. Even if the food source is unlimited, the colony is of limited size and the number of ants visiting the feeder will stabilize once every available foraging ant is collecting food. Negative feedback leads to homeostasis, stable output in the face of varied input. For the ants, changes in food distribution leads to a smooth adjustment of the distribution of ants between food sources.
(d) Response thresholds
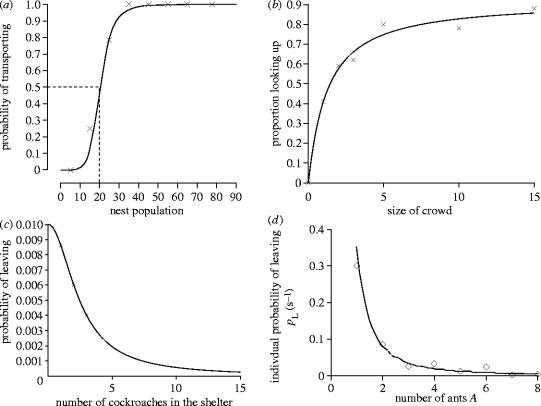
Animals often change their behaviour in response to a stimulus reaching some threshold. For example, bumble bees begin fanning (to cool down the nest) when the temperature concentration inside the nest exceeds a particular threshold level, this level of fanning often being variable between individuals (Weidenmuller 2004). Threshold responses are also seen in between individual interactions. Figure 7 gives four examples of response thresholds where the stimulus is other individuals. For Temnothorax ants the stimulus is the density of ants within a nest cavity (figure 7a). When the density reaches at least 20.2 ants in a 24 cm2 nest, the ants switch from tandem run recruitment to transporting (Pratt 2005, in press). In this sense the response threshold acts as a quorum; above the quorum a more rapid form of recruitment begins (Pratt et al. 2002).
Figure 7.
Examples of response thresholds. (a) The probability of a recruiting Temnothorax albipennis ant performing a transport rather than a tandem run, as a function of the mean nest population (N) of the destination site on her immediately previous visit there (data from Pratt 2005, in press). The fitted line is . (b) The mean proportion of passer-by's looking up as a function of the size of a group (C) of people stood in the street looking up at a sixth floor window (taken from Milgram 1992). Fitted line is , giving a threshold of 1.22. (c) The probability of a cockroach leaving a shelter as a function of the number of other cockroaches (C) on the site (taken from Ame et al. 2004). Fitted line is . (d) The probability per second of an ant moving away from a stopped group of ants as a function of the number of stopped ants (A; taken from Depickere et al. 2004). Fitted line is .
Milgram et al. (1969), reported in Milgram (1992), planted a stimulus group of people, all of whom looked up at a supposed point of interest on the pavement on a busy street. The proportion of naïve passers-by who looked up at the point was then observed as a function of the size of the stimulus group (figure 7b). The proportion of those looking up increased as a function of group size in a form similar to that seen in the response of the ants to nest mates. The larger the group the larger the probability of stopping. The same behaviour is observed in ants (figure 7c) and cockroaches (figure 7d). In these cases the amount of time spent sitting still increases as a threshold like function of group size. In all these cases, response thresholds can interact with positive feedback to generate local aggregations. If only one person stops in the street there is only a 40% chance of another following their gaze and a group is unlikely to build up. If by chance five people stop and look up then the probability increases to 80% and the group has a higher probability of becoming stable, even in the absence of any true stimulus.
(e) Leadership
The notion of self-organization seems somehow incompatible with the notion of leadership. In insect societies, however, there are some key individuals that catalyse and organize the group (Robson & Traniello 1999). A classic example of catalyst behaviour is the shaking signal in honey bees. When foraging begins in the morning some of the first foraging bees to find food shake the other bees deep in the hive (Seeley 1995), serving to inform those bees that a period of greater activity is about to begin (Seeley et al. 1998). The distribution of number of shaking signal bouts across individual bees during their lifetime is highly skewed, indicating that it is certain bees that act as catalysts (Biesmeijer 2003). Often leadership is assumed by the possession of particular information. In fish, a small number of informed individuals can lead the migration of larger numbers of individuals (Reebs 2000). Couzin et al. (2005) used an SPP model to show that the larger the group, the smaller the proportion of informed individuals required to lead it. This result relies on the fact that followers are subject to positive feedback. As a few individuals follow a leader, other individuals follow these followers and the group takes a single direction. Rather than being opposites, self-organized positive feedback and leadership are instead powerfully combined to produce co-ordinated collective migration.
(f) Inhibition
Members of a group exhibiting one type of behaviour can inhibit the behaviour of others. When this inhibition is passive it is indistinguishable from negative feedback. For example, as ants are recruited to one food source the number available to visit another is reduced. Inhibition can also be active, whereby members of one group actively try to reduce another type of behaviour. For example, when choosing a new nest Temnothorax albipenis ants who have begun transporting to one nest site will, if they encounter an alternative nest site that they rank as inferior, begin transporting away from that nest to the nest they first transported to (Mallon et al. 2001; Pratt et al. 2002). If, as often happens during Temnothorax emigrations, transport begins to more than one potential nest site, these between site transports provide between site inhibition. Transports away from a potential nest site will reduce the population and the probability the nest will reach quorum, and thus the incidence of splitting between nest sites.
(g) Redundancy
Very little is known about the value of redundancy in animal groups or even our own society, but it is often implicated in the observation that, as Francis Ratnieks has put it, ‘insect societies never crash’. Unlike computers, which are constructed of many specialized and essential components, insect societies consist, with the possible exception of the queen, of vast numbers of replaceable units: 20–30% of honey bee workers rest at any one time (Seeley 1995). If one type of honey bee workers, for example foragers, are removed, these are rapidly replaced by a new cohort of younger bees (Lindauer 1952). The apparent redundancy in the system allows it to continue to function even when faced with a major reduction in its workforce.
(h) Synchronization
Audience applause and other synchronization phenomena are achieved through small adjustments by individuals of their own frequency towards that of some local average. Essentially, synchronization is an example of positive feedback in time rather than space. The coupling of synchronization in time with positive feedback in space may have an important role in determining the productivity of animal groups. For example, some ant colonies synchronize bouts of resting and activity (Cole 1991). If by all being active at the same time the ants can disproportionably increase their ‘output’ (as in figure 4b), then being synchronized will see an overall increase in output compared to constant activity levels.
(i) Selfishness
Tucked away here, as the last subheading in a section on components of collective behaviour, is the question that would spring to many biologists' mind even before I began giving different examples of collective animal behaviour: ‘how did these collective behaviours evolve through natural selection?’ I have made no attempt up to now to address the problem of the ‘ultimate’ reason for cockroach aggregation, fish schools, ant trail networks or audience applause. In the light of my discussion of a universal theorem this omission may be considered neglectful. There is only one theory of biological systems that has any claim on universality and that is natural selection. In relation to group co-operation, natural selection produces Hamilton's rule: the relatedness of the individual that profits from the altruistic act of the focal individual must be higher than the cost/benefit ratio this act imposes (Hamilton 1964). All collective animal behaviour involves some form of co-operation, in the sense that individuals interact to form a pattern that is larger in scale than one individual. Hamilton's rule can explain many of the sophisticated forms of co-operation that have evolved in social insects, where inter-individual relatedness is high (Trivers & Hare 1976; Crozier & Pamilo 1996). Similar reasoning often explains helping behaviour in mammals and birds (Griffin & West 2003). However, many studies have failed to detect the levels of relatedness predicted for highly co-operative insect societies (reviewed by Korb & Heinze 2004), and at all levels of biological organization helping behaviour can be uncorrelated with relatedness (Griffin & West 2002).
In cases where collective patterns are generated by individuals with low inter-individual relatedness, it could be the collective properties of the animal groups that are subject to natural selection. For example, if working as part of a group means that the group becomes more than the sum of its parts, then unrelated individuals can increase their own fitness by being part of the group. Also, genetically diverse groups may benefit from inter-individual variability in terms of better informed collective decisions (Fuchs & Schade 1994; but see evidence to the contrary in Neumann & Moritz 2000) and disease resistance (Baar & Schmid-Hempel 1999). There is observational evidence for such possibilities; many of the behavioural interactions observed in ant colonies, such as positive feedback and threshold response, are also seen in cockroaches (Deneubourg et al. 2002) and locusts (Simpson et al. 1999). The problem with invoking natural selection on the collective properties of groups is that individuals should act to maximize their own fitness. If one individual can benefit from the other individuals' co-operation, without paying any cost associated with the resulting collective pattern, then it can pass on its genes more effectively than those paying the cost. Ultimately, such reasoning can predict changes in the shape or even the collapse of collective patterns that can be exploited.
Collapse of co-operation need not always occur just because individuals are expected to act only in pursuit of their own reproductive interests or economic gain. The last thirty years has seen an explosion of theories and experimental tests of theories of why selfish individuals ‘apparently’ co-operate: aggregations may result from individuals attempting to put others between themselves and danger to create a selfish herd (Hamilton 1971) or to minimize the cost of disintegration (Parrish & Edelstein-Keshet 1999); co-operation can evolve where individuals interact repeatedly with, or are spatially located close to, each other (Nowak & May 1992; Doebeli & Hauert 2005); and in some cases, such as worker policing by honey bees (Ratnieks 1988; Ratnieks & Visscher 1989), group members actively search out and stop cheaters. In all cases, the argument for why acting alone or cheating does not out-compete co-operation first requires a full understanding of the other components of collective behaviour. Likewise, rather than being an alternative to the mechanistic approach of studying the components of collective behaviour, the selfishness idea provides us with useful constraints on the possible behavioural algorithms evolved by individuals in groups.
6. Vision of the future
This paper is part of a series of special issues by young scientists looking into the future of their fields. In order to think about the future I started by reading the books by the pioneers of self-organization—von Bertanfly, Weiner and Nicolis & Prigogine—to find out how they saw the future when they first had the ideas that have inspired our work today. What struck me most was how much their vision for the future was also the vision for the future that is common to all aspects of modern biology, from post-human genome genetics to biodiversity. The emphasis then was on ‘systems biology’, on understanding how different parts of biological systems work together, moving away from reductionism and establishing principles for biological organization. Pick up a recent copy of Science or Nature and these same issues fill the discussion pages. How do we integrate all we know about the parts of biological systems to understand how they function at a collective level?
At first thought, it sounds depressing that the vision so many of us have for the future is the same as that of scientists writing 30 or even 50 years ago. It appears that the pioneers of self-organization were wrong when they proclaimed the immediate future as a future of ‘systems biology’. Instead, biology became a field of super-efficient stamp collecting with research projects, most notably the genome projects, mapping out the detailed structure of the units making up the biological world. 30 years on we have a lot more information, but we are only a little wiser as to how we should put it all together. Gradually, however, we are beginning to understand how the parts of biological systems make up the whole. The examples I have presented here illustrate how through a combination of mathematical modelling, experimental manipulation and observation we can begin to understand collective animal behaviour. Similar progress has been made in the study of other complex biological systems from human organs (Hunter & Borg 2003) to ecosystems (Levin 2000). Often such understanding relies on technology, such as digital tracking and computer simulations, that was not available even 10 years ago. The technological tools may finally be available for us to begin to disentangle biological complexity.
So my primary vision for the future is the further use of technology and mathematics for finally doing ‘systems biology’. We now have a large number of the technological tools for performing data analysis and building detailed mathematical models. The work I have described here has applied these tools mainly to the study of insect societies, but they can also be used to look at locust swarms, fish schools, human crowds, commercial farm animals and many other animal groups. The immediate future should see greater emphasis on application of some of the theories developed over the last 30 years. With more applications will also come a greater pragmatism on the part of the theoreticians and an increased understanding of the use of mathematics on the part of experimentalists.
I hope the term self-organized will be replaced gradually by something akin to the principles I describe here. Describing a system as self-organized tells us little about how it actually works, while providing a slight sense of mysticism. From a practical point of view it is better to say that the behaviour of a system arises from a particular combination of, for example, positive feedback, response thresholds and negative feedback. Such description allows for more detailed between system comparisons, not only between different types of collective animal behaviour but across all complex systems. In this article I have avoided using any mathematics in the main text, but nearly all of the systems I describe have been understood with the help of a mathematical model of some sort. I would like to see the development of mathematics that is appropriate for describing different principles of collective behaviour, but does not insist that the whole world should fit in to a certain type of equation. If there are so many principles underlying collective behaviour it is unlikely that a single formalism can describe them all. Instead, mathematics should help us formulate and test hypotheses, but our understanding of experimental results should be brought together by a common non-mathematical language.
A slightly more controversial vision I have for the future is that the division between evolutionary biology and mechanistic biology will narrow. At present researchers can often be classified as looking at ‘why’ questions of the evolution of collective behaviour and the ‘how’ questions of the way animal groups function. This division is, however, narrowing as evolutionary biologists become interested in how, for example, benefits of being in a group changes with group size. This important function in calculating optimal group joining strategy can only be found by detailed knowledge of how a group is made up of its parts. Likewise, biologists whose interest in collective behaviour comes from a background in mathematics or physics cannot ignore the importance of selfishness behaviour in group situations. The view I have taken in this article is that the primary aim of the study of collective animal behaviour is to determine the algorithms that produce the collective behaviour and to understand the principles that underlie these algorithms. Natural selection and the ultimate reasons for and evolutionary origin of this behaviour becomes just one of the principles in obtaining such understanding.
If natural selection is to lose its central importance in animal behaviour, then rationality is also to lose its grip on the study of human behaviour. Rationality has long been considered the null model for economics (Popper 1957), and has assumed importance in the understanding of how humans will behave in group situations. Like natural selection, rationality is also only one of the principles that govern collective behaviour in humans (Schelling 1978; Milgram 1992). The positive feedback in consumer choice, the synchronization of applause and the threshold response to a crowd are also important principles. I have endeavoured in this article to show that many collective human behaviours are similar to their animal counterparts. In fact, they are so similar that the same mathematical models can be used to describe collective patterns in both humans and animals. Such understanding could be invaluable in designing the spaces in which we live and work, or in developing product advertising campaigns. It would not tell us what it feels like to be part of a football crowd, but it could tell us how to construct an easily evacuated football ground. While I can never be sure what students might talk about together after their morning lectures, I might one day be able to predict how long they have to queue for a cup of coffee.
Acknowledgments
I would like to thank Madeleine Beekman, David Broomhead, Anders Johansson, Stephen Pratt, Francis Ratnieks, Max Reuter, Nicole Saam and Lovisa Sumpter for many interesting conversations that have contributed to this article. Thank you to Hedi Emmerig and Iain Couzin for careful readings, and to the two reviewers for their comments. This research was funded by the Royal Society.
References
- Ame J.M, Rivault C, Deneubourg J.L. Cockroach aggregation based on strain odour recognition. Anim. Behav. 2004;68:793–801. doi:10.1016/j.anbehav.2004.01.009 [Google Scholar]
- Anderson C, McShea D.W. Individual versus social complexity, with particular reference to ant colonies. Biol. Rev. 2001;76:211–237. doi: 10.1017/s1464793101005656. doi:10.1017/S1464793101005656 [DOI] [PubMed] [Google Scholar]
- Ashby W.R. Principles of the self-organizing dynamic system. J. Gen. Psychol. 1947:125–128. doi: 10.1080/00221309.1947.9918144. [DOI] [PubMed] [Google Scholar]
- Baar B, Schmid-Hempel P. Experimental variation in polyandry affects parasite load and fitness in a bumble bee. Nature. 1999;397:151–153. doi:10.1038/16451 [Google Scholar]
- Bak P. Copernicus Books; New York: 1996. How nature works: the science of self-organized criticality. [Google Scholar]
- Beckers R, Goss S, Deneubourg J.L, Pasteels J.M. Colony size, communication and ant foraging strategy. Psyche. 1989;96:239–256. [Google Scholar]
- Beckers R, Deneubourg J.L, Goss S. Trails and U-turns in the selection of a path by the ant Lasius–Niger. J. Theor. Biol. 1992;159:397–415. [Google Scholar]
- Beckers R, Deneubourg J.L, Goss S. Modulation of trail laying in the ant Lasius–Niger (hymenoptera, formicidae) and its role in the collective selection of a food source. J. Insect Behav. 1993;6:751–759. doi:10.1007/BF01201674 [Google Scholar]
- Beekman M, Sumpter D.J.T, Ratnieks F.L.W. Phase transition between disordered and ordered foraging in Pharaoh's ants. Proc. Natl Acad. Sci. USA. 2001;98:9703–9706. doi: 10.1073/pnas.161285298. doi:10.1073/pnas.161285298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesmeijer J.C. The occurrence and context of the shaking signal in honey bees (Apis mellifera) exploiting natural food sources. Ethology. 2003;109:1009–1020. doi:10.1046/j.0179-1613.2003.00939.x [Google Scholar]
- Biesmeijer J.C, de Vries H. Exploration and exploitation of food sources by social insect colonies: a revision of the scout-recruit concept. Behav. Ecol. Sociobiol. 2001;49:89–99. doi:10.1007/s002650000289 [Google Scholar]
- Boi S, Couzin I.D, Del Buono N, Franks N.R, Britton N.F. Coupled oscillators and activity waves in ant colonies. Proc. R. Soc. B. 1999;266:371–378. doi:10.1098/rspb.1999.0647 [Google Scholar]
- Bonabeau E, Theraulaz G, Deneubourg J.L, Aron S, Camazine S. Self-organization in social insects. Trends Ecol. Evol. 1997;12:188–193. doi: 10.1016/s0169-5347(97)01048-3. doi:10.1016/S0169-5347(97)01048-3 [DOI] [PubMed] [Google Scholar]
- Bonabeau E, Theraulaz G, Deneubourg J.L, Franks N.R, Rafelsberger O, Joly J.L, Blanco S. A model for the emergence of pillars, walls and royal chambers in termite nests. Phil. Trans. R. Soc. B. 1998;353:1561–1576. doi:10.1098/rstb.1998.0310 [Google Scholar]
- Bortkiewicz, L. J. 1898 The law of small numbers
- Buck J, Buck E. Synchronous fireflies. Sci. Am. 1976;234:74–85. doi: 10.1038/scientificamerican0576-74. [DOI] [PubMed] [Google Scholar]
- Camazine S, Visscher P.K, Finley J, Vetter R.S. House-hunting by honey bee swarms: collective decisions and individual behaviors. Insect. Soc. 1999;46:348–360. doi:10.1007/s000400050156 [Google Scholar]
- Camazine S, Deneubourg J.L, Franks N.R, Sneyd J, Theraulaz G, Bonabeau E. Princeton studies in complexity. Princeton University Press; Princeton, NJ: 2001. Self-organization in biological systems. [Google Scholar]
- Chretien, L. 1996 Organisation spatiele du materiel provenant de l'excavation du nid chez Messor barbarus et des cadavres d'ouvrieres chez Lasius niger (Hymenoptera: Formicidae). In Center for nonlinear phenomena and complex systems, Ph.D. thesis, Universite Libre de Bruxelles, Brussles.
- Cole B.J. Short-term activity cycles in ants: generation of periodicity through worker interaction. Am. Nat. 1991;137:244–259. doi:10.1086/285156 [Google Scholar]
- Collett M, Despland E, Simpson S.J, Krakauer D.C. Spatial scales of desert locust gregarization. Proc. Natl Acad. Sci. USA. 1998;95:13 052–13 055. doi: 10.1073/pnas.95.22.13052. doi:10.1073/pnas.95.22.13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin I.D, Krause J. Self-organization and collective behavior in vertebrates. Adv. Study Behav. 2003;32:1–75. doi:full_text [Google Scholar]
- Couzin I.D, Krause J, James R, Ruxton G.D, Franks N.R. Collective memory and spatial sorting in animal groups. J. Theor. Biol. 2002;218:1–11. doi: 10.1006/jtbi.2002.3065. doi:10.1006/jtbi.2002.3065 [DOI] [PubMed] [Google Scholar]
- Couzin I.D, Krause J, Franks N.R, Levin S.A. Effective leadership and decision-making in animal groups on the move. Nature. 2005;433:513–516. doi: 10.1038/nature03236. doi:10.1038/nature03236 [DOI] [PubMed] [Google Scholar]
- Crozier R.H, Pamilo P. Oxford series in ecology and evolution. Oxford University Press; Oxford, UK: 1996. Evolution of social insect colonies: sex allocation and kin selection. [Google Scholar]
- Czirok A, Vicsek T. Collective behavior of interacting self-propelled particles. Physica A. 2000;281:17–29. [Google Scholar]
- Czirok A, Barabasi A.L, Vicsek T. Collective motion of self-propelled particles: kinetic phase transition in one dimension. Phys. Rev. Lett. 1999;82:209–212. [Google Scholar]
- Deneubourg J.L. Application de l'ordre par fluctuations à la description de certaines étapes de la construction du nid chez les termites. Insect. Soc. 1977;24:117–130. doi:10.1007/BF02227166 [Google Scholar]
- Deneubourg J.L, Goss S. Collective patterns and decision-making. Ethol. Ecol. Evol. 1989;1:295–311. [Google Scholar]
- Deneubourg J.L, Goss S, Franks N, Pasteels J.M. The blind leading the blind: modeling chemically mediated army ant raid patterns. J. Insect Behav. 1989;2:719–725. doi:10.1007/BF01065789 [Google Scholar]
- Deneubourg J.L, Lioni A, Detrain C. Dynamics of aggregation and emergence of cooperation. Biol. Bull. 2002;202:262–267. doi: 10.2307/1543477. [DOI] [PubMed] [Google Scholar]
- Depickere S, Fresneau D, Deneubourg J.L. Dynamics of aggregation in Lasius niger (Formicidae): influence of polyethism. Insectes Sociaux. 2004;51:81–90. [Google Scholar]
- Detrain C, Deneubourg J.L. Scavenging by Pheidole pallidula: a key for understanding decision-making systems in ants. Anim. Behav. 1997;53:537–547. doi:10.1006/anbe.1996.0305 [Google Scholar]
- Detrain C, Deneubourg J.L. Complexity of environment and parsimony of decision rules in insect societies. Biol. Bull. 2002;202:268–274. doi: 10.2307/1543478. [DOI] [PubMed] [Google Scholar]
- Detrain C, Deneubourg J.L, Pasteels J.M. Decision-making in foraging by social insects. In: Detrain C, Deneubourg J.L, Pasteels J.M, editors. Information processing in social insects. Birkhäuser Verlag; Basel: 1999. pp. 331–354. [Google Scholar]
- Doebeli M, Hauert C. Models of cooperation based on the prisoner's dilemma and the snowdrift game. Ecol. Lett. 2005;8:748–766. doi:10.1111/j.1461-0248.2005.00773.x [Google Scholar]
- Farkas I, Helbing D, Vicsek T. Social behaviour: Mexican waves in an excitable medium—The stimulation of this concerted motion among expectant spectators is explained. Nature. 2002;419:131–132. doi: 10.1038/419131a. doi:10.1038/419131a [DOI] [PubMed] [Google Scholar]
- Farkas I, Helbing D, Vicsek T. Human waves in stadiums. Physica A—Stat. Mech. Appl. 2003;330:18–24. doi:10.1016/j.physa.2003.08.014 [Google Scholar]
- Fewell J.H. Social insect networks. Science. 2003;301:1867–1870. doi: 10.1126/science.1088945. doi:10.1126/science.1088945 [DOI] [PubMed] [Google Scholar]
- Franks N.R, Bryant S, Griffiths R, Hemerik L. Synchronization of the behaviour within nests of the ant Lepothorax acervorum (Fabricius): I. Discovering the phenomenon and its relation to the level of starvation. Bull. Math. Biol. 1990;52:597–612. doi:10.1016/S0092-8240(05)80368-4 [Google Scholar]
- Franks N.R, Gomez N, Goss S, Deneubourg J.L. The blind leading the blind in army ant raid patterns: testing a model of self-organization (Hymenoptera: Formicidae) J. Insect Behav. 1991;4:583–607. doi:10.1007/BF01048072 [Google Scholar]
- Franks N.R, Dornhaus A, Fitzsimmons J.P, Stevens M. Speed versus accuracy in collective decision making. Proc. R. Soc. B. 2003a;270:2457–2463. doi: 10.1098/rspb.2003.2527. doi:10.1098/rspb.2003.2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N.R, Mallon E.B, Bray H.E, Hamilton M.J, Mischler T.C. Strategies for choosing between alternatives with different attributes: exemplified by house-hunting ants. Anim. Behav. 2003b;65:215–223. doi:10.1006/anbe.2002.2032 [Google Scholar]
- Fuchs S, Schade V. Lower performance in honey bee colonies of uniform paternity. Apidologie. 1994;25:155–168. [Google Scholar]
- Galton F. Vox populi. Nature. 1907;75:450–451. [Google Scholar]
- Gregoire G, Chate H. Onset of collective and cohesive motion. Phys. Rev. Lett. 2004:92. doi: 10.1103/PhysRevLett.92.025702. [DOI] [PubMed] [Google Scholar]
- Gregoire G, Chate H, Tu Y.H. Moving and staying together without a leader. Physica D—Nonlin. Phenom. 2003;181:157–170. doi:10.1016/S0167-2789(03)00102-7 [Google Scholar]
- Griffin A.S, West S.A. Kin selection: fact and fiction. Trends Ecol. Evol. 2002;17:15–21. doi:10.1016/S0169-5347(01)02355-2 [Google Scholar]
- Griffin A.S, West S.A. Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science. 2003;302:634–636. doi: 10.1126/science.1089402. doi:10.1126/science.1089402 [DOI] [PubMed] [Google Scholar]
- Gueron S, Levin S.A, Rubenstein D.I. The dynamics of mammalian herds: from individual to aggregations. J. Theor. Biol. 1996;182:85–98. doi:10.1006/jtbi.1996.0144 [Google Scholar]
- Hamilton W.D. The genetical evolution of social behaviour. I, II. J. Theor. Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. doi:10.1016/0022-5193(64)90038-4 [DOI] [PubMed] [Google Scholar]
- Hamilton W.D. Geometry for the selfish herd. J. Theor. Biol. 1971;31:295–311. doi: 10.1016/0022-5193(71)90189-5. doi:10.1016/0022-5193(71)90189-5 [DOI] [PubMed] [Google Scholar]
- Helbing D, Huberman B.A. Coherent moving states in highway traffic. Nature. 1998;396:738–740. doi:10.1038/25499 [Google Scholar]
- Helbing D, Molnar P. Social force model for pedestrian dynamics. Phys. Rev. E. 1995;51:4282–4286. doi: 10.1103/physreve.51.4282. doi:10.1103/PhysRevE.51.4282 [DOI] [PubMed] [Google Scholar]
- Helbing D, Treiber M. Traffic theory—jams, waves, and clusters. Science. 1998;282:2001–2003. doi:10.1126/science.282.5396.2001 [Google Scholar]
- Helbing D, Farkas I, Vicsek T. Simulating dynamical features of escape panic. Nature. 2000;407:487–490. doi: 10.1038/35035023. doi:10.1038/35035023 [DOI] [PubMed] [Google Scholar]
- Hoare D.J, Couzin I.D, Godin J.G.J, Krause J. Context-dependent group size choice in fish. Anim. Behav. 2004;67:155–164. doi:10.1016/j.anbehav.2003.04.004 [Google Scholar]
- Hölldobler B, Wilson E.O. Belknap Press of Harvard University Press; Cambridge, MA: 1990. The ants. [Google Scholar]
- Hunter P.J, Borg T.K. Integration from proteins to organs: the physiome project. Nat. Rev. (Mol. Cell Biol.) 2003;4:237–243. doi: 10.1038/nrm1054. doi:10.1038/nrm1054 [DOI] [PubMed] [Google Scholar]
- Jackson D.E, Holcombe M, Ratnieks F.L.W. Knowing which way to go—trail geometry gives polarity to ant foraging networks. Nature. 2004;432:907–909. doi: 10.1038/nature03105. doi:10.1038/nature03105 [DOI] [PubMed] [Google Scholar]
- Jackson, D. E., Martin, S. J., Holcombe, M., Ratnieks, F. L. W. In press. Longevity and detection of persistent foraging trails in Pharaoh's ants, Monomorium pharaonis (L.). Anim. Behav.
- Jeanne R.L, editor. Interindividual behavioral variability in social insects. Westview Press; Boulder, CO: 1988. [Google Scholar]
- Jeanson R, Deneubourg J.L, Theraulaz G. Discrete dragline attachment induces aggregation in spiderlings of a solitary species. Anim. Behav. 2004;67:531–537. doi:10.1016/j.anbehav.2003.06.013 [Google Scholar]
- Jeanson R, Rivault C, Deneubourg J.L, Blanco S, Jost C, Theraulaz G. Self-organized aggregation in cockroaches. Anim. Behav. 2005;69:167–180. doi:10.1016/j.anbehav.2004.02.009 [Google Scholar]
- Kauffman S.A. Oxford University Press; Oxford, UK: 1993. The origins of order. [Google Scholar]
- Kitano H. Computational systems biology. Nature. 2002;420:206–210. doi: 10.1038/nature01254. doi:10.1038/nature01254 [DOI] [PubMed] [Google Scholar]
- Korb J, Heinze J. Multilevel selection and social evolution of insect societies. Naturwissenschaften. 2004;91:291–304. doi: 10.1007/s00114-004-0529-5. doi:10.1007/s00114-004-0529-5 [DOI] [PubMed] [Google Scholar]
- Kuramoto Y. Self-entrainment of a population of coupled oscillators. In: Araki H, editor. Int. Symp. on Mathematical Problems in Theoretical Physics. vol. 39. Springer; Berlin: 1975. [Google Scholar]
- Kuramoto Y. Springer; Berlin: 1984. Chemical oscillations, waves and turbulence. [Google Scholar]
- Levin S.A. Helix books; Reading, MA: 2000. Fragile dominion: complexity and the commons. [Google Scholar]
- Lindauer M. Ein Beitrag zur Frage der Arbeitsteilung im Bienenstaat. Z. Vergl. Physiol. 1952;34:299–345. doi:10.1007/BF00298048 [Google Scholar]
- Mackinson S. Variation in structure and distribution of pre-spawning Pacific herring shoals in two regions of British Columbia. J. Fish Biol. 1999;55:972–989. [Google Scholar]
- Mailleux A.C, Jean-Louis D, Detrain C. How do ants assess food volume? Anim. Behav. 2000;59:1061–1069. doi: 10.1006/anbe.2000.1396. [DOI] [PubMed] [Google Scholar]
- Mallon E.B, Pratt S.C, Franks N.R. Individual and collective decision-making during nest site selection by the ant Leptothorax albipennis. Behav. Ecol. Sociobiol. 2001;50:352–359. doi:10.1007/s002650100377 [Google Scholar]
- Milgram S. McGraw-Hill; New York: 1992. The individual in the social world. [Google Scholar]
- Milgram S, Toch H. Collective behaviour: crowds and social movements. In: Lindzey G, Aronson E, editors. The handbook of social psychology. vol. IV. Addison-Wesley; Reading, MA: 1969. [Google Scholar]
- Milgram S, Bickman L, Berkowitz L. Note on the drawing power of crowds of different size. J. Pers. Soc. Psychol. 1969;13:79–82. [Google Scholar]
- Möglich M. Social organization of nest emigration in Leptothorax (Hym., Form.) Insect. Soc. 1978;25:205–225. doi:10.1007/BF02224742 [Google Scholar]
- Neda Z, Ravasz E, Brechet Y, Vicsek T, Barabasi A.L. The sound of many hands clapping. Nature. 2000a;403:849. doi: 10.1038/35002660. doi:10.1038/35002660 [DOI] [PubMed] [Google Scholar]
- Neda Z, Ravasz E, Vicsek T, Brechet Y, Barabasi A.L. Physics of the rhythmic applause. Phys. Rev. E. 2000b;61:6987–6992. doi: 10.1103/physreve.61.6987. doi:10.1103/PhysRevE.61.6987 [DOI] [PubMed] [Google Scholar]
- Neumann P, Moritz R.F.A. Testing genetic variance hypotheses for the evolution of polyandry in the honeybee (Apis mellifera capensis Escholtz) Insect. Soc. 2000;41:271–279. [Google Scholar]
- Nicolis G, Prigogine I. Wiley; New York: 1977. Self-organization in nonequilibrium systems. [Google Scholar]
- Nowak M.A, May R.M. Evolutionary games and spatial chaos. Nature. 1992;359:826–829. doi:10.1038/359826a0 [Google Scholar]
- Okubo A. Dynamical aspects of animal grouping. Adv. Biophys. 1986;22:1–94. doi: 10.1016/0065-227x(86)90003-1. doi:10.1016/0065-227X(86)90003-1 [DOI] [PubMed] [Google Scholar]
- Ormerod P. Pantheon books; New York: 1998. Butterfly economics. [Google Scholar]
- Pankiw T, Page R.E. Response thresholds to sucrose predict foraging division of labor in honeybees. Behav. Ecol. Sociobiol. 2000;47:265–267. doi:10.1007/s002650050664 [Google Scholar]
- Parrish J.K, Edelstein-Keshet L. Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science. 1999;284:99–101. doi: 10.1126/science.284.5411.99. [DOI] [PubMed] [Google Scholar]
- Parrish J.K, Viscido S.V, Grunbaum D. Self-organized fish schools: An examination of emergent properties. Biol. Bull. 2002;202:296–305. doi: 10.2307/1543482. [DOI] [PubMed] [Google Scholar]
- Partridge B.L.J. The structure and function of fish schools. Scientific American. 1982;246 doi: 10.1038/scientificamerican0682-114. [DOI] [PubMed] [Google Scholar]
- Partridge B.L.J, Johansson J, Kalisk J. Structure of schools of giant bluefin tuna in Cape Cod Bay. Environ. Biol. Fish. 1983;9:253–262. [Google Scholar]
- Popper K. Routledge/Abingdon; London/USA: 1957. The poverty of historicism. [Google Scholar]
- Pratt S.C. Detection of a nestmate quorum by encounter rates in the ant Leptothorax albipennis. Behav. Ecol. 2005;16:488–496. doi:10.1093/beheco/ari020 [Google Scholar]
- Pratt, S. C. In press. Behavioural mechanisms of collective nest-site choice by the ant Temnothorax curvispinosis Insect. Soc.
- Pratt S.C, Pierce N.E. The cavity-dwelling ant Leptothorax curvispinosus uses nest geometry to discriminate between potential homes. Anim. Behav. 2001;62:281–287. doi:10.1006/anbe.2001.1777 [Google Scholar]
- Pratt S.C, Mallon E.B, Sumpter D.J.T, Franks N.R. Quorum sensing, recruitment, and collective decision-making during colony emigration by the ant Leptothorax albipennis. Behav. Ecol. Sociobiol. 2002;52:117–127. doi:10.1007/s00265-002-0487-x [Google Scholar]
- Pratt S.C, Sumpter D.J.T, Mallon E.B, Franks N.R. An agent-based model of collective nest site choice by the ant Leptothorax albipennis. Anim. Behav. 2005;70:1023–1036. [Google Scholar]
- Ratnieks F.L.W. Reproductive harmony via mutual policing by workers in Eusocial hymenoptera. Am. Nat. 1988;132:217–236. doi:10.1086/284846 [Google Scholar]
- Ratnieks F.L.W, Visscher P.K. Worker policing in the honeybee. Nature. 1989;342:796–797. doi:10.1038/342796a0 [Google Scholar]
- Reebs S.G. Can a minority of informed leaders determine the foraging movements of a fish shoal? Anim. Behav. 2000;59:403–409. doi: 10.1006/anbe.1999.1314. doi:10.1006/anbe.1999.1314 [DOI] [PubMed] [Google Scholar]
- Reynolds C.W. Flocks, herds and schools: a distributed behavioural model. Comp. Graph. 1987;21:25–33. [Google Scholar]
- Robson S.K, Traniello J.F.A. Key individuals and the organisation of labor in ants. In: Detrain C, Deneubourg J.L, Pasteels J.M, editors. Information processing in social insects. Birkhauser; Basel: 1999. pp. 239–259. [Google Scholar]
- Schelling T. Norton; New York: 1978. Micromotives and macrobehaviour. [Google Scholar]
- Seeley T.D. Belknap Press of Harvard University Press; Cambridge, MA: 1995. The wisdom of the hive. [Google Scholar]
- Seeley T.D. Thoughts on information and integration in honey bee colonies. Apidologie. 1998;29:67–80. [Google Scholar]
- Seeley T.D. When is self-organization used in biological systems? Biol. Bull. 2002;202:314–318. doi: 10.2307/1543484. [DOI] [PubMed] [Google Scholar]
- Seeley T.D. Consensus building during nest-site selection in honey bee swarms: the expiration of dissent. Behav. Ecol. Sociobiol. 2003;53:417–424. [Google Scholar]
- Seeley T.D, Buhrman S.C. Nest-site selection in honey bees: how well do swarms implement the “best-of-N” decision rule? Behav. Ecol. Sociobiol. 2001;49:416–427. doi:10.1007/s002650000299 [Google Scholar]
- Seeley T.D, Visscher P.K. Choosing a home: how the scouts in a honey bee swarm perceive the completion of their group decision making. Behav. Ecol. Sociobiol. 2003;54:511–520. doi:10.1007/s00265-003-0664-6 [Google Scholar]
- Seeley T.D, Visscher P.K. Quorum sensing during nest-site selection by honeybee swarms. Behav. Ecol. Sociobiol. 2004;56:594–601. doi:10.1007/s00265-004-0814-5 [Google Scholar]
- Seeley T.D, Camazine S, Sneyd J. Collective decision-making in honey bees: how colonies choose among nectar sources. Behav. Ecol. Sociobiol. 1991;28:277–290. doi:10.1007/BF00175101 [Google Scholar]
- Seeley T.D, Weidenmuller A, Kuhnholz S. The shaking signal of the honey bee informs workers to prepare for greater activity. Ethology. 1998;104:10–26. [Google Scholar]
- Simpson S.J, McCaffery A.R, Hagele B.F. A behavioural analysis of phase change in the desert locust. Biol. Rev. 1999;74:461–480. doi:10.1017/S000632319900540X [Google Scholar]
- Simpson S.J, Despland E, Hagele B.F, Dodgson T. Gregarious behavior in desert locusts is evoked by touching their back legs. Proc. Natl Acad. Sci. USA. 2001;98:3895–3897. doi: 10.1073/pnas.071527998. doi:10.1073/pnas.071527998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sornette D. Springer; Berlin: 2004. Critical phenomena in natural sciences. [Google Scholar]
- Stern K, McClintock M.K. Regulation of oculation by human pheromones. Nature. 1998;392:177–179. doi: 10.1038/32408. doi:10.1038/32408 [DOI] [PubMed] [Google Scholar]
- Strogatz S. Penguin books; London: 2003. Sync: the emerging science of spontaneous order. [Google Scholar]
- Sumpter D.J.T, Beekman M. From nonlinearity to optimality: pheromone trail foraging by ants. Anim. Behav. 2003;66:273–280. doi:10.1006/anbe.2003.2224 [Google Scholar]
- Surowiecki J. Little Brown; New York: 2004. The wisdom of crowds. [Google Scholar]
- Theraulaz G, et al. Spatial patterns in ant colonies. Proc. Natl Acad. Sci. USA. 2002;99:9645–9649. doi: 10.1073/pnas.152302199. doi:10.1073/pnas.152302199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theraulaz G, Gautrais J, Camazine S, Deneubourg J.L. The formation of spatial patterns in social insects: from simple behaviours to complex structures. Philos. Trans. R. Soc. A. 2003;361:1263–1282. doi: 10.1098/rsta.2003.1198. doi:10.1098/rsta.2003.1198 [DOI] [PubMed] [Google Scholar]
- Trivers R.L, Hare H. Haplodiploidy and the evolution of the social insects. Science. 1976;191:249–263. doi: 10.1126/science.1108197. [DOI] [PubMed] [Google Scholar]
- von Bertalanffy L. George Braziller; New York: 1968. General system theory. [Google Scholar]
- von Dassow G, Meir E, Munro E, Odell G. The segment polarity network is a robust development module. Nature. 2000;406:188–192. doi: 10.1038/35018085. doi:10.1038/35018085 [DOI] [PubMed] [Google Scholar]
- Weidenmuller A. The control of nest climate in bumblebee (Bombus terrestris) colonies: interindividual variability and self reinforcement in fanning response. Behav. Ecol. 2004;15:120–128. doi:10.1093/beheco/arg101 [Google Scholar]
- Wiener N. Wiley; New York: 1948. Cybernetics. [Google Scholar]
- Wilson E.O. Belknap Press of Harvard University Press; Cambridge, MA: 1971. The insect societies. [Google Scholar]
- Wolfram S. Wolfram Media; Champaign, IL: 2002. A new kind of science. [Google Scholar]