Abstract
For centuries scientists have been fascinated with the question of how the brain works. Investigators have looked at both where different functions are localized and how the anatomical microstructure varies across the brain surface. Here we discuss how advances in magnetic resonance imaging (MRI) have allowed in vivo visualization of the fine structure of the brain that was previously only visible in post-mortem brains. We present data showing the correspondence between definitions of the primary visual cortex defined anatomically using very high-resolution MRI and functionally using functional MRI. We consider how this technology can be applied to allow the investigation of brains that differ from normal, and what this ever-evolving technology may be able to reveal about in vivo brain structure in the next few years.
Keywords: structure and function, myeloarchitecture, high-resolution imaging, functional magnetic resonance imaging
One of the most fascinating aspects of the brain is the range of functions that it can perform. While most other major organs of the body are specialized to perform a specific role, the brain is responsible for processing everything that we can see, hear, touch and think. For hundreds of years scientists have been interested in how this large variety of functions is represented in the brain. The first attempt at localizing function to particular regions of the brain came from the study of phrenology by Gall & Spurzheim at the end of the eighteenth century (Gall & Spurzheim 1810). Human characteristics were assigned to different regions of the head according to the bumps on the surface of the skull with the belief that regions used frequently would increase in size, while those not used would atrophy. This division of function is shown in figure 1a. Clearly, the types of attributes that are represented in this division reflect the views of that time, and are no longer considered to have scientific value. Moreover, it is clear that differences in the shape of the skull do not necessarily reflect underlying differences in brain structure.
Figure 1.
Different approaches to dividing the brain into distinct areas. (a) Phrenology divisions, based on the pattern of bumps on the skull. (b) The lateral and medial views of the brain with the divisions of Brodmann.
In the era when phrenology was becoming increasingly popular, neurologists were just beginning to investigate the structure of the post-mortem brain: the beginning of modern histology. The brain is made up of grey matter, white matter and cerebrospinal fluid (CSF). The grey matter contains the cell bodies and dendrites of the neurons and forms the cerebral cortex. White matter consists of the axons of the neurons and appears white due to the presence of a fatty substance known as myelin. Myelin surrounds the axons in order to increase the speed of transmission of information. Finally, the brain is bathed in a solution known as CSF that provides cushioning for the brain within the skull.
Fransisco Gennari (Gennari 1782) was the first to identify a white line lying within the grey matter that was more predominant in the posterior regions of the brain. Several decades later, Baillarger (1840) investigated the cortex by cutting slices, putting them between glass slides and viewing them with a light shining through. With this more sensitive method he discovered that, in fact, this white line described by Gennari could be traced in all parts of the cortex. Although, these observations were made in the eighteenth and nineteenth centuries, it was not until 1907 that Elliott Smith used a lens to divide the cortex into over 30 regions based on the differences in the patterns of the white lines present in the cortex (Elliott Smith 1907). These white lines identified by Gennari, Baillarger and Elliott Smith are actually myelin that exists within the cortex, and these patterns that occur in the cortex are known as myeloarchitecture.
At a similar time, Korbinian Brodmann, one of the best known anatomists, was also working to subdivide the human brain into distinct regions. However, rather than using the presence of the white lines within the cortex, he meticulously divided the cortex into ‘areas’ on the basis of the cell size, type and density as seen under a microscope (Brodmann 1909; Garey 1999). This type of classification is termed cytoarchitecture. Although, several alternative classifications schemes have since been suggested (e.g. von Economo & Koskinas 1929), these 52 ‘Brodmann's areas’ are still very much used in modern neuroscience. These cytoarchitectonic maps of Brodmann are shown in figure 1b.
It is, of course, true that identifying regions based on their visual appearance does not necessarily mean that the regions also have distinct functions. At a similar time to these histological divisions of the cortex, investigations were also taking place into the localization of different brain functions using patients who had suffered damage to their brain. One of the first functions to be localized in this way was language. In the late nineteenth century, Paul Broca studied a patient who could no longer produce any fluent speech, although he could understand spoken language (Broca 1861). A few years later, Wernicke described a patient who could not understand language, and, although his speech was fluent, it was meaningless. Following the death of these patients, the location of the damage to their brains was identified. The two distinct regions noted in the patients are now known as Broca's area and Wernicke's area.
While looking at the specific deficits that arise from brain damage can indicate some type of functional localization, one of the problems is that the type of damage resulting from either strokes or impact tends to be quite extensive. Thus, actually locating the region of the brain that is critical for the lost function is very difficult.
Over the past decade, the use of functional brain imaging has allowed the investigation of functional localization in the brains of normal subjects. In this type of scanning, subjects are asked to perform a particular task, such as generating words or looking at a particular type of visual pattern, while changes in blood flow to the brain are measured. A localized change in blood flow indicates that a particular area is involved in the processing of that task.
While non-invasive methods for identifying the function of a particular brain region are now well established, there is still a challenge to relate this location to the architecture visible under the microscope. Here, we describe how imaging technology can now be used to identify anatomical regions based on the underlying myeloarchitecture in the living human brain. This has allowed a comparison between underlying anatomy and functional specialization in the living human for the first time. We then discuss, with two examples, how this anatomical imaging can provide additional, important information about damaged brains. Finally, we consider how this technology may be improved over the next few years to provide an even more sensitive method of investigating the anatomy underlying brain function.
1. Finding the myelin using magnetic resonance imaging
Most people are familiar with magnetic resonance imaging (MRI) scans of the brain and the way they beautifully depict the internal structure of this fascinating organ. The most striking feature in such scans, apart from the overall shape of the persons head, is the distinction between the convoluted surface of the brain—the cerebral cortex—and the white matter connections that wire up different parts of the brain to each other. While MRI is understood to map the concentration of water in the structure being imaged, this distinction between white matter and grey matter arises in a much more subtle way. In nuclear magnetic resonance (NMR), the phenomenon that MRI is based upon, a characteristic property of the hydrogen atoms being imaged is the freedom of motion that the water molecules they are attached to have. This property is characterized numerically as the ‘relaxation time’ T1. MR images can be made sensitive to T1, such that water molecules in free solution could appear dark on an image when compared to water molecules that are in the vicinity of large fatty molecules, such as the myelin that coats the axons in white matter. Such imaging methods have been used for many years to generate in vivo images of the human brain, and diagnose disorders of the white matter, such as multiple sclerosis. However, as described earlier, the white matter is not the only place where myelin is present in the brain. In certain regions of the cerebral cortex, connections within the cortex are also myelinated, giving rise to the patterns of myeloarchitecture used to make anatomical distinctions between brain areas. One of the most striking examples of this is in the primary visual cortex where there is a very dense band of myelination in the middle of the grey matter. This band, first described by Gennari, gives rise to this structure's alternate name—the striate cortex. Attempts to image cortical myelination in living humans, however, have not been so straightforward. The major obstacle to imaging this myeloarchitecture is its size. Most imaging of the brain is performed at a resolution of around 1×1×1 mm3. However, since the thickness of the myelin band within the cortex is only around 0.25 mm, it would not be visible at that resolution. Therefore, it is necessary to increase the resolution of the scans. The first attempt to do this was by Clark et al. (1992). Imaging on a standard 1.5 T clinical scanner, they observed a dark band within the cortex on MRI scans. In this work, they aligned the imaging slices to be perpendicular to the plane of the cortex so that their high in-plane resolution of 0.39 mm would stand a chance of detecting the myelinated band. While these images were promising, it took considerable developments in imaging technology to provide images that unequivocally displayed the myelination of the striate cortex. The challenge in such imaging is to overcome the huge reductions in the ratio of ‘signal’ to ‘noise’ that occur when the resolution of the images is increased. By using innovations such as high magnetic field (3 T) specialist MRI machines, novel detection hardware and repeated measurements over a period of up to an hour, several research groups have been able to clearly demonstrate the ability to image the cortical myelin pattern of the striate cortex in vivo (Barbier et al. 2002; Clare et al. 2002; Walters et al. 2003; Bridge et al. 2005). Examples of such images are shown in figure 2. On these images, myelin appears dark relative to non-myelinated grey matter (the CSF that bathes the brain appears brightest). This is opposite to what is seen on a classical T1-weighted image, due to the particular parameters used. The cortical myelination can be seen as a dark line within the grey matter as indicated by the white arrows. In each image, the thickness of the ‘slice’ that is imaged is considerably larger than the in-plane resolution of 0.3×0.3 mm2, meaning that as the highly convoluted cerebral cortex curves in and out of the slice, the stria of Gennari appears and disappears. In order to increase the detection of the striate cortex, our approach has been to image the cortex at three different orientations, as shown in the three rows of figure 2. Thus, by combining the information from the three scans it should be possible to detect the stria of Gennari throughout its length.
Figure 2.

Viewing the stria of Gennari with high-resolution imaging. In these images, the white matter appears as dark and the CSF as white. The three rows represent the three different orientations at which the brain was scanned in a single subject. The right column shows a magnified view of the region around the primary visual cortex. The stria of Gennari can be seen as a dark line in the middle of the grey matter, as indicated by the white arrows. These scans were acquired at a resolution of 0.3×0.3 mm2.
2. Comparing structure and function in the living brain
One of the problems with the histology performed in post-mortem brains is that there is no opportunity to investigate whether the divisions based on the anatomy correspond to divisions that can be measured functionally. The ability to detect myelin within the cortex of living human subjects provides us with an ideal opportunity to investigate whether the myeloarchitecture-based divisions correspond to those determined functionally.
The visual system lends itself particularly well to investigating the relationship between functional and structural definitions, as it is possible to subdivide the occipitial lobe into several different functional areas. Zeki (1970) showed that the primate visual cortex could be divided into multiple distinct areas, each with its own representation of the visual field, i.e. every point in the visual world is represented once in each visual area. Furthermore, these maps are arranged ‘retinotopically’ such that objects adjacent in space are also represented by adjacent bits of cortex. This neural arrangement has been exploited by visual scientists using functional MRI (fMRI) to identify several distinct visual areas in human subjects (Engel et al. 1994, 1997; Sereno et al. 1995; DeYoe et al. 1996). These fMRI experiments use two types of flashing stimuli. An expanding ring stimulus, as shown in figure 3a, is used to measure the response to different eccentricities of the stimulus. The subject views the centre of the screen, while the flashing ring stimulus is increased in size from very small in the centre to the large annulus shown in the figure. This causes a travelling wave of activity to move across the surface of the visual cortex. Figure 3b shows a rendering of the activity on the visual cortex of one subject. It can be seen that the activity corresponding to the stimulus being at the centre of the visual field is at the back of the brain (red/yellow) and as the stimulus gets larger, the focus of the neural activity moves forward in the brain.
Figure 3.
Mapping individual visual areas in the visual cortex. The top row shows the flashing expanding ring stimulus used to map the eccentricity of the visual field (a). (b) The neural activity to central stimuli is represented at the back of the brain and eccentric stimuli activate more anterior regions of the cortex. (c) This activity superimposed on a flattened view of the left hemisphere. The lower row shows the representation of the angular dimension. (d) A flashing wedge stimulus moves around the visual field. The resulting activity is shown on the rendering of the visual cortex in (e), and it can be seen, with reference to the key, that one hemisphere has a representation of the opposite side of visual space. (f) The border between the functionally defined primary and secondary visual cortices (V1 and V2).
The angular dimension is mapped using a wedge of flashing stimulation, as shown in figure 3d. The wedge is moved around the visual field as the subject looks at the centre of the screen. Each hemisphere only has a representation of one-half of visual space, leading to the pattern of activation seen in figure 3e. Since multiple visual areas exist, and each has a representation of the visual field, a pattern of stripes can be seen on the cortex. When the cortical surface is computationally flattened, this pattern of stripes becomes more obvious. Figure 3c shows the eccentricity activity on a flattened representation of the visual cortex. Figure 3f shows the activity due to the change in angle as the flashing wedge moves around the visual field. The primary visual cortex is marked V1 on figure 3f, and contains a representation of one-half of visual space. V1 is bordered on both sides by the secondary visual cortex (V2). These borders occur at the vertical midline, as can be seen by the flattened view. One-half of V2 represents the upper visual field, and the other half represents the lower visual field, such that the two parts of V2 can be added together to give a representation of the whole hemifield. In order to obtain this type of flattened representation, it is necessary to be able to determine regions of grey matter. This is because the cortical surface has many folds (leading to sulci and gyri) so, although two parts of the brain may look as though they are adjacent, in fact they may represent areas of visual space that are quite far apart. Figure 4 illustrates this problem. The physical distance between X and Y is very small. However, the distance along the cortical surface, shown by the dashed white and black line, is considerably longer. Therefore, regions X and Y may represent quite different regions of the visual field. In order to produce flattened cortical maps, such as those in figure 3, on which the functional data can be overlaid, it is critical to have an excellent method to distinguish the grey matter from the white matter and CSF.
Figure 4.

The importance of identifying grey matter. The two points X and Y are physically very close together on the brain. However, this is due to the folding of the cortex, and, in fact, along the cortical surface the distance between the two points is considerably longer (shown by the white and black dashed line).
This method of mapping the functionally defined primary visual cortex allows us to investigate whether the anatomical definition of the striate cortex corresponds with the functional definition. The demonstration of correspondence between these two measures can validate these two independent methods of defining V1.
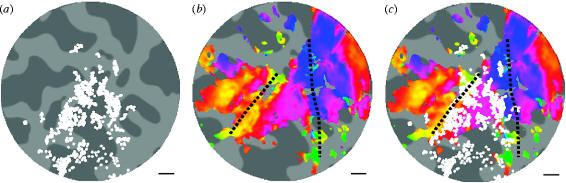
We set out to test how well the striate cortex defined anatomically corresponded with this functional definition of V1. We scanned five subjects with both high-resolution anatomical imaging and fMRI to identify the location of the border between V1 and V2. The high-resolution imaging was performed in three separate scanning sessions, using three different slice orientations (shown in figure 2). To obtain a map of the cortical region where the myelination could be visualized, three observers independently marked on each of the three high-resolution scans where they could see the dark line through the cortex. For the final images, any region of cortex where two of the three observers could see the myelination was considered to contain the striate cortex. These regions of cortex were then transformed from the three individual scans to a scan of the whole brain that was taken for each subject, allowing the data to be combined from the different scan orientations. An illustration of the region identified as containing the myelination pattern on a flattened brain for a single subject is shown in figure 5a.
Figure 5.
Anatomical and functional definitions of striate cortex. (a) The regions of the visual cortex on the flattened cortex in which the myelination has been identified (from all three different orientations). (b) The same region of cortex, with the retinotopic map superimposed. The dotted black lines show the functionally defined border between V1 and V2. In (c), these two datasets are combined to show the correspondence between the functional and the anatomical data.
We hypothesized that the cortex showing myelination should only be found within the primary visual cortex, and not outside. In order to measure this, we performed a retinotopic mapping experiment using fMRI in the same subjects to measure the location of the functional border between V1 and V2. The retinotopic map from the same subject is shown in figure 5b. The border between V1 and V2 is shown in black. When the anatomical and functional definitions of the striate cortex/primary visual cortex are combined, it is clear that they correspond well. In figure 5c, it can be seen that the majority of the region where the striate cortex is identified (white region) lies within the region designated V1 by the retinotopic mapping. However, it is also clear that although a considerable amount of myelination can be identified within the functional definition of V1, it is not fully covered. In fact, the proportion of this functionally defined V1 in which the myelination could be identified was 81%. Across all five subjects that were scanned in this experiment, the mean proportion was 56%. One of the challenges with this high-resolution scanning technique is improving the data quality such that this proportion can be increased to nearer 100%. Possible methodological improvements will be discussed later.
There are two major obstacles to the comparison of the high-resolution structural images with the functional images. First, there is a significant difference in the resolution of the images. The structural images are 0.3×0.3×1.5 mm3 compared to 2×2×2 mm3 for the functional images. This difference in values is necessary because the structural imaging requires very high spatial resolution, while the functional imaging requires very high temporal resolution. However, this difference should not lead to any systematic offset between the two datasets. Second, the technique used to gather the fMRI data, known as echo planar imaging (EPI) can lead to image distortions. Such distortions are not present in the structural imaging. However, in the visual cortex, distortion of EPI images is low because there are no large areas of CSF, a major cause of distortion. A third possibility is that the particular pattern of blood vessels in the region of the boundaries between visual areas will affect the fMRI signal. However, it is not the magnitude of the neural response that provides the location of the boundary, but the pattern of the response. Therefore, although the signal magnitude may vary considerably due to the vasculature, there should not be any consistent effect on the boundary location measured with fMRI.
To summarize, although there are major differences in spatial resolution and distortion between the structural and functional techniques, neither should consistently affect the location of visual area boundaries. This is reflected in the close correspondence between the boundaries measured with the two techniques.
A second visual area that has been investigated is that known as human V5 or middle temporal area (MT). This area is particularly sensitive to moving stimuli, and when a subject is presented with a field of moving dots compared to stationary dots, this region will activate (Zeki et al. 1991; Watson et al. 1993; Tootell & Taylor 1995; Huk et al. 2002). Furthermore, patients with damage in this region often show ‘motion blindness’ such that they are impaired at tasks that require the detection of motion, but unaffected on those that do not (Marcar 1997). Studies in the macaque monkey have shown that the homologous motion sensitive region (V5/MT) has a distinctive pattern of myelination within the cortex. Walters et al. (2003) tested the relationship between the structural region detected with the high-resolution MRI and the region of functional activation to moving visual stimuli. They found that the area believed to show the myeloarchitecture characteristic of V5 was in good spatial alignment with the activated region.
A detailed study of the myeloarchitecture of the human visual cortex by Clarke & Miklossy (1990) has raised the possibility that it may be possible to distinguish further visual areas on the basis of their pattern of myelination. However, it is likely that further methodological improvement will be necessary before such a study is attempted.
3. Application of the methodology to abnormal brains
The data presented above illustrate that the myelination as imaged using high-resolution MRI can indicate the region of cortex that represents the primary visual cortex. There are several useful ways in which this technique can be applied. In particular, this methodology is useful in patients with abnormal brain structure. For example, if there is a tumour or lesion in the visual cortex, it may be difficult to locate V1 from the normal position of the calcarine sulcus due to movement of or damage to the brain. Furthermore, while it may be possible to produce a retinotopic map to functionally define different visual areas, this is considerably more difficult when the brain structures are not in their usual place. This difficulty arises, in part, because it is necessary to identify the grey matter of the cortex and distinguish it from the white matter and CSF in order to produce a retinotopic map, from which the visual areas can be defined. In a normal brain this is reasonably straightforward, because the T1 values for these three types of brain matter are quite discrete and distinction is not too difficult. However, where there is additional matter present, such as a tumour, determining the entire surface of grey matter is considerably more difficult. Any errors in this process mean that when the cortical surface is subsequently flattened out, points that are adjacent on the cortical surface are not necessarily adjacent on the flattened map. The boundaries between visual areas are therefore likely to be considerably more difficult to detect.
Although, it is necessary to distinguish the grey matter in order to define the striate cortex in the high-resolution image, it does not require a continuous cortical surface as is necessary for the retinotopic mapping. Therefore, this technique could help localization in abnormal brains where there is a need to identify the striate cortex. We have performed high-resolution scanning in two patients in order to try to detect the striate cortex as a method for locating V1 in affected hemispheres, one in a patient with a large occipital lesion and the other in a patient with an occipital tumour.
Subject GY has been studied extensively over the past 20 years (Barbur et al. 1980, 1993; Baseler et al. 1999; Cowey & Stoerig 2004). Following a car accident at the age of 8 years, GY suffered an extensive lesion in the region of the left primary visual cortex (figure 6a) and in the right parietal lobe (not shown). Lesions of V1 render subjects blind on the affected side, and in fact the region of the visual field that is affected can be predicted from the area that is damaged. GY has been studied due to a phenomenon that he shows, termed ‘blindsight’. This means that, despite not being able to ‘see’ in his blind field, GY can perform several tasks above chance levels, such as detecting the direction of a moving stimulus. When doing these tasks GY feels that he is guessing, and therefore is unaware that his choices are often correct. One of the popular explanations for this is the existence of a direct pathway from the subcortical visual areas to the motion area, human MT/V5. However, it is important in experiments such as these to ensure that there are no remaining regions of V1 that underlie this ability. Barbur et al. (1993) measured the visual field of GY to investigate the regions in which his vision was affected, and found that his central visual field (less than 2.5°) was spared, as was a small strip of the lower visual field. Therefore, in these regions GY can consciously see objects. The spared region of V1 corresponding to central vision can be seen on a normal MRI scan of the brain, as it is clear that the damage did not extend all the way to the back of the brain. Baseler et al. (1999) used fMRI to produce a retinotopic map for patient GY in both his affected and unaffected hemispheres. While the retinotopic map showed activation in the spared central region of the visual field, activity in the dorsal part of primary visual cortex, which would correspond to the strip in the lower visual field, was not obvious. However, Baseler et al. noted that in higher visual areas that receive input from V1, there was activity that could have come from the dorsal part of V1.
Figure 6.
Scans from ‘blindsight’ patient GY. The scan in (a) shows the large lesion in the left occipital lobe of this patient. (b) Two slices from a high-resolution scan of the occipital lobe. Again, in all these scans, white matter is dark and the CSF is bright. The lesion on the right-hand side of these pictures shows up white. Adjacent to the lesion is a small portion of cortex that appears to contain the stria of Gennari, indicated by the arrows. This dark band can be seen if multiple cross-sections are taken through the grey matter, shown by the black lines in the upper panel of (c). When the change in intensity across the cortex is calculated, it is clear that there is a decrease in intensity in the middle of the grey matter. (c, lower panel).
We applied our high-resolution technique in an attempt to locate remaining regions of striate cortex. Figure 6a shows a single slice from a scan of GY's brain showing the large lesion in the occipital cortex (indicated by the white arrow). Figure 6b shows two slices from the high-resolution scans that show the lesion (and CSF) as bright white. Adjacent to the lesioned region is a section of dorsal visual cortex (representing the lower visual field) that appears to show the myelination predicted for primary visual cortex. In this case, we do not have the functional data to test whether this region lies within the central spared vision or not. However, it is clear that the combination of this technique, that has the potential to detect ‘islands’ of residual striate cortex, with a carefully constructed retinotopic map should provide a complete plan of striate cortex regions that remain following damage to the visual cortex.
Our second patient was referred to a neurologist complaining of blurring in the visual field. CT scanning revealed a tumour in the posterior region of the right occipital lobe. This tumour can be seen in figure 7a as a white region at the back of the brain. In order to provide an idea of the risks of surgical removal of the tumour, we were asked to investigate the cortical tissue surrounding the tumour to suggest what visual function could be lost if this tissue were damaged during surgery. As described above, since the majority of retinal output is projected to V1, and then on to higher visual areas, loss of any of this region will render the subject effectively blind in some or all of one visual field. As an initial step, we used a standard retinotopic mapping fMRI experiment to attempt to identify V1 based on its functional organization. However, two factors affected our ability to achieve this mapping. First, the level of response to the stimulation was considerably weaker in the side with the tumour. Second, the identification of the grey matter was very difficult due to the presence of the tumour, as it was not always clear how to classify a particular piece of tissue. This functional data gave an initial estimate of where V1 was located and its relationship to the tumour location. In order to attempt to establish conclusively that this functionally defined region did indeed correspond to V1, we performed a high-resolution scan to locate the striate cortex. Two slices from this scan are shown in figure 7b. The tumour can be seen on these scans circled in white. The presence of myelination is indicated with the white arrows. Since we were only able to perform this high-resolution scanning in one orientation, it was not possible to obtain a complete map of the region that could be classified as striate cortex. However, by combining the regions where the myelination could be detected with the functional data from the retinotopic mapping, we were able to demonstrate that this tumour was surrounded by primary visual cortex, and therefore surgical removal could lead to cortical blindness in some regions of the left visual field.
Figure 7.
High-resolution scanning of the visual cortex of a patient with a tumour. (a) The location of the tumour at the posterior part of the right hemisphere. (b) Two slices from the high-resolution anatomical scan with the identified myelination shown with the white arrows. The tumour is outlined in white.
4. Towards in vivo histology
The work presented here illustrates the ability to image the myelin patterns within the cortex. The main advantage of using this method is that it is directly comparable with post-mortem histological staining methods. The bands of myelination that are imaged using MRI are exactly the same as those that are revealed by staining. This is important in being able to link the work using classical neuroscience methods with the more recent neuroimaging methods. However, there are several problems with attempting to characterize the cortex in this way. First, while considerable improvements in the scanning technology have made the reliable detection of the stria of Gennari in the striate cortex possible, the technique is still not yet sensitive enough to detect all the subtle myelin patterns that the cortex contains. For this to happen, there needs to be a significant increase in the sensitivity of the MRI method. The most basic, and most expensive, way to increase the sensitivity is to increase the strength of the magnet used for the MRI. Although 1.5 T is still the most common field strength in the clinical domain, there are now many 3 T scanners being used in the research arena. There are also a small but increasing number of scanners built around 7 T magnets and the highest human imaging system operates at 9.4 T. At present, the large technological and cost overheads of scanning at such high field means that this will not be very widely available for some time. However, it is applications like the potential for carrying out in vivo histology that may make these machines less the preserve of highly specialist labs, and more an essential tool for neuroscience (Robitaille et al. 2000; Wiggins et al. 2005). Much cheaper methods for increasing the sensitivity, however, should soon be available on many scanners, even those operating at 1.5 T. The design of MRI signal detectors (radiofrequency coils) has advanced considerably over the past 5 years. Specifically, by using an array of multiple detectors, rather than just a single detector, the sensitivity can be significantly increased. While eight channel detectors are now common on new MRI machines, 16, 32 and even 64 channel detectors are rapidly becoming available and can improve the sensitivity by at least a factor of 4 (Zhu et al. 2004; de Zwart et al. 2004). These major technological developments will also need to be accompanied by some more basic developments in the way that images are acquired and analysed.
It is important to ensure that the MRI signals used to measure histology correspond to the histological properties found in sections of cortex viewed through a microscope. To measure this correspondence Zilles and colleagues have scanned post-mortem brains with MRI at high-resolution and compared the patterns found with histologically stained sections of cortex (Amunts et al. 2000; Eickhoff et al. 2005). The use of post-mortem brains means that the MRI scans can be long, which means that the quality of the images can be considerably higher than in the shorter scans that must be used in living subjects. Obviously, this type of work with post-mortem brains is not suitable for the ultimate goal of correlating structure and function.
Even, if the MRI methods could be improved sufficiently to reliably image all the myelin patterns in the cortex, this would still not be sufficient to distinguish as many brain regions as is currently done under the microscope, since not all cortical regions have a distinct myeloarchitecture. Histological maps, such as that of Brodmann, rely on many microscopic features of the cerebral cortex such as cell size, shape and density to distinguish regions. These features are far too small to be imaged directly by MRI. However, there are promising signs that it may be possible to find MRI markers that identify the underlying cellular structure. One that is already established and validated is the measurement of the thickness of the cerebral cortex. In the primary motor cortex, for example, the cells are significantly larger and less densely spaced than those in the adjacent sensory cortex (Rademacher et al. 2001). This microscopic feature results in a bulk change in the thickness of the cortex in this region. Several researchers have now demonstrated that by measuring the thickness of the cortex from MRI scans, it is possible to find such boundaries (Fischl & Dale 2000), and this method may provide valuable markers for the cellular structure.
The ability to directly probe cell density or type itself would be the optimal way to image histology. As described earlier, some of the fundamental NMR parameters that govern the appearance of MRI scans are themselves sensitive to the molecular environment of the water being imaged. The NMR ‘relaxation times’ T1 and T2 are examples of these, and by measuring these values and how they vary over the cerebral cortex, some indication of cell structure may be obtained. There is already some evidence that T1 is not uniform over the cortex (Fischl et al. 2004), but the structural features to which T1 would correlate are not yet clear. Similarly, T2 is sensitive to the neural biochemistry, such as the water and iron content of different tissue types.
Even more promising is measuring the changes in how free water molecules are to diffuse among the microscopic structures of the brain. One of the great advances in MRI as a tool for neuroscience in the last few years has been using water diffusion imaging to infer the direction and strength of brain connections. If the axons in the white matter that carry the signals between neurons are thought of as long tubes, it is perhaps intuitive that individual water molecules are freer to move along these tubes than across them. By using MRI to map the direction that water molecules are most commonly moving, it is possible to predict the direction of the axon at any point. This technique has been successfully been applied to determining connections through the white matter, and it may also be possible to use the sensitivity of this bulk measure to probe the microscopic structural differences within the grey matter.
The range of possible measures for in vivo cortical histology presented here demonstrates the huge versatility of MRI as a technique. Much of this versatility comes from the fact that the method is based on the NMR phenomenon, which is well established as a very powerful technique for evaluating chemical composition and molecular structure. While issues of sensitivity and technical complexity mean that it is not clear how the full characterization power of NMR can be brought to image the human brain, the field is not short of mechanisms to tap that may be able to answer some of the most important questions of neuroscience.
5. Concluding remarks
We have presented data illustrating the ability to detect myeloarchitecture patterns in the cortex of living human beings. Although, the technology has been applied predominantly in the visual system, the rapid advances in methodology are likely to allow the investigation of other brain regions over the next few years.
While being able to understand the relationship between brain structure and function is likely to be crucial in attaining a complete understanding of the brain, the potential of this in vivo technique for understanding diseases of the brain is considerable. It is currently very difficult to detect many neurodegenerative diseases such as Alzheimer's and Parkinson's until there has been considerable damage to the brain and patients have begun to show symptoms. If these diseases could be detected at an earlier stage, the opportunities for pre-symptomatic treatment are much greater. In the future, high-resolution MRI could become a standard method of detecting pathological changes in the brain at the earliest possible occasion, leading to treatment and the minimization of irreversible damage.
Acknowledgments
We thank Prof. Alan Cowey and Natalie Voets for help with scanning of the two patients presented here, and Dr Kate Watkins for helpful comments on the manuscript.
References
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. Brodmann's areas 17 and 18 brought into stereotaxic space—where and how variable? Neuroimage. 2000;11:66–84. doi: 10.1006/nimg.1999.0516. doi:10.1006/nimg.1999.0516 [DOI] [PubMed] [Google Scholar]
- Baillarger J.G.F. Recherches sur la structure de la couche corticale des circonvolutions du cerveau. Mem. Acad. R. Med. 1840;8:149–183. [Google Scholar]
- Barbier E.L, Marrett S, Danek A, Vortmeyer A, van Gelderen P, Duyn J, Bandettini P, Grafman J, Koretsky A.P. Imaging cortical anatomy by high-resolution MR at 3.0t: detection of the stripe of Gennari in visual area 17. Magn. Reson. Med. 2002;48:735–738. doi: 10.1002/mrm.10255. doi:10.1002/mrm.10255 [DOI] [PubMed] [Google Scholar]
- Barbur J.L, Ruddock K.H, Waterfield V.A. Human visual responses in the absence of the geniculo-calcarine projection. Brain. 1980;103:905–928. doi: 10.1093/brain/103.4.905. [DOI] [PubMed] [Google Scholar]
- Barbur J.L, Watson J.D, Frackowiak R.S, Zeki S. Conscious visual perception without v1. Brain. 1993;116:1293–1302. doi: 10.1093/brain/116.6.1293. [DOI] [PubMed] [Google Scholar]
- Baseler H.A, Morland A.B, Wandell B.A. Topographic organization of human visual areas in the absence of input from primary cortex. J. Neurosci. 1999;19:2619–2627. doi: 10.1523/JNEUROSCI.19-07-02619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge H, Clare S, Jenkinson M, Jezzard P, Parker A.J, Matthews P.M. Independent anatomical and functional measures of the V1/V2 boundary in human visual cortex. J. Vis. 2005;5:93–102. doi: 10.1167/5.2.1. doi:10.1167/5.2.1 [DOI] [PubMed] [Google Scholar]
- Broca M.P. Perte de la parole, ramollissement chronique et desstruction partielle du lob anterieur gauche de cerveau. Bull. Soc. Anthropol. 1861;62:235–238. [Google Scholar]
- Brodmann K. Barth; Leipzig: 1909. Vergleichende lokalisationslehre der großhirnrinde in ihren prinzipeien dargestellt auf grund des zellenbaues. [Google Scholar]
- Clare, S., Jezzard, P. & Matthews, P. M. 2002 Identification of the myelinated layers in striate cortex on high resolution mri at 3 tesla. Paper presented at the Proc. Int. Soc. Magnetic Resonance in Medicine
- Clark V.P, Courchesne E, Grafe M. In vivo myeloarchitectonic analysis of human striate and extrastriate cortex using magnetic resonance imaging. Cereb. Cortex. 1992;2:417–424. doi: 10.1093/cercor/2.5.417. [DOI] [PubMed] [Google Scholar]
- Clarke S, Miklossy J. Occipital cortex in man: organization of callosal connections, related myelo- and cytoarchitecture, and putative boundaries of functional visual areas. J. Comp. Neurol. 1990;298:188–214. doi: 10.1002/cne.902980205. doi:10.1002/cne.902980205 [DOI] [PubMed] [Google Scholar]
- Cowey A, Stoerig P. Stimulus cueing in blindsight. Prog. Brain Res. 2004;144:261–277. doi: 10.1016/S0079-6123(03)14418-4. doi:10.1016/S0079-6123(03)14418-4 [DOI] [PubMed] [Google Scholar]
- DeYoe E.A, Carman G.J, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc. Natl Acad. Sci. USA. 1996;93:2382–2386. doi: 10.1073/pnas.93.6.2382. doi:10.1073/pnas.93.6.2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zwart J.A, Ledden P.J, van Gelderen P, Bodurka J, Chu R, Duyn J.H. Signal-to-noise ratio and parallel imaging performance of a 16-channel receive-only brain coil array at 3.0 Tesla. Magn. Reson. Med. 2004;51:22–26. doi: 10.1002/mrm.10678. doi:10.1002/mrm.10678 [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Walters N.B, Schleicher A, Kril J, Egan G.F, Zilles K, Watson J.D.G, Amunts K. High-resolution MRI reflects myeloarchitecture and cytoarchitecture of human cerebral cortex. Hum. Brain Mapp. 2005;24:206–215. doi: 10.1002/hbm.20082. doi:10.1002/hbm.20082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott Smith G. A new topographical survey of the human cerebral cortex being an account of the distribution of the anatomically distinct cortical areas and their relationship to the cerebral sulci. J. Anat. Physiol. 1907;41:237–254. [PMC free article] [PubMed] [Google Scholar]
- Engel S.A, Rumelhart D.E, Wandell B.A, Lee A.T, Glover G.H, Chichilnisky E.J, Shadlen M.N. fMRI of human visual cortex. Nature. 1994;369:525. doi: 10.1038/369525a0. doi:10.1038/369525a0 [DOI] [PubMed] [Google Scholar]
- Engel S.A, Glover G.H, Wandell B.A. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb. Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. doi:10.1093/cercor/7.2.181 [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl Acad. Sci. USA. 2000;97:11 050–11 055. doi: 10.1073/pnas.200033797. doi:10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat D.H, van der Kouwe A.J.W, Makris N, Segonne F, Quinn B.T, Dale A.M. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. doi:10.1016/j.neuroimage.2004.07.016 [DOI] [PubMed] [Google Scholar]
- Gall F.J, Spurzheim J.G. Anatomie et physiologie du systeme nerveux en general, et du cerveau en particulier, avec des observations sur la possibilite de reconnoitre plusieurs dispositions intellectuelles et morales de l'homme et des animaus, par la configuration de leurs tetes. vol. 1. 1810. Paris. [Google Scholar]
- Garey L.J. 2nd edn. Imperial College Press; London: 1999. Brodmann's localisation in the cerebral cortex. [Google Scholar]
- Gennari F. Regio Typographeo; Parma, Italy: 1782. Francisci gennari parmensis medicinae doctoris collegiati de peculiari structura cerebri nonnullisque eius morbis–paucae aliae anatom. Observat. Accedunt. [Google Scholar]
- Huk A.C, Dougherty R.F, Heeger D.J. Retinotopy and functional subdivision of human areas MT and MST. J. Neurosci. 2002;22:7195–7205. doi: 10.1523/JNEUROSCI.22-16-07195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcar V.L, Zihl J, Cowey A. Comparing the visual deficits of a motion blind patient with the visual deficits of monkeys with area MT removed. Neuropsychologia. 1997;35:1459–1465. doi: 10.1016/s0028-3932(97)00057-2. doi:10.1016/S0028-3932(97)00057-2 [DOI] [PubMed] [Google Scholar]
- Rademacher J, Burgel U, Geyer S, Schormann T, Schleicher A, Freund H.J, Zilles K. Variability and asymmetry in the human precentral motor system. A cytoarchitectonic and myeloarchitectonic brain mapping study. Brain. 2001;124:2232–2258. doi: 10.1093/brain/124.11.2232. doi:10.1093/brain/124.11.2232 [DOI] [PubMed] [Google Scholar]
- Robitaille P.L, Abduljalil A.M, Kangarlu A. Ultra high resolution imaging of the human head at 8 Tesla. J. Comput. Assist. Tomogr. 2000;24:2–8. doi: 10.1097/00004728-200001000-00002. doi:10.1097/00004728-200001000-00002 [DOI] [PubMed] [Google Scholar]
- Sereno M.I, Dale A.M, Reppas J.B, Kwong K.K, Belliveau J.W, Brady T.J, Rosen B.R, Tootell R.B. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Tootell R.B, Taylor J.B. Anatomical evidence for MT and additional cortical visual areas in humans. Cereb. Cortex. 1995;5:39–55. doi: 10.1093/cercor/5.1.39. [DOI] [PubMed] [Google Scholar]
- von Economo C.F, Koskinas G.N. Oxford University Press; London: 1929. The cytoarchitectonics of the human cerebral cortex (S. Parker, Transl.) [Google Scholar]
- Walters N.B, Egan G.F, Kril J.J, Kean M, Waley P, Jenkinson M, Watson J.D.G. In vivo identification of human cortical areas using high-resolution MRI: an approach to cerebral structure–function correlation. Proc. Natl Acad. Sci. USA. 2003;100:2981–2986. doi: 10.1073/pnas.0437896100. doi:10.1073/pnas.0437896100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J.D, Myers R, Frackowiak R.S, Hajnal J.V, Woods R.P, Mazziotta J.C, Shipp S, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb. Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Wiggins G.C, Potthast A, Triantafyllou C, Wiggind C.J, Wald L.L. Eight-channel phased array coil and detunable TEM volume coil for 7 T brain imaging. Magn. Reson. Med. 2005;54:235–240. doi: 10.1002/mrm.20547. doi:10.1002/mrm.20547 [DOI] [PubMed] [Google Scholar]
- Zeki S.M. Interhemispheric connections of prestriate cortex in monkey. Brain Res. 1970;19:63–75. doi: 10.1016/0006-8993(70)90237-4. doi:10.1016/0006-8993(70)90237-4 [DOI] [PubMed] [Google Scholar]
- Zeki S, Watson J.D, Lueck C.J, Friston K.J, Kennard C, Frackowiak R.S. A direct demonstration of functional specialization in human visual cortex. J. Neurosci. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, et al. Highly parallel volumetric imaging with a 32-element RF coil array. Magn. Reson. Med. 2004;52:869–877. doi: 10.1002/mrm.20209. doi:10.1002/mrm.20209 [DOI] [PMC free article] [PubMed] [Google Scholar]