Abstract
Objective
To evaluate the phenotype, proliferative, and differentiation capacities in vitro of stromal cells derived from peritoneal, ovarian, and deeply infiltrating endometriosis.
Design
Experimental study using phase contrast microscopy, immunocytochemistry, and functional bioassays.
Setting
University-based laboratory.
Patient(s)
Women with and without endometriosis undergoing surgery for benign indications.
Intervention(s)
None.
Main Outcome Measure(s)
The stability in vitro of stromal cells derived from peritoneal (n = 18), ovarian (n = 29), and deeply infiltrating (n = 14) endometriotic lesions, as well as endometrium from women with (n = 5) and without endometriosis (n = 5) was evaluated by detection of endometrial markers. The proliferative and differentiation capacity of the cells was assessed by the use of cell doubling estimation and in vitro decidualization assays.
Result(s)
The expression of the progesterone receptor and CD10 in stromal cells derived from the three types of endometriotic lesions is retained in culture up to passage 10. The doubling time of stromal cells from deeply infiltrating lesions is lower than that of endometrial stromal cells. Levels of prolactin and insulin-like growth factor binding protein-1 (IGFBP-1) are reduced in supernatants from stromal cells derived from the three types of lesions and from the endometrium of women with endometriosis.
Conclusion(s)
The peritoneal, ovarian, and deeply infiltrating endometriotic stromal cell lines we describe retain in vivo tissue markers. Loss of differentiation capacity of the endometriotic cell lines and endometrial cells from women with endometriosis may influence the capacity for proliferation and survival of these cells in the ectopic environment.
Keywords: Endometriosis, endometrium, endometriotic cell lines, in vitro decidualization
Despite decades of research the pathogenesis of endometriosis remains poorly understood and therapies are limited. The disease is manifested as lesions containing ectopic endometrial epithelia and stroma that usually occur in the peritoneal cavity. According to Sampson's theory (1), endometriosis results from the transport of endometrial cells by retrograde menstruation into the peritoneal cavity where they attach to the peritoneum, proliferate and differentiate, and invade the underlying tissue. The assumption is that this process establishes small, early lesions, and subsequent growth and invasion lead to more progressive disease.
An alternative theory is that endometriosis results from metaplasia of Müllerian-type epithelium (2), by which cellular modification due to epigenetic or genetic alterations results in transformation of a specific Müllerian tissue type into endometrial tissue. Whatever the cause, the three different types of endometriotic lesions (i.e., peritoneal surface lesions, endometrioma, and deeply infiltrating lesions) are likely to have discrete aetiologies (3).
Development of new and more effective treatments for endometriosis will depend upon the determination of the molecular and cellular mechanisms that underlie the etiology of the disease. This has been hampered in part by the paucity of reproducible in vitro models of endometriosis. A number of different model systems have been exploited to study the function of cytokines, growth factors, peritoneal fluid, and immune factors in the pathogenesis of endometriosis. These include isolated epithelial and stromal cells (4-7), cultured menstrual efflux (8-11), transformed cell lines derived from peritoneal lesions (12), and cocultures of endometrial cells and peritoneal mesothelium (13-15).
One factor that may influence the severity of the disease is whether the cells undergo proliferation or differentiation in ectopic sites. Here, we report the use of an experimental culture system of endometriotic stromal cells to investigate the differentiation capacity of cells derived from different lesions. We establish the reliability and integrity of the system by determining expression with time in culture of endometrial markers in cultured stromal cell lines derived from peritoneal surface lesions, ovarian endometriomas, and deeply infiltrating lesions. We further determine the cell doubling times, as an indicator of proliferative capacity, of stromal cells derived from the different lesions, and women with and without endometriosis and the extent to which they retain the capacity to differentiate.
MATERIALS AND METHODS
Tissue Samples
All tissue samples were obtained with informed consent in accordance with the requirements of the Oxfordshire Research Ethics Committee. Samples of peritoneal surface lesions (n = 18), ovarian endometriomas (n = 29), and deeply infiltrating lesions (n = 14) were obtained from women 21–48 years old undergoing laparoscopy for pain (n = 13) or other benign indications (n = 28). Endometrium at different stages of the menstrual cycle was obtained by Pipelle biopsy from fertile women undergoing diagnostic laparoscopy or sterilization who were 20–49 years old with (n = 5) and without (n = 5) endometriosis (the latter comprising the normal control group), or by endometrial curettage of the bisected uteri obtained at hysterectomy for benign indications. Endometriotic samples were obtained from two of the five women with endometriosis from whom endometrium was obtained. None of the women had received hormonal medication in the preceding 3 months. Endometriotic tissue was dissected away from the adjacent host tissue, and diagnosis was confirmed by histological examination.
Isolation of Endometriotic and Endometrial Stromal Cells and Cell Culture
Endometriotic and endometrial stromal cells were isolated as described previously (16). The purified stromal cells were plated into 75-cm2 tissue culture flasks (106 cells per flask) and maintained in Dulbecco's modified essential medium (DMEM; Invitrogen, United Kingdom) supplemented with 10% heat-inactivated fetal bovine serum and 50 IU/mL–50 μg/mL penicillin-streptomycin (DMEM complete) at 37°C in a humidified environment with 5% CO2 in air. Stromal cell viability was assessed by Trypan blue exclusion and was similar in endometriotic and endometrial cell lines (approximately 84.2% and 86.9%, respectively). Stromal cells were used between passages 2 and 10. Human foreskin fibroblasts (FS2; a gift from P. Handford, Department of Biochemistry, University of Oxford) were used between passages 10 and 15. Human myometrial myocytes (a gift from S. Phaneuf, Department of Obstetrics and Gynaecology, University of Oxford) were used between passages 6 and 10 (17).
Cytospins
Cytospins (Shandon Southern Products Ltd., United Kingdom) of endometriotic and endometrial stromal cells were prepared at passages 2, 4, 6, and 10. Antibodies against cytokeratin, THY-1, vimentin, CD45, or CD68 (Table 1), were used with the alkaline phosphatase antialkaline phosphatase (APAAP) detection method (Dako, United Kingdom) according to the manufacturer's instructions. Cells (400 in each cytospin) were scored for positive or negative staining and the results were expressed as percentage positive.
TABLE 1.
Details of the antibodies used to detect marker expression.
| Marker | Clone | Working dilution | Source | Reference no. |
|---|---|---|---|---|
| Cytokeratin 18 | JMB2 | 1:2 | In-house | (18) |
| THY-1 | F 15-42-1 | 7.6 μg/mL | Serotec | (19) |
| Vimentin | V9 | 1:100 | Sigma | (20) |
| CD45 | F 10/89/4 | 0.4 μg/mL | Serotec | (18) |
| CD68 | Ki-M6 | 10 μg/mL | Serotec | (21) |
| Estrogen receptor α | 1 D5 | 14 mg/mL | Dako | (22) |
| Progesterone receptor | PgR636 | 4.7 μg/mL | Dako | (23) |
| CD10 | MEM78 | 1:10 | Novocastra | (24) |
| Mouse IgG | 2T8-2F5 | 10 μg/mL | Coulter |
Note: IgG = immunoglobulin G.
Immunocytochemistry
Cultures of endometriotic and endometrial stromal cells were seeded onto 13-mm diameter glass coverslips size 0 (Chance, United Kingdom), cultured to confluence, and fixed and stained by the use of immunofluorescent techniques as described previously (16). Specific antigens (Table 1) were detected by incubation with antibodies to estrogen receptor-α (ER-α), progesterone receptor (PR), CD10, or mouse immunoglobulin G (IgG), followed by incubation with 15 μg/mL of donkey anti-mouse fluorescein isothiocyanate (FITC)-conjugated IgG (Jackson ImmunoResearch Laboratories, Inc., PA). Staining was assessed using a Leitz DMRBE microscope (Leica Corp., Germany) and Openlab imaging software (Improvision, United Kingdom).
Cell Doubling Assays
Subconfluent human endometriotic and endometrial stromal cells were plated into 25-cm2 flasks (2 × 105 cells/flask) in DMEM complete and incubated for 24, 48, 72, or 96 hours, dissociated with 1x trypsin-EDTA, and Trypan blue excluded cells were counted with a haemocytometer. Cell doubling time (TG) was calculated as TG = log(2)*T/log(Y) − log(X) with incubation time (T), final cell count (Y), and inoculation cell count (X). All cell doubling times were expressed as TG ± SEM.
In Vitro Decidualization of Stromal Cells
Cultures of endometriotic and endometrial stromal cells were seeded into four-well plates (0.5 × 105 cells per well) in DMEM/F12 (Invitrogen, United Kingdom) containing 10% charcoal-stripped calf serum (Sigma, United Kingdom) and grown until confluent. Decidualization was induced by the addition of 0.5 mM of 8-Bromoadenosine 3′:5′-cyclic Monophosphate (8-Br-cAMP; Sigma, United Kingdom). The morphology of the stromal cells was assessed, and duplicate samples of cells and the supernatants from cells cultured in the presence or absence of 8-Br-cAMP were collected on days 3, 6, 9, 13, 16, and 20. Prolactin (PRL) and insulin-like growth factor-binding protein-1 (IGFBP-1) levels in the culture supernatants were measured with the PRL Immunolite Kit (DPC, United Kingdom) and DuoSet ELISA Kit (R&D Systems, United Kingdom), respectively, according to the manufacturer's instructions.
Cells were lysed with 50 mM of sodium hydroxide, and the total protein concentration was measured using the Coomassie Plus assay (Pierce, United Kingdom) at a wavelength of 600 nm using a MRX Microplate Reader (Dynex, United Kingdom). Levels of secreted PRL and IGFBP-1 were normalized to the amount of total protein present in each well and values were expressed as ng ± SEM/100 μg total protein. The detection levels of PRL and IGFBP-1 were 0.5 ng/mL and 60 ρg/mL, respectively.
Statistical Analyses
The decidualization responses were subjected to one-way analysis of variance (ANOVA) followed by a Tukey's Multiple Comparison posttest. Results with an α level of <0.05 were considered statistically significant.
RESULTS
Endometriotic and Endometrial Stromal Cells Retain Endometrial Markers in Culture
In the absence of a specific marker for endometriosis, a number of cellular markers were used to confirm the stromal phenotype and the exclusion of contaminating cells such as smooth muscle, endothelial, and haematopoietic cells (Table 1). Immunohistology performed on sections of endometrium and endometriosis confirmed that glandular epithelium stained positive for cytokeratin 18, and endometrial and endometriotic stromal cells were cytokeratin-negative, and positive for vimentin and THY-1 (data not shown), thus confirming previous reports (5-7, 12). In addition, expression of the common acute lymphoblastic leukemia antigen CD10, which, according to recent reports, is expressed in endometrial and endometriotic stroma (17), was investigated.
Cytospins were prepared from endometriotic and endometrial stromal cell lines at passages 2, 4, 6, and 10, and screened with antibodies to cytokeratin, THY-1, vimentin, CD45, or CD68 (Table 1) to assess the stability of the stromal cell phenotype over time in culture (Fig. 1). Endometriotic stromal cells were >95% THY-1 and vimentin-positive with <2% contaminating epithelial and bone-marrow-derived cells, as revealed by staining with cytokeratin and CD45, respectively, at passage 2. This pattern was retained throughout the culture period up to passage 10 and was comparable to the expression of markers by cultured endometrial stromal cells from women with and without endometriosis.
FIGURE 1.
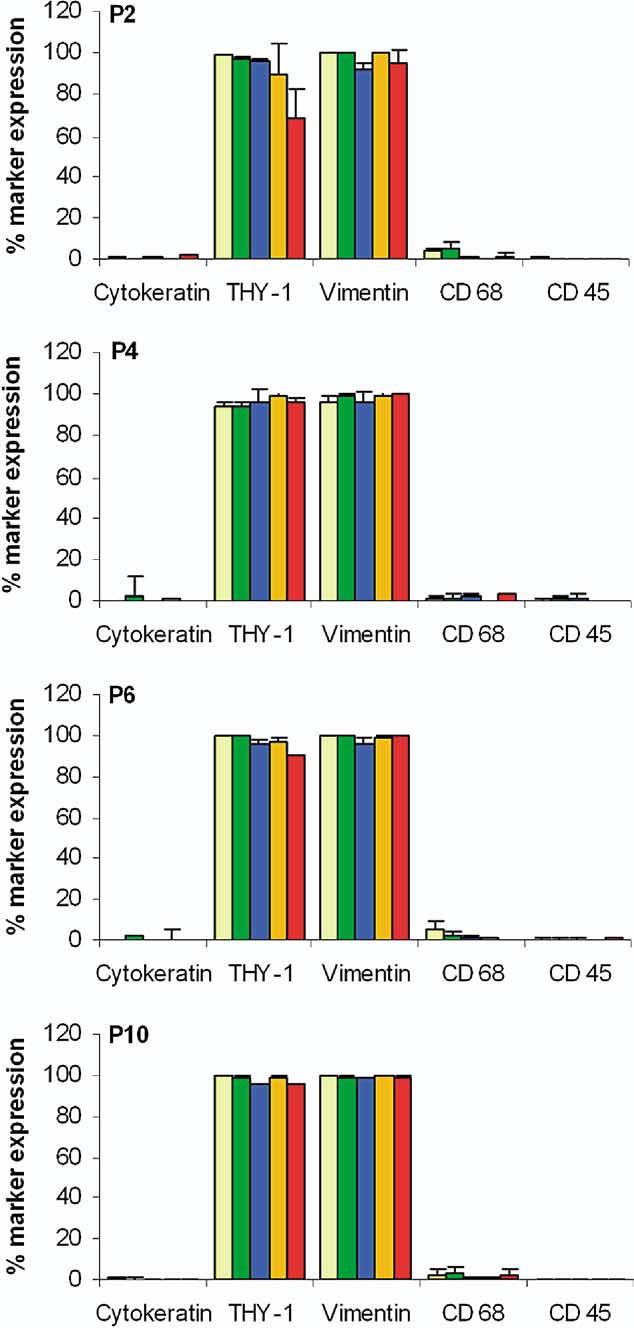
Expression of markers in cultured stromal cells. Quantitative immunocytochemistry of tissue marker expression in endometriotic and endometrial stromal cells at passages 2, 4, 6, and 10. Data are expressed as percentage positive cells and bars represent mean ± SEM. Endometriotic stromal cells express >95% THY-1 and vimentin and <2% cytokeratin, CD45 and CD68. The level of purity and tissue marker expression is comparable to endometrial controls and remained stable with time in culture. (PSL, n = 8) peritoneal surface lesion; (DIL, n = 8) deeply infiltrating lesion; (Eoma, n = 8) ovarian endometrioma; (EME, n = 3) endometrium from women with endometriosis; (EM, n = 3) endometrium; and (P) passage of cells.
Endometriotic tissue in situ is thought to be hormone-responsive. We therefore investigated the expression of hormone receptors in the cultured endometriotic and endometrial stromal cell lines. Stromal cell lines derived from ovarian endometriomas, peritoneal surface lesions, and deeply infiltrating lesions; endometrial stromal cells from women with and without endometriosis (n = 3 each) were stained for ER-α, PR and CD10 at primary culture, as well as passages 2, 4, and 6 (Fig. 2). Stromal cells derived from peritoneal surface and deeply infiltrating lesions retained expression of ER-α and PR with passaging.
FIGURE 2.
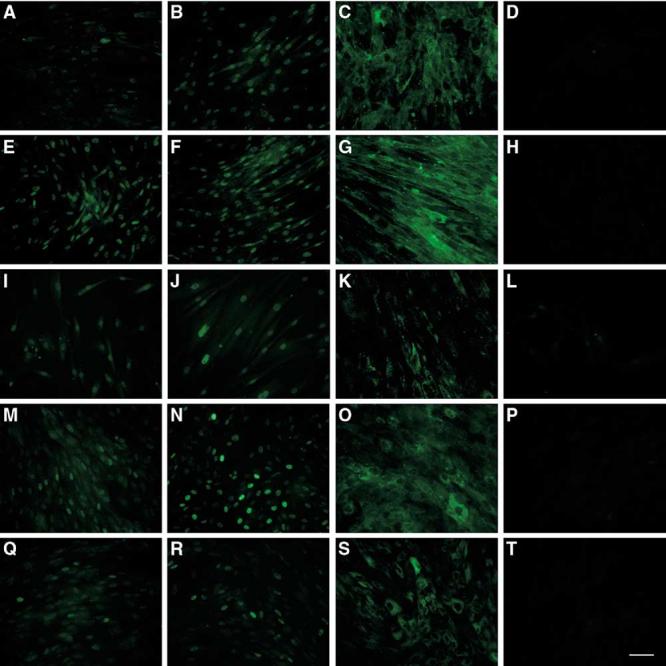
Expression of hormone receptors and CD10 in endometriotic and endometrial stromal cells. Endometriotic stromal cells (n = 3 each) from peritoneal surface lesions (A–D), deeply infiltrating lesions (E–H), ovarian endometrioma (I–L), and endometrial stromal cells (n = 3 each) from women with (M–P) and without endometriosis (Q–T) were grown on glass coverslips to confluence and stained for ER-α (A, E, I, M, Q), PR (B, F, J, N, R), and CD10 (C, G, K, O, S) expression and with the negative control mouse IgG (D, H, L, P, T) using immunofluorescent technique. The PSL and DIL stromal cell expressed ER-α at each passage tested, whereas Eoma stromal cells expressed ER-α only at primary culture. Expression of PR was detectable in all three endometriotic lesions at each passage tested. Endometrial stromal cells expressed both ER-α and PR. Endometriotic and endometrial stromal cells expressed CD10 in the cytoplasm. PSL, peritoneal surface lesion; DIL, deeply infiltrating lesion; Eoma, ovarian endometrioma. Magnification ×20, scale bar 100 μm.
Ovarian-endometrioma-derived stromal cells displayed a low expression of ER-α at primary culture, which was subsequently lost with passaging, and retained PR expression up to passage 6. Endometrial stromal cells from women with and without endometriosis retained expression of ER-α and PR with passaging over the period monitored. Furthermore, cultured stromal cells derived from all of the endometriotic lesions and endometrial tissue samples expressed CD10.
Endometriotic Stromal Cells Divide More Rapidly Than Endometrial Stromal Cells
Normal human cells can have different growth characteristics in vitro depending on the donor, donor age, and origin of tissue (18). We therefore investigated the growth properties of the three different types of endometriotic lesions and endometrium from women with and without endometriosis (Fig. 3). Stromal cells isolated from endometrium from women without and with endometriosis had similar cell doubling times of 3.9 ± 0.9 days and 3.2 ± 0.7 days respectively. These cell doubling times were comparable to stromal cells derived from ovarian endometrioma and peritoneal surface lesions (3.0 ± 0.4 days and 2.7 ± 0.2 days, respectively). Stromal cells isolated from deeply infiltrating lesions had a significantly shorter cell doubling times of 1.9 ± 0.4 days compared with those isolated from endometrium from women with and without endometriosis and endometrioma.
FIGURE 3.
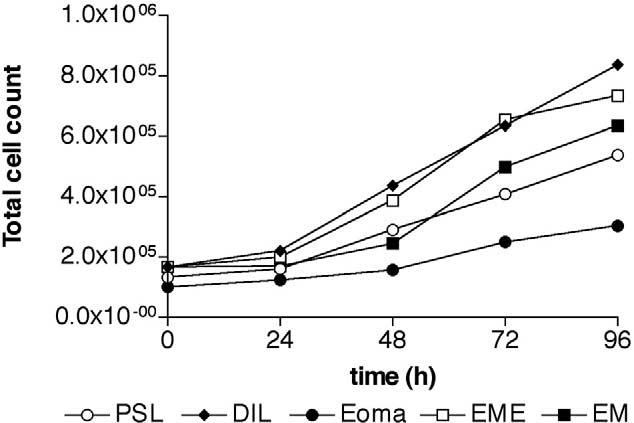
Growth curve of endometriotic and endometrial stromal cells. Endometriotic and endometrial stromal cells were plated in DMEM complete, and total cell counts were determined after 24, 48, 72, and 96 hours of culture. (open circle, PSL, n = 3) peritoneal surface lesion; (diamond, DIL, n = 3) deeply infiltrating lesion; (filled circle, Eoma, n = 3) ovarian endometrioma; (open square, EME, n = 3) endometrium from women with endometriosis; (filled square, EM, n = 4) endometrium.
Endometriotic and Endometrial Stromal Cells From Women With Endometriosis Exhibit a Reduced Capacity for Decidualization
Endometrial stromal cells undergo decidualization in response to steroid hormones during the late secretory stage of the menstrual cycle and early pregnancy, and can be induced to decidualize in vitro (19). We investigated whether endometriotic stromal cells retain this capacity for differentiation in response to 8-Br-cAMP, as assessed by morphology and measurement of PRL and IGFBP-1 production.
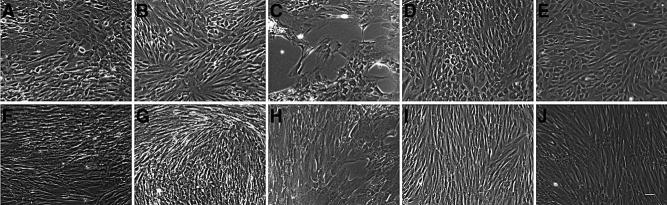
We tested stromal cells derived from ovarian endometriomas, peritoneal surface lesions, and deeply infiltrating lesions, as well as endometrial stromal cell lines from fertile women with and without endometriosis (n = 5 each), FS2 cells, and human myometrial cells. Cells derived from endometrial tissues exhibited a characteristic change in morphology from bipolar fibroblast to polygonal decidual cell from day 3 onward (Fig. 4). Endometriotic stromal cells also exhibited morphological changes, but these changes were not evident until day 6.
FIGURE 4.
Morphology of in vitro decidualized endometriotic and endometrial stromal cells. Confluent stromal cells from peritoneal surface lesions (A, F), deeply infiltrating lesions (B, G), ovarian endometrioma (C, H), and endometrial stromal cells from women with (D, I) and without endometriosis (E, J) were treated with or without 0.5 mM of 8 Br-cAMP for 9 days. Endometriotic stromal cells (A–C) underwent morphological changes from bipolar fibroblasts into polygonal decidual cells, but these were delayed (day 6 onward) and not as widespread compared with endometrial stromal cells that exhibited the characteristic change in morphology from day 3 onward (D, E). Untreated cells (F–J) retained a fibroblast-like, spindle shape appearance. Magnification ×10, scale bar 100 μm.
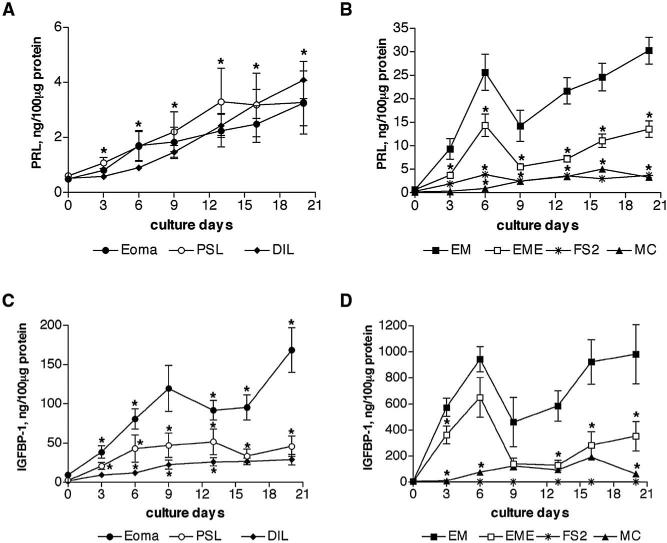
We measured PRL and IGFBP-1 levels as a more sensitive quantitative assessment of decidualization (Fig. 5). The levels of PRL and IGFBP-1 secretion in supernatants of endometrial stromal cells from women with and without endometriosis increased until day 6, were then lower on day 9, and subsequently increased until day 20. In contrast, PRL and IGFBP-1 secretion in supernatants of endometriotic stromal cells, although significantly lower than endometrial cells, increased without a peak until day 20.
FIGURE 5.
Time-dependent secretion of PRL and IGFBP-1 in endometriotic and endometrial stromal cells. Endometriotic stromal cells (A, C) and endometrial stromal cells (B, D) were allowed to decidualize in vitro, in response to 0.5 mM of 8-Br-cAMP for 20 days. The PRL and IGFBP-1 secretion by stromal cells into supernatants was measured every 3–4 days and normalized to total protein contents. Secretion of PRL was similar by all three types of endometriotic stromal cells, dermal fibroblasts, and myometrial myocytes. Endometrial stromal cells secreted tenfold more PRL in comparison with endometriotic stromal cells (*P<.001 throughout culture period). The PRL secretion by endometrial stromal cells from women with endometriosis was also reduced by half in comparison with normal endometrial stromal cells (*P<.05, B). (C) Secretion of IGFBP-1 was >threefold higher in stromal cells derived from ovarian endometrioma compared with peritoneal surface lesions and deeply infiltrating lesions. The difference between endometrioma and deeply infiltrating lesions was significant throughout the culture period. (D) Endometrial stromal cells secreted 20-fold more IGFBP-1 in comparison with endometriotic stromal cells (*P<.001 throughout the culture period). Secretion of IGFBP-1 was also lower in endometrial stromal cells from women with endometriosis compared with those from women without endometriosis (*P<.05). (○, PSL, n = 5) peritoneal surface lesion; (diamond, DIL, n = 5) deeply infiltrating lesion; (filled circle, Eoma, n = 5) ovarian endometrioma; (asterisk, FS2, n = 1) foreskin fibroblasts; (closed square, EM, n = 5) endometrium; (open square, EME, n = 5) endometrium from women with endometriosis; (triangle, MC, n = 1) myometrial myocytes.
Stromal cells derived from ovarian endometriomas, peritoneal surface and deeply infiltrating lesions, foreskin fibroblasts, and myocytes secreted similar PRL levels into the supernatants (at day 6: 1.7 ± 0.5, 1.7 ± 0.5, 1 ± 0.1, 3.8 ± 0.1, and 1 ± 0.04 ng/100 μg protein, respectively) (Fig. 5A and 5B). Cumulative PRL levels were also comparable (12.8 ± 0.4, 15.3 ± 0.4, 13.2 ± 0.5, 18.7 ± 0.5 and 16.1 ± 0.8 ng/100 μg protein, respectively). Levels of IGFBP-1 in culture supernatants from stromal cells derived from ovarian endometriomas were at least twofold higher than those from peritoneal surface and deeply infiltrating lesions (at day 6: 80.8 ± 12.9, 43.01 ± 17.5 and 12.1 ± 3.2 ng/100 μg protein, respectively) (Fig. 5C). Foreskin fibroblasts did not secrete detectable levels of IGFBP-1. Cumulative IGFBP-1 secretion by stromal cells derived from ovarian endometriomas, peritoneal surface and deeply infiltrating lesions, and myocytes was 747.1 ± 24.9, 244.6 ± 6.7, 127.5 ± 4 and 564.2 ± 24.7 ng/100 μg protein, respectively.
Levels of PRL in endometrial stromal cell supernatants from normal controls were twofold higher than in those from women with endometriosis (at day 6: 25.7 ± 3.8 ng/100 μg protein and 14.4 ± 2.4 ng/100 μg protein, respectively) (Fig. 5B). Levels of IGFBP-1 were also higher in endometrial stromal cell supernatants from women without endometriosis than those from affected women (905 ± 94 and 651 ± 152 ng/100 μg protein, respectively, at day 6) (Fig. 5D). Cumulative PRL and IGFBP-1 levels were reduced in supernatants from stromal cells derived from women with endometriosis (56 ± 2 and 1923 ± 79.9 ng/100 μg protein, respectively) compared with those derived from unaffected women (126.4 ± 4 and 4690 ± 132.3 ng/100 μg protein, respectively). The levels of PRL and IGFBP-1 from endometriotic and endometrial stromal cells in the absence of 8-Br-cAMP was close to the minimum the detection level of 0.5 ng/mL and 60 pg/mL in all samples.
The levels of PRL and IGFBP-1 in supernatants from stromal cells derived from ovarian endometriomas, peritoneal surface lesions, and deeply infiltrating lesions correlated well (r2 = 0.8540, r2 = 0.6178, and r2 = 0.7886, respectively), and in endometrial stromal cells from women with and without endometriosis (r2 = 0.9614 and r2 = 0.6945, respectively).
DISCUSSION
Endometriosis is a significant women's healthcare problem worldwide, and determination of the molecular and cellular process that lead to endometriosis remains a challenging clinical and scientific problem. The manipulation of cell lines derived from endometriotic lesions offers a valuable experimental system with which to study the molecular and cellular processes underlying the pathogenesis of the disease. However, it is important to demonstrate that such cell lines retain endometrial integrity if they are to be a useful tool for investigating how processes that are likely to be involved in the pathogenesis of the disease, such as proliferation and differentiation, are regulated in endometriotic cells. Here, we demonstrate that [1] cultured stromal cells derived from the three different types of endometriotic lesions exhibit sustained expression of endometrial markers in in vitro culture; [2] compared with endometrial stromal cells, endometriotic stromal cells from deeply infiltrating lesions exhibit increased proliferative potential; and [3] stromal cells derived from endometriotic lesions and endometrium from women with endometriosis have a reduced capacity for decidualization.
Previously, various markers have been used to confirm the purity of isolated endometriotic cells including cytokeratin 18 and vimentin, negative markers such as van Willebrand factor VIII, and the leukocyte markers CD3, CD11b, CD14, and CD45 (6, 7, 20). The cell lines from the three different types of lesions we describe here retain expression of vimentin, and lack cytokeratin, CD45, and CD68 with time in culture. In addition, expression of THY-1, a marker of endometrial stromal fibroblasts (5, 21), and CD10, a distinguishing marker for endometriosis (22), in stromal cells from the three types of endometriotic lesions is also sustained with passage in culture.
Endometriosis occurs in women during their reproductive years, and the condition is likely to be hormone-dependent. We observe expression of ER-α in stromal cells derived from peritoneal surface and deeply infiltrating lesions and expression of PR in stromal cells derived from all endometriotic lesions.
Hormone receptor levels in endometriotic lesions in vivo are reportedly lower than in normal endometrium, and the cycle-specific changes of hormone receptor expression observed in the endometrium are not always evident in endometriotic lesions (23-25). Thus, the pattern of hormone receptor expression we observe in the endometriotic stromal cell lines is consistent with that in vivo.
Differentiation of endometrial stromal cells into predecidual cells occurs in the late secretory phase of the cycle (26, 27). We investigated the possibility that endometrial cells capable of forming an endometriotic lesion are reprogrammed and lose their capacity for differentiation. Our experimental data suggest that stromal cells derived from ovarian endometriomas, peritoneal surface lesions, and deeply infiltrating lesions retain the capacity to differentiate morphologically and to secrete biochemical markers of decidualization, PRL, and IGFBP-1, but at a much lower level than endometrial stromal cells. Decidualization of endometriotic stromal cells has been observed in women (28-31) and nonhuman primates (32-34) in vivo. In addition, PRL and IGFBP-1 levels in peritoneal fluid and serum have been found previously to be similar in women with and without endometriosis (35-37).
These reports suggest that, although morphological changes associated with decidualization occur in endometriotic lesions, levels of biochemical markers are either not increased or are being cleared rapidly from the bodily fluids.
Although endometriotic lesions are benign, they share certain characteristics with malignancies, indicating that some of the processes involved in the aetiology of both pathologies may be similar. We show that, in common with tumors, endometriotic stromal cells have a reduced capacity for cellular differentiation. We speculate that this in turn may influence the capacity for proliferation of the cells in the ectopic environment.
The maximum number of cell doublings in endometrial stromal cells is variable but is remarkably high for cells derived from adult human tissues and may reflect the extraordinary proliferative capacity of the endometrium (18, 38). It has been reported previously that endometrial stromal cells have a doubling time of 4–5 days and stromal cells derived from ovarian endometriosis 5–6 days depending on the culture conditions (7). However, we observe shorter cell doubling times for endometrial stromal cells (3–4 days) in our culture conditions. Endometriotic stromal cells have a further reduced doubling time (3 days) with deeply infiltrating endometriotic stromal cells having the shortest doubling time (2 days), which is comparable to previously reported transformed endometriotic cells (12). These observations support the notion that the proliferative capacity of stromal cells is increased in the ectopic environment.
We report that levels of prolactin and IGFBP-1 secreted by decidualizing endometrial stromal cells derived from women with endometriosis are reduced in comparison with women without endometriosis. Recent studies demonstrate that the endometrium from women with endometriosis displays morphologically normal but biochemically abnormal responses during the window of implantation (reviewed in [39] and references therein). In this context, abnormal remodeling of the extracellular matrix of endometrial stroma and aberrant integrin expression have been associated with implantation defects. Our data suggest that in women with endometriosis, the signaling cascade leading to decidualization might be impaired, potentially decreasing the biochemical maturation required for correct implantation.
In conclusion, we have shown that stromal cells derived from different endometriotic lesions exhibit sustained expression of endometrial markers with culture and decreased capacity for differentiation. We speculate that the reduced capacity for differentiation of endometriotic cells may be associated with an increased capacity for survival and proliferation of stromal cells in the ectopic environment. The characterized endometriotic stromal cell cultures we describe provide a relevant experimental system that will allow further dissection of the molecular basis of the processes involved in the pathogenesis of distinct endometriotic lesions.
Acknowledgments
This research was funded by a studentship (to P.K.) from The National Endometriosis Society, The Wellcome Trust, and The Medical Research Council. We are extremely grateful to David Barlow, Enda McVeigh, and the clinical staff of the Women's Centre, John Radcliffe Hospital for their valuable contribution to this work.
Footnotes
Supported by the National Endometriosis Society, Wellcome Trust, and the Medical Research Council.
REFERENCES
- 1.Sampson J. The development of the implantation theory for the origin of peritoneal endometriosis. Am J Obstet Gynecol. 1940;40:549–56. [Google Scholar]
- 2.Fujii S. Secondary Müllerian system and endometriosis. Am J Obstet Gynecol. 1991;165:219–25. doi: 10.1016/0002-9378(91)90255-p. [DOI] [PubMed] [Google Scholar]
- 3.Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68:585–96. doi: 10.1016/s0015-0282(97)00191-x. [DOI] [PubMed] [Google Scholar]
- 4.Osteen KG, Hill GA, Hargrove JT, Gorstein F. Development of a method to isolate and culture highly purified populations of stromal and epithelial cells from human endometrial biopsy specimens. Fertil Steril. 1989;52:965–72. doi: 10.1016/s0015-0282(16)53160-4. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Shaw S, Shorter SC, Naish CE, Barlow DH, Starkey PM. Isolation and purification of human endometrial stromal and glandular cells using immunomagnetic microspheres. Hum Reprod. 1992;7:156–61. doi: 10.1093/oxfordjournals.humrep.a137609. [DOI] [PubMed] [Google Scholar]
- 6.Matthews CJ, Redfern CP, Hirst BH, Thomas EJ. Characterization of human purified epithelial and stromal cells from endometrium and endometriosis in tissue culture. Fertil Steril. 1992;57:990–7. doi: 10.1016/s0015-0282(16)55014-6. [DOI] [PubMed] [Google Scholar]
- 7.Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994;78:642–9. doi: 10.1210/jcem.78.3.8126136. [DOI] [PubMed] [Google Scholar]
- 8.Koks CA, Groothuis PG, Dunselman GA, de Goeij AF, Evers JL. Adhesion of shed menstrual tissue in an in-vitro model using amnion and peritoneum: a light and electron microscopic study. Hum Reprod. 1999;14:816–22. doi: 10.1093/humrep/14.3.816. [DOI] [PubMed] [Google Scholar]
- 9.Koks CA, Groothuis PG, Dunselman GA, de Goeij AF, Evers JL. Adhesion of menstrual endometrium to extracellular matrix: the possible role of integrin alpha(6)beta(1) and laminin interaction. Mol Hum Reprod. 2000;6:170–7. doi: 10.1093/molehr/6.2.170. [DOI] [PubMed] [Google Scholar]
- 10.Nisolle M, Casanas-Roux F, Donnez J. Early-stage endometriosis: adhesion and growth of human menstrual endometrium in nude mice. Fertil Steril. 2000;74:306–12. doi: 10.1016/s0015-0282(00)00601-4. [DOI] [PubMed] [Google Scholar]
- 11.Witz CA, Allsup KT, Montoya-Rodriguez IA, Vaughn SL, Centonze VE, Schenken RS. Culture of menstrual endometrium with peritoneal explants and mesothelial monolayers confirms attachment to intact mesothelial cells. Hum Reprod. 2002;17:2832–8. doi: 10.1093/humrep/17.11.2832. [DOI] [PubMed] [Google Scholar]
- 12.Zeitvogel A, Baumann R, Starzinski-Powitz A. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am J Pathol. 2001;159:1839–52. doi: 10.1016/S0002-9440(10)63030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witz CA, Monotoya-Rodriguez IA, Schenken RS. Whole explants of peritoneum and endometrium: a novel model of the early endometriosis lesion. Fertil Steril. 1999;71:56–60. doi: 10.1016/s0015-0282(98)00400-2. [DOI] [PubMed] [Google Scholar]
- 14.Witz CA, Thomas MR, Montoya-Rodriguez IA, Nair AS, Centonze VE, Schenken RS. Short-term culture of peritoneum explants confirms attachment of endometrium to intact peritoneal mesothelium. Fertil Steril. 2001;75:385–90. doi: 10.1016/s0015-0282(00)01699-x. [DOI] [PubMed] [Google Scholar]
- 15.Witz CA, Dechaud H, Montoya-Rodriguez IA, Thomas MR, Nair AS, Centonze VE, et al. An in vitro model to study the pathogenesis of the early endometriosis lesion. Ann NY Acad Sci. 2002;955:296–307. doi: 10.1111/j.1749-6632.2002.tb02790.x. discussion 340–2, 396–406. [DOI] [PubMed] [Google Scholar]
- 16.Chobotova K, Muchmore ME, Carver J, Yoo HJ, Manek S, Gullick WJ, et al. The mitogenic potential of heparin-binding epidermal growth factor in the human endometrium is mediated by the epidermal growth factor receptor and is modulated by tumor necrosis factor-alpha. J Clin Endocrinol Metab. 2002;87:5769–77. doi: 10.1210/jc.2002-020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asboth G, Phaneuf S, Europe-Finner G, Toth M, Bernal A. Prostaglandin E2 activates phospholipase C and elevates intracellular calcium in cultured myometrial cells: involvement of EP1 and EP3 receptor subtypes. Endocrinology. 1996;137:2572–9. doi: 10.1210/endo.137.6.8641211. [DOI] [PubMed] [Google Scholar]
- 18.Holinka CF. Growth and hormonal responsiveness of human endometrial stromal cells in culture. Hum Cell. 1988;1:207–17. [PubMed] [Google Scholar]
- 19.Tang B, Guller S, Gurpide E. Cyclic adenosine 3′,5′-monophosphate induces prolactin expression in stromal cells isolated from human proliferative endometrium. Endocrinology. 1993;133:2197–203. doi: 10.1210/endo.133.5.8404671. [DOI] [PubMed] [Google Scholar]
- 20.Iwabe T, Harada T, Tsudo T, Nagano Y, Yoshida S, Tanikawa M, et al. Tumor necrosis factor-alpha promotes proliferation of endometriotic stromal cells by inducing interleukin-8 gene and protein expression. J Clin Endocrinol Metab. 2000;85:824–9. doi: 10.1210/jcem.85.2.6335. [DOI] [PubMed] [Google Scholar]
- 21.Koumas L, King AE, Critchley HO, Kelly RW, Phipps RP. Fibroblast heterogeneity: existence of functionally distinct Thy 1(+) and Thy 1(−) human female reproductive tract fibroblasts. Am J Pathol. 2001;159:925–35. doi: 10.1016/S0002-9440(10)61768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onda T, Ban S, Shimizu M. CD10 is useful in demonstrating endometrial stroma at ectopic sites and in confirming a diagnosis of endometriosis. J Clin Pathol. 2003;56:79. doi: 10.1136/jcp.56.1.79-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujishita A, Nakane PK, Koji T, Masuzaki H, Chavez RO, Yamabe T, et al. Expression of estrogen and progesterone receptors in endometrium and peritoneal endometriosis: an immunohistochemical and in situ hybridization study. Fertil Steril. 1997;67:856–64. doi: 10.1016/s0015-0282(97)81397-0. [DOI] [PubMed] [Google Scholar]
- 24.Bergqvist A, Ferno M. Oestrogen and progesterone receptors in endometriotic tissue and endometrium: comparison of different cycle phases and ages. Hum Reprod. 1993;8:2211–7. doi: 10.1093/oxfordjournals.humrep.a138005. [DOI] [PubMed] [Google Scholar]
- 25.Nisolle M, Casanas-Roux F, Wyns C, de Menten Y, Mathieu PE, Donnez J. Immunohistochemical analysis of estrogen and progesterone receptors in endometrium and peritoneal endometriosis: a new quantitative method. Fertil Steril. 1994;62:751–9. doi: 10.1016/s0015-0282(16)57000-9. [DOI] [PubMed] [Google Scholar]
- 26.Hulboy DL, Rudolph LA, Matrisian LM. Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod. 1997;3:27–45. doi: 10.1093/molehr/3.1.27. [DOI] [PubMed] [Google Scholar]
- 27.Tang B, Guller S, Gurpide E. Mechanism of human endometrial stromal cells decidualization. Ann N Y Acad Sci. 1994;734:19–25. doi: 10.1111/j.1749-6632.1994.tb21731.x. [DOI] [PubMed] [Google Scholar]
- 28.McCluggage WG, Kirk SJ. Pregnancy associated endometriosis with pronounced stromal myxoid change. J Clin Pathol. 2000;53:241–2. doi: 10.1136/jcp.53.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toki T, Obinata M, Nakayama K, Oguchi O, Fujii S. Ovarian pregnancy associated with microscopic decidualized endometriosis of the ovary: report of a case. J Obstet Gynaecol Res. 1998;24:45–8. doi: 10.1111/j.1447-0756.1998.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 30.Sakaki M, Hirokawa M, Sano T, Takahashi H, Tezuka K, Abe K, et al. Ovarian endometriosis showing decidual change and Arias-Stella reaction with biotin-containing intranuclear inclusions. Acta Cytol. 2003;47:321–4. [PubMed] [Google Scholar]
- 31.Skidmore RA, Woosley JT, Katz VL. Decidualized umbilical endometriosis. Int J Gynaecol Obstet. 1996;52:269–73. doi: 10.1016/0020-7292(95)02612-6. [DOI] [PubMed] [Google Scholar]
- 32.Frasor J, Gaspar CA, Donnelly KM, Gibori G, Fazleabas AT. Expression of prolactin and its receptor in the baboon uterus during the menstrual cycle and pregnancy. J Clin Endocrinol Metab. 1999;84:3344–50. doi: 10.1210/jcem.84.9.5948. [DOI] [PubMed] [Google Scholar]
- 33.Usborne AL, Bolton IB, Slukvin I. Stromal decidualization of endometriosis in the rhesus macaque (Macaca mulatta): a case report. Comp Med. 2002;52:167–70. [PubMed] [Google Scholar]
- 34.Fazleabas AT, Kim JJ, Srinivasan S, Donnelly KM, Brudney A, Jaffe RC. Implantation in the baboon: endometrial responses. Semin Reprod Endocrinol. 1999;17:257–65. doi: 10.1055/s-2007-1016233. [DOI] [PubMed] [Google Scholar]
- 35.Haney AF, Handwerger S, Weinberg JB. Peritoneal fluid prolactin in infertile women with endometriosis: lack of evidence of secretory activity by endometrial implants. Fertil Steril. 1984;42:935–8. [PubMed] [Google Scholar]
- 36.Martinez LB, Leyva MZ, Romero IC. Prolactin receptor in human endometriotic tissues. Acta Obstet Gynecol Scand. 2002;81:5–10. doi: 10.1034/j.1600-0412.2002.810102.x. [DOI] [PubMed] [Google Scholar]
- 37.Taskin O, Giudice L, Mangal R, Dunn RC, Dsupin BA, Poindexter AN, et al. Insulin-like growth factor binding proteins in peritoneal fluid of women with minimal and mild endometriosis. Hum Reprod. 1996;11:1741–6. doi: 10.1093/oxfordjournals.humrep.a019479. [DOI] [PubMed] [Google Scholar]
- 38.Holinka CF, Gurpide E. Proliferative potential and polymorphism of human endometrial stromal cells. Gynecol Endocrinol. 1987;1:71–81. doi: 10.3109/09513598709082698. [DOI] [PubMed] [Google Scholar]
- 39.Giudice LC, Telles TL, Lobo S, Kao L. The molecular basis for implantation failure in endometriosis: on the road to discovery. Ann NY Acad Sci. 2002;955:252–64. doi: 10.1111/j.1749-6632.2002.tb02786.x. discussion 293–5, 396–406. [DOI] [PubMed] [Google Scholar]