Abstract
IL-11 signaling is critical for decidualization of the endometrial stroma in early pregnancy in the mouse. In this study, we investigate the function of IL-11 signaling in cAMP-induced decidualization of human endometrial stromal cells. We show that treatment of endometrial stromal cells with 8-bromo-cAMP (8-Br-cAMP) results in an increase in the levels of secreted IL-11, whereas levels of cell surface IL-11 receptor α are similar with or without 8-Br-cAMP treatment. The production of IL-11 correlates with the production of molecular markers of decidualization, prolactin and IGF-binding protein-1. The expression of these markers is inhibited when IL-11 signaling is specifically blocked in decidualizing endometrial stromal cells by the IL-11 antagonist W147A. We demonstrate that 8-Br-cAMP-induced endometrial stromal cells derived from patients with primary infertility produce lower levels of prolactin, IGF-binding protein-1, and IL-11 than cells derived from fertile women. Our results suggest that IL-11 expression is critically important during decidualization in the human endometrium, and that aberrant regulation of endometrial IL-11 production may be associated with some types of infertility.
THE FAILURE OF the embryo to implant into the endometrium is a major cause of infertility. After the initial stages of implantation in which the embryo attaches to and penetrates the luminal epithelium, the trophoblast invades the endometrial stroma, which then undergoes extensive differentiation to form the decidua. The process of decidualization is essential for a successful pregnancy and is likely to be regulated by locally acting soluble, and extracellular matrix-incorporated, signaling molecules. The morphological differentiation of endometrial stromal cells from elongated spindle-shaped fibroblasts into larger polygonal decidual cells is associated with the expression of a variety of specific secreted molecules, including prolactin and IGF-binding protein-1 (IGFBP-1) (1, 2).
Endometrial stromal cells can differentiate in vitro into decidual cells in response to exogenous factors such as estrogen (E) and progesterone (P) and to analogs of cAMP, exhibiting morphological and biochemical changes similar to those observed in vivo (3-6). In addition, molecules such as relaxin (5), activin A (7), prostaglandin E2 (8), epidermal growth factor (9), and CRH (10) that are expressed in the human endometrium in vivo have been shown to facilitate the process of endometrial stromal cell decidualization in vitro. However, the molecular mechanisms of decidualization and the signaling cascades that drive it remain to be fully elucidated.
Evidence from knockout and transgenic mouse experiments suggests that IL-11 signaling via its interaction with the IL-11 receptor α-subunit (IL-11Rα) is an essential component of the decidualization process in the mouse (11, 12). Female mice lacking the IL-11Rα gene are infertile due to a defective uterine response to the implanting blastocyst, which is characterized by severely limited stromal differentiation, followed by uncontrolled trophoblast invasion and embryo resorption.
IL-11 is a soluble cytokine that has pleiotropic functions in a variety of tissues and cells (reviewed in Ref. 13). It belongs to the glycoprotein 130 (gp130) family of cytokines that activate a common transmembrane signaling receptor, gp130. A functional high-affinity IL-11 signaling receptor is formed in a complex of two gp130 signaling subunits, two ligand molecules, and two ligand-binding IL-11Rα (14).
In the human endometrium, IL-11 and IL-11Rα mRNA and protein are coexpressed throughout the menstrual cycle (15-18). Levels of IL-11 have been reported to be elevated in predecidual cells of late secretory endometrium (16, 18) and in first-trimester decidua and chorionic villi in humans and primates (19, 20). There is also evidence that IL-11 advances the process of in vitro P-induced decidualization of human endometrial stromal cells (21). The mechanisms of regulation of IL-11 expression in the decidualizing endometrium are not known, although the expression of IL-11 by nondecidualized endometrial stromal cells in vitro has been shown to be modulated by a number of factors (15, 18).
More recently, microarray analyses have revealed that IL-11 mRNA is strongly up-regulated in endometrial stromal cells induced to decidualize in vitro with cAMP (22). In this study, we examine the production and function of IL-11 in decidualized human endometrial stromal cells in vitro in response to 8-bromo-cAMP (8-Br-cAMP). We also investigate the capacity of endometrial stromal cells derived from fertile patients and patients with primary infertility to produce IL-11.
Materials and Methods
Tissue collection and in vitro culture of human endometrial stromal cells
Endometrial tissues were obtained with informed consent from patients undergoing sterilization, hysterectomy for benign conditions, or laparoscopy for infertility investigation (primary infertility) in accordance with the requirements of the Oxfordshire clinical research ethics committee. Patients had regular menstrual cycles of 26–30 d and had not used hormonal medication for the 3 months before surgery. Fertile patients were women with normal endometrium and proven fertility, and represented the control group of patients (mean age, 33.7 yr). Samples were obtained from both proliferative (n = 2) and secretory (n = 4) stage endometrium. The infertile patient group (n = 10; mean age, 31.8 yr) was comprised of women who had been diagnosed with primary, unexplained infertility for more than 2 yr and excluded those patients who were infertile due to tubal ligation or male factor infertility. Five of these patients had evidence of endometriosis. Samples were obtained from proliferative (n = 5) and secretory (n = 5) stage endometrium. The tissue was staged according to the last menstrual period and standard histological criteria (23).
Endometrial stromal cells were prepared according to a protocol described previously (24). Cells were used between passages 2 and 5 and were at least 84% and 92% positive for Thy-1 and vimentin, respectively. Cells were maintained in DMEM (Invitrogen Life Technologies, Paisley, UK) supplemented with 10% fetal calf serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37 C in 5% CO2 and passaged when confluent.
In vitro decidualization of human endometrial stromal cells
Endometrial stromal cell lines (n = 3) from the control group of patients were plated at 1 × 105 cells/well in duplicate into four-well tissue culture plates in DMEM supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% charcoal-stripped fetal calf serum (Sigma-Aldrich Corp., Poole, UK) and incubated until confluent. Decidualization was induced by incubating the cells in phenol red-free DMEM/Ham's F-12 (Invitrogen Life Technologies) supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, and 0.5 mm 8-Br-cAMP (Sigma-Aldrich Corp.) with or without 250 ng/ml or 1 μg/ml of the specific IL-11 antagonist W147A (25) for 16 d. The culture media were changed every 3 d. The culture supernatants were collected, centrifuged, and stored at −20 C. The cells were lysed with 0.05 m sodium hydroxide, and total protein concentrations were measured using the Coomassie Plus assay (Pierce Ltd., Tattenhall, UK). Decidualization of endometrial stromal cells from fertile women (n = 6) and patients with primary infertility (n = 10) was induced as described above. Cells were used at passages 2 (n = 3 fertile; n = 7 infertile), 3 (n = 1 fertile; n = 2 infertile), or 5 (n = 2 fertile; n = 1 infertile). Levels of secreted prolactin, IGFBP-1, and IL-11 were measured in culture supernatants as described below. Phase contrast microscopy was used to observe morphological changes associated with decidualization.
Cell viability assay
Cells were plated in 96-well plates and allowed to reach confluence. Decidualization was induced as described above with various concentrations of W147A in replicates of six. The medium was changed every 3 d, and cell viability was assessed on d 14 using the WST-1 colorimetric assay (Roche, Lewes, UK) according to the manufacturer's instructions.
Measurement of prolactin, IGFBP-1, and IL-11
Culture supernatants were analyzed for prolactin, IGFBP-1, and IL-11 using the Immulite Prolactin Kit (Diagnostic Products Corp., Llanberis, UK) or Duoset ELISAs with anti-IGFBP-1 or anti-IL-11 antibodies (R&D Systems, Abingdon, UK), respectively, according to the manufacturer's instructions. The concentrations were normalized to the amount of total protein in each well. The minimum detection limits for prolactin, IGFBP-1, and IL-11 were 0.5 ng/ml, 60 pg/ml, and 16 pg/ml, respectively.
Quantitation of IL-11Rα by flow cytometry
Endometrial stromal cell lines from control (n = 8) and infertile (n = 6) women were cultured in the presence of 8-Br-cAMP in DMEM/Ham's F-12 in 75-ml tissue culture flasks as described above for 6 d. Cells were then detached by incubation with 0.25% trypsin/1 mm EDTA (Sigma-Aldrich Corp.), centrifuged, and fixed in 3% paraformaldehyde in PBS for 30 min at room temperature. Nonspecific antibody-binding sites were blocked by incubation in 10% normal human serum and 2% goat serum in PBS for 20 min at 4 C, washed three times in PBS, and incubated in 10 μg/ml rabbit anti-IL-11Rα (N-20, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), or 10 μg/ml rabbit IgG (DakoCytomation, Ely, UK) in blocking buffer for the controls. Cells were washed three times in PBS and incubated in 25 μg/ml fluorescein isothiocyanate-conjugated anti-rabbit IgG (DakoCytomation). All incubations were performed for 1 h at 4 C. Cells were resuspended in PBS and analyzed using a Coulter EPICS Altra flow cytometer that was precalibrated before each experiment with Flow Set Beads (Beckman Coulter, Inc., Fullerton, CA). Fluorescein isothiocyanate fluorescence was detected at 525 nm. The x-mean brightness of each cell sample analyzed represents the levels of IL-11Rα in that sample.
Statistical analyses
The data were analyzed with one-way ANOVA, followed by post hoc Dunnett's multiple comparison test for multiple groups using PRISM version 3 software (GraphPad, Inc., San Diego, CA). Differences were considered significant when P ≤ 0.05. The direction and magnitude of correlation were quantified by the correlation coefficient (r) or the coefficient of determination (r2), which is a relative measure of the goodness of fit of the observed data points to the regression line (ranging from 0–1, where 1 is the measure of best fit).
Results
cAMP induces IL-11 secretion in decidualized human endometrial stromal cells
Human endometrial stromal cells were induced to decidualize in response to 0.5 mm 8-Br-cAMP, and the levels of decidualization markers produced by cells were similar at different passages. The 8-Br-cAMP-treated stromal cells displayed typical morphological changes associated with decidualization, from elongated fibroblasts to polygonal, plump cells (compare Fig. 1, A and C). Cells from patients with primary infertility also underwent a morphological change similar to that observed in the control group (Fig. 1B). Prolactin and IGFBP-1 exhibited characteristic and reproducible profiles of expression during decidualization (Fig. 2A). After stimulation with 8-Br-cAMP, the levels of both markers rapidly increased, reaching a peak of IGFBP-1 on d 3 (1367.55 ± 279.12 ng/100 μg protein) and a peak of prolactin on d 6 (28.07 ± 10.74 ng/100 μg protein). Levels of both molecules decreased on d 9 and reached a second peak on d 12 (23.4 ± 4.33 ng prolactin/100 μg protein and 758.38 ± 294.15 ng IGFBP-1/100 μg protein). The levels of IL-11 secreted into culture supernatants by decidualized (8-Br-cAMP-treated) and nondecidualized (medium only) cells were measured by ELISA. After stimulation with 8-Br-cAMP, the levels of IL-11 increased throughout the culture period (Fig. 2A). The 8-Br-cAMP-treated stromal cells secreted significantly elevated levels of IL-11 (4.82 ± 2.17 pg/μg protein on d 0; 547 ± 114 pg/μg protein on d 3; 778 ± 49 pg/μg protein on d 16) compared with nondecidualized control cells (2.3 ± 1.5 pg/μg protein on d 3; 6.8 ± 5.3 pg/μg protein on d 16).
Fig. 1.
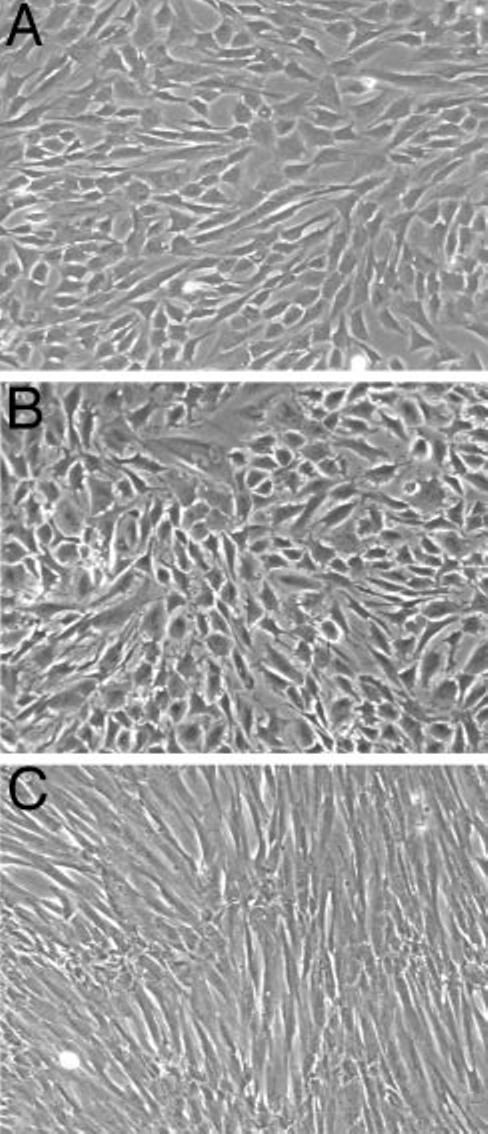
Morphology of 8-Br-cAMP-treated human endometrial stromal cells from fertile and primary infertility patients. Confluent cells from the fertile control group (A) and primary infertility patients (B) were treated with 8-Br-cAMP and similarly displayed typical decidual morphology of large polygonal cells. Untreated cells (C) retained a fibroblast-like, spindle-shaped appearance. Photographs are representative of several experiments and were taken on d 4 of the treatment (magnification, ×40).
Fig. 2.
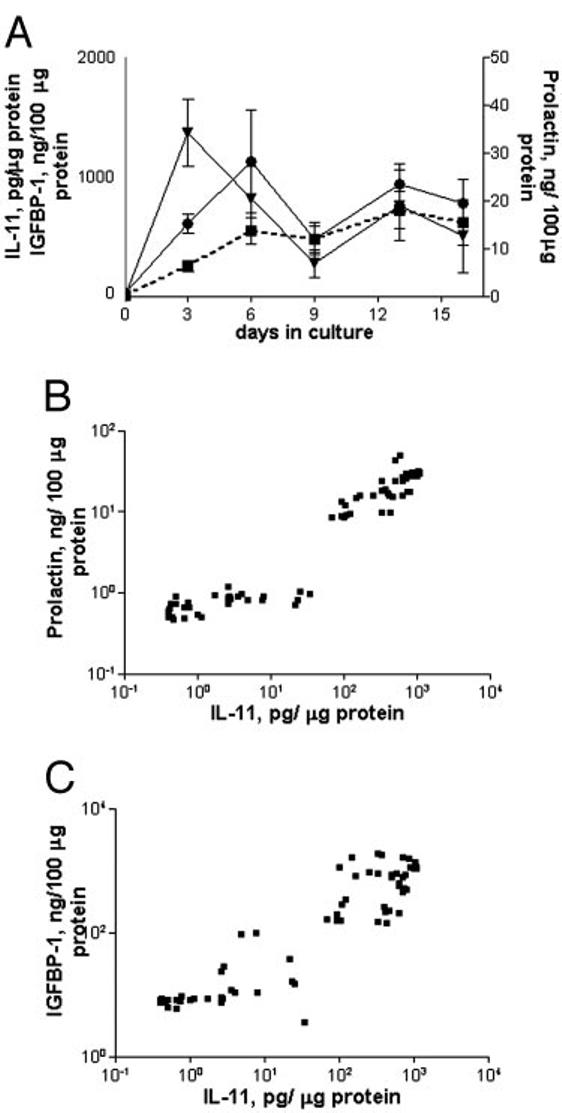
Production of prolactin, IGFBP-1, and IL-11 by human endometrial stromal cells during 8-Br-cAMP-induced decidualization. A, Culture supernatants from stromal cells (n = 3) treated with 0.5 mm 8-Br-cAMP for 16 d were collected and assayed for prolactin ([unk]), IGFBP-1 (▼), and IL-11 (■; dotted line). The concentrations were normalized to the amount of total protein in each well. Data are expressed as the mean ± sem. B, IL-11 concentrations in supernatants plotted against corresponding concentrations of prolactin (r2 = 0.76; P < 0.0001). C, IL-11 concentrations plotted against corresponding concentrations of IGFBP-1 (r2 = 0.48; P < 0.0001).
The concentrations of IL-11 in the culture supernatants were plotted against the concentrations of corresponding prolactin and IGFBP-1 and revealed a good degree of correlation between the secretion of IL-11 and prolactin (Fig. 2B; r = 0.87; r2 = 0.76; P < 0.0001) and, to a lesser extent, IGFBP-1 (Fig. 2C; r = 0.69; r2 = 0.48; P < 0.0001).
IL-11Rα expression is similar in decidualized and nondecidualized endometrial stromal cells
The production of IL-11Rα in 8-Br-cAMP-treated decidualized endometrial stromal cells was analyzed by flow cytometry. Decidualization was assessed morphologically and by measurement of secreted prolactin as described above. The brightness of fluorescence (x-mean) of cAMP-treated cells was higher than that of untreated endometrial stromal cells from some individuals, but overall the difference was not statistically significant (Fig. 3). Levels of IL-11Rα were similar in cells derived from the fertile control group and patients with primary infertility.
Fig. 3.
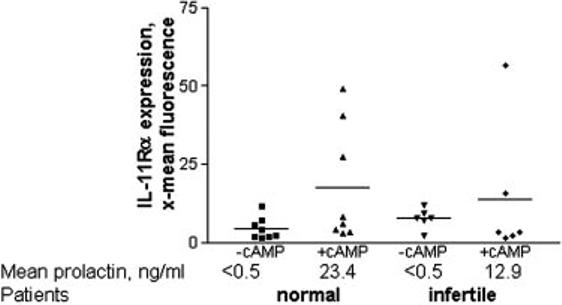
Levels of IL-11Rα are similar in decidualizing cultures of human endometrial stromal cells from fertile and infertile patients. Measurement of IL-11Rα on endometrial stromal cells derived from the fertile control group (n = 8) and primary infertility (n = 6) patients treated with 0.5 mm 8-Br-cAMP for 6 d by flow cytometry. Data are expressed as the mean channel brightness. There was no significant difference between the expression of IL-11Rα in the control and infertile patient groups and under different culture conditions. Data are expressed individually, and the mean is represented by the horizontal line.
W147A inhibits secretion of prolactin and IGFBP-1 by cAMP-treated human endometrial stromal cells
We have recently demonstrated that a high-affinity IL-11 antagonist, W147A, competitively disrupts the formation of multimeric receptor complexes, blocking IL-11 signaling via signal transducer and activator of transcription-3 phosphor-ylation in cultured human endometrial cells (25). The function of IL-11 produced by decidualizing human endometrial stromal cells in vitro was investigated by coincubating the cells with 8-Br-cAMP and increasing concentrations of W147A.
Levels of prolactin and IGFBP-1 in culture supernatants of 8-Br-cAMP-treated cells were lower in the presence of W147A from d 3 onward (Fig. 4, A and B). Addition of W147A led to an approximately 3- to 4-fold reduction of prolactin and an approximately 4- to 5-fold reduction of IGFBP-1 to nearly background levels on d 16 (Fig. 4C), at which point levels of IGFBP-1 were also reduced 4- to 5-fold (Fig. 4C). Addition of W147A to 8-Br-cAMP-treated endometrial stromal cells did not affect cell viability relative to the control (Fig. 4D).
Fig. 4.
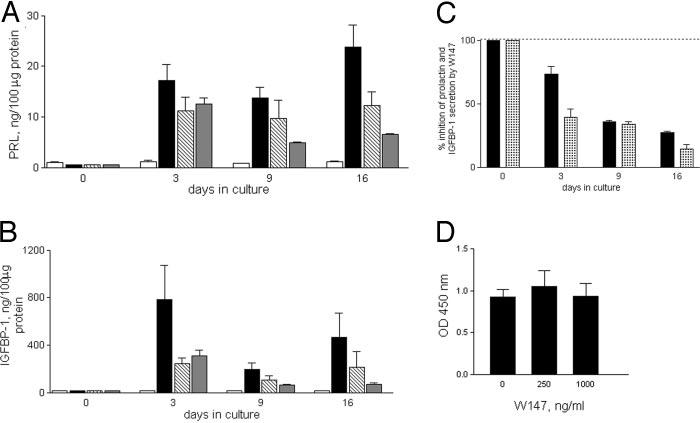
W147A inhibits prolactin and IGFBP-1 production by decidualizing human endometrial stromal cells. Levels of prolactin (A) and IGFBP-1 (B) in supernatants from confluent stromal cells (n = 3) treated for 16 d without (□) or with (■) 0.5 mm 8-Br-c AMP, or 8-Br-cAMP with increasing concentrations of W147A ([unk], 250 ng/ml; [unk], 1 μg/ml). Data are expressed as the mean ± sem. C, Inhibition of cAMP-induced secretion of prolactin (■) and IGFBP-1 ([unk]) in the presence of 1 μg/ml W147A, expressed as a percentage of the cAMP-treated control. Prolactin and IGFBP-1 levels are significantly lower in the presence of W147A (P < 0.05 on d 3–16). D, Cell viability in the presence of 8-Br-cAMP and W147A assessed using the WST-1 colorimetric assay and expressed as the mean OD450nm ± sem. PRL, Prolactin.
Levels of prolactin, IGFBP-1, and IL-11 are decreased in decidualizing cells from women with primary infertility
Decidualization of endometrial stromal cells from the normal patients and those with primary infertility was initially assessed by morphological changes. The morphologies of the cAMP-treated cells were similar for both infertile and normal groups and were typical of decidual cells (Fig. 1, A and B). However, we found major differences in the production of IL-11 and decidualization markers in the two groups (Fig. 5). Culture supernatants of endometrial stromal cells derived from the infertile group contained significantly lower levels of prolactin, IGFBP-1, and IL-11 (e.g. d 6: 6.7 ± 2.0 ng/100 μg, 350.0 ± 151.5 ng/100 μg, and 82.65 ± 21.3 pg/μg, respectively) than those from cells derived from the fertile group (e.g. d 6: 22.9 ± 5.4 ng/100 μg, 807.3 ± 130.1 ng/100 μg, and 464 ± 115.9 pg/μg, respectively) in the presence of 8-Br-cAMP.
Fig. 5.
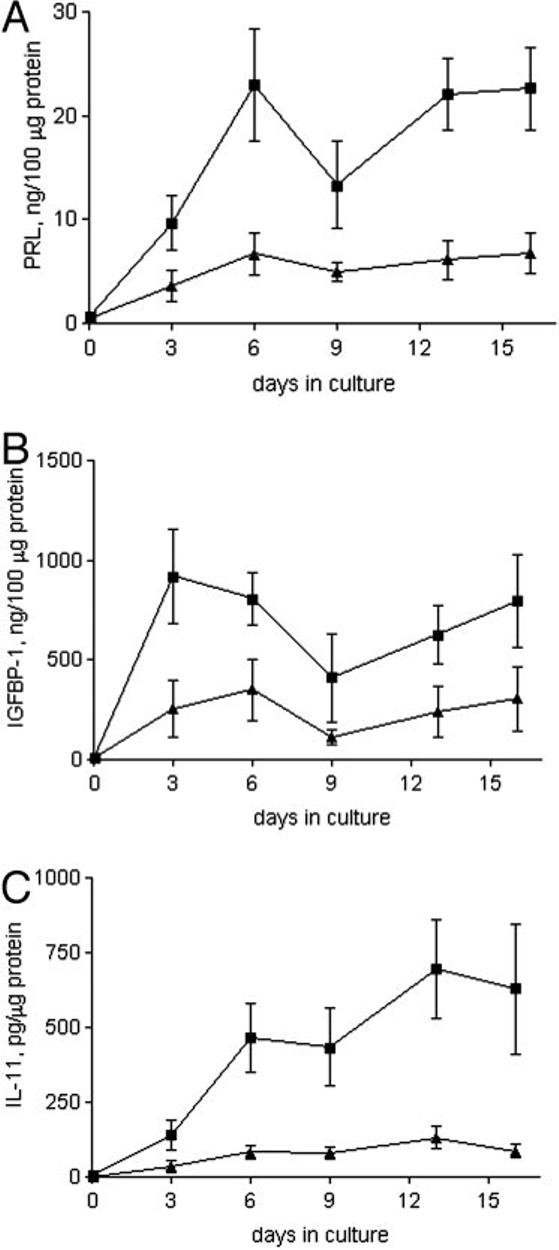
Prolactin, IGFBP-1, and IL-11 production by decidualizing endometrial stromal cells from fertile and infertile women. Levels of prolactin (A), IGFBP-1 (B), and IL-11 (C) in culture supernatants from confluent stromal cells isolated from fertile (■; n = 6) and primary infertility (▲;n = 10) patients treated with 8-Br-cAMP for 16 d. Data are expressed as the mean ± sem. P < 0.05 for fertile vs. primary infertility groups at each time point. PRL, Prolactin.
Discussion
Decidualization of endometrial stroma during implantation of the human embryo is likely to involve complex, regulated interaction of signaling pathways. The importance of IL-11 signaling in decidualization during embryo implantation in the mouse has been established in IL-11Rα gene knockout experiments (11, 12). In this study we have investigated the function of IL-11 signaling in 8-Br-cAMP-induced decidualization of human endometrial stromal cells. We show that 1) IL-11 is produced by 8-Br-cAMP-induced human endometrial stromal cells; 2) specific blocking of IL-11Rα activation by the endogenous IL-11 inhibits decidualization; and 3) IL-11 production and decidualization capacity are considerably reduced in endometrial stromal cells from patients with primary infertility.
We have directly demonstrated that IL-11 is produced by 8-Br-cAMP-induced endometrial stromal cells and has an important function in decidualization. The levels of IL-11 we detect in these cells are higher than those in E plus P-induced decidualized cells, in which levels of secreted IL-11 are similar to those in untreated cells (21). This discrepancy suggests that induction of IL-11 and decidualization are mediated by factors in addition to E and P. Indeed, IL-11 production by cells can be up-regulated by analogs of cAMP or cAMP inducers, such as forskolin, via an activating protein-1 transcription site in the IL-11 promoter, as demonstrated for breast carcinoma cells (26) and osteoblasts (27, 28). We also note that the decidual response to 8-Br-cAMP is rapid, occurring within a few days, as shown here and reported by others (29), whereas the response to E plus P, in the absence of many other factors that may be relevant physiologically, is much slower (30).
Our data demonstrate that the IL-11 antagonist W147A reduces decidualization of endometrial stromal cells. However, even at the highest concentration of W147A used in our study (1 μg/ml), which was previously shown to block IL-11-induced phosphorylation of signal transducer and activator of transcription-3 in endometrial stromal cells (25), the secretion of neither marker was completely abolished. These data suggest an important autocrine/juxtacrine function for IL-11 signaling in the process of 8-Br-cAMP-induced decidualization and point to additional factors that participate in and facilitate the decidualization process.
Other molecules have been shown to have a function in decidualization in vitro (5, 7-10, 31). Indeed, recent microarray profiling data from cAMP-induced endometrial stromal cells predict that there are a large number of cAMP-modulated factors that participate in decidualization, including molecules involved in cytokine and hormone signaling, cell cycle regulation, and extracellular matrix remodeling (22), and these may, in turn, regulate IL-11 production and decidualization. Such molecules may include TGFβ and TNFα, which are increased early in 8-Br-cAMP-induced decidualization of human endometrial stromal cells (22, 32) and are potent stimulators of IL-11 production in nondecidualized endometrial stromal and epithelial cells (18). In addition, and similar to the scenario with IL-11, expression of activin A by stromal cells is increased during 8-Br-cAMP-induced decidualization, and its specific inhibition, in turn, inhibits the production of decidualizing markers in this system (31). Decidualization is thus a finely tuned, complex process that is likely to occur as a result of a cascade of signaling events, in which IL-11-receptor interactions have a regulatory function.
Our results indicate that modulation of IL-11, rather than its receptor, is critical in the decidualization process, because the levels of IL-11-Rα are similar in nondecidualized and decidualized stromal cells. Similar conclusions can be drawn from in vivo studies in mice, which suggest that although IL-11 levels are maximal at the time of stromal differentiation, the levels of IL-11Rα remain constant throughout the cycle (11). Furthermore, IL-11 expression is reduced in gestational tissues in human anembryonic, compared with normal, pregnancy, whereas IL-11Rα levels are the same (19).
One of the major findings of our study is that the decidualization capacity of endometrial stromal cells isolated from patients with primary infertility is reduced compared with that of cells derived from the normal group. Although the morphological changes of decidualization are similar in cells from both groups, cells derived from infertility patients secrete markedly reduced levels of prolactin, IGFBP-1, and IL-11 compared with cells derived from fertile women during 8-Br-cAMP-mediated decidualization. Furthermore, similar expression of IL-11Rα in decidualized stromal cells from infertile and fertile patients is observed, reinforcing the idea that modulation of IL-11 expression, and not its receptor, is important in controlling the process of decidualization in humans.
In conclusion, the data presented here demonstrate that IL-11 has an important function in in vitro decidualization of human endometrial stromal cells and provide additional evidence that in humans, as in mice, IL-11 is crucial for correct decidualization. We show that decidualization of endometrial stromal cells derived from women with primary infertility is defective, and that the production of IL-11 is compromised in these cells. This raises the possibility that defects in endometrial IL-11 signaling may be associated with some types of infertility, and if so, this marks it as a potential target for future therapeutic strategies in the treatment of infertility.
Acknowledgments
We are extremely grateful to the following for their expert assistance: Richard Branton for performing flow cytometry, Joan Bellinger for performing prolactin assays, Dorothea Brosi for tissue collection, and Janet Carver for help with generation of cell lines.
Abbreviations
- 8-Br-cAMP
8-Bromo-cAMP
- E
estrogen
- gp130
glycoprotein 130
- IGFBP-1
IGF-binding protein-1
- IL-11Rα
IL-11 receptor α-subunit
- P
progesterone
Footnotes
This research was funded by a National Endometriosis Society Studentship (to P.K.), the Medical Research Council, and the Wellcome Trust.
Contributor Information
Jung Hye Hwang, Nuffield Department of Obstetrics and Gynecology, University of Oxford, John Radcliffe Hospital , Oxford OX3 9DU, United Kingdom; Department of Obstetrics and Gynecology, Hanyang University College of Medicine , Seoul, Korea.
John K. Heath, Cancer Research UK Growth Factor Group, University of Birmingham School of Biosciences , Birmingham B15 2TT, United Kingdom
References
- 1.Irwin JC, Kirk D, King RJ, Quigley MM, Gwatkin RB. Hormonal regulation of human endometrial stromal cells in culture: an in vitro model for decidualization. Fertil Steril. 1989;52:761–768. doi: 10.1016/s0015-0282(16)61028-2. [DOI] [PubMed] [Google Scholar]
- 2.Bell SC, Jackson JA, Ashmore J, Zhu HH, Tseng L. Regulation of insulin-like growth factor-binding protein-1 synthesis and secretion by progestin and relaxin in long term cultures of human endometrial stromal cells. J Clin Endocrinol Metab. 1991;72:1014–1024. doi: 10.1210/jcem-72-5-1014. [DOI] [PubMed] [Google Scholar]
- 3.Daly DC, Maslar IA, Riddick DH. Prolactin production during in vitro decidualization of proliferative endometrium. Am J Obstet Gynecol. 1983;145:672–678. doi: 10.1016/0002-9378(83)90572-0. [DOI] [PubMed] [Google Scholar]
- 4.Mizuno K, Tanaka T, Umesaki N, Ogita S. Establishment and characterization of in vitro decidualization in normal human endometrial stromal cells. Osaka City Med J. 1998;44:105–115. [PubMed] [Google Scholar]
- 5.Huang JR, Tseng L, Bischof P, Janne OA. Regulation of prolactin production by progestin, estrogen, and relaxin in human endometrial stromal cells. Endocrinology. 1987;121:2011–2017. doi: 10.1210/endo-121-6-2011. [DOI] [PubMed] [Google Scholar]
- 6.Tang B, Guller S, Gurpide E. Cyclic adenosine 3′,5′-monophosphate induces prolactin expression in stromal cells isolated from human proliferative endometrium. Endocrinology. 1993;133:2197–2203. doi: 10.1210/endo.133.5.8404671. [DOI] [PubMed] [Google Scholar]
- 7.Jones RL, Salamonsen LA, Findlay JK. Activin A promotes human endometrial stromal cell decidualization in vitro. J Clin Endocrinol Metab. 2002;87:4001–4004. doi: 10.1210/jcem.87.8.8880. [DOI] [PubMed] [Google Scholar]
- 8.Frank GR, Brar AK, Cedars MI, Handwerger S. Prostaglandin E2 enhances human endometrial stromal cell differentiation. Endocrinology. 1994;134:258–263. doi: 10.1210/endo.134.1.7506205. [DOI] [PubMed] [Google Scholar]
- 9.Irwin JC, de las Fuentes L, Giudice LC. Growth factors and decidualization in vitro. Ann NY Acad Sci. 1994;734:7–18. doi: 10.1111/j.1749-6632.1994.tb21730.x. [DOI] [PubMed] [Google Scholar]
- 10.Zoumakis E, Margioris AN, Stournaras C, Dermitzaki E, Angelakis E, Makrigiannakis A, Koumantakis E, Gravanis A. Corticotrophin-releasing hormone (CRH) interacts with inflammatory prostaglandins and interleukins and affects the decidualization of human endometrial stroma. Mol Hum Reprod. 2000;6:344–351. doi: 10.1093/molehr/6.4.344. [DOI] [PubMed] [Google Scholar]
- 11.Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- 12.Bilinski P, Roopenian D, Gossler A. Maternal IL-11Rα function is required for normal decidua and fetoplacental development in mice. Genes Dev. 1998;12:2234–2243. doi: 10.1101/gad.12.14.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du X, Williams DA. Interleukin-11: review of molecular, cell biology, and clinical use. Blood. 1997;89:3897–3908. [PubMed] [Google Scholar]
- 14.Barton VA, Hall MA, Hudson KR, Heath JK. Interleukin-11 signals through the formation of a hexameric receptor complex. J Biol Chem. 2000;275:36197–36203. doi: 10.1074/jbc.M004648200. [DOI] [PubMed] [Google Scholar]
- 15.Karpovich N, Chobotova K, Carver J, Heath JK, Barlow DH, Mardon HJ. Expression and function of interleukin-11 and its receptor α in the human endometrium. Mol Hum Reprod. 2003;9:75–80. doi: 10.1093/molehr/gag012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimitriadis E, Salamonsen LA, Robb L. Expression of interleukin-11 during the human menstrual cycle: coincidence with stromal cell decidualization and relationship to leukaemia inhibitory factor and prolactin. Mol Hum Reprod. 2000;6:907–914. doi: 10.1093/molehr/6.10.907. [DOI] [PubMed] [Google Scholar]
- 17.Cork BA, Tuckerman EM, Li TC, Laird SM. Expression of interleukin (IL)-11 receptor by the human endometrium in vivo and effects of IL-11, IL-6 and LIF on the production of MMP and cytokines by human endometrial cells in vitro. Mol Hum Reprod. 2002;8:841–848. doi: 10.1093/molehr/8.9.841. [DOI] [PubMed] [Google Scholar]
- 18.Cork BA, Li TC, Warren MA, Laird SM. Interleukin-11 (IL-11) in human endometrium: expression throughout the menstrual cycle and the effects of cytokines on endometrial IL-11 production in vitro. J Reprod Immunol. 2001;50:3–17. doi: 10.1016/s0165-0378(00)00089-9. [DOI] [PubMed] [Google Scholar]
- 19.Chen HF, Lin CY, Chao KH, Wu MY, Yang YS, Ho HN. Defective production of interleukin-11 by decidua and chorionic villi in human anembryonic pregnancy. J Clin Endocrinol Metab. 2002;87:2320–2328. doi: 10.1210/jcem.87.5.8478. [DOI] [PubMed] [Google Scholar]
- 20.Dimitriadis E, Robb L, Liu YX, Enders A, Martin H, Stoikos C, Wallace E, Salamonsen L. IL-11 and IL-11Rα immunolocalisation at primate implantation sites supports a role for IL-11 in placentation and fetal development. Reprod Biol Endocrinol. 2003;1:34. doi: 10.1186/1477-7827-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimitriadis E, Robb L, Salamonsen LA. Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Mol Hum Reprod. 2002;8:636–643. doi: 10.1093/molehr/8.7.636. [DOI] [PubMed] [Google Scholar]
- 22.Tierney EP, Tulac S, Huang ST, Giudice LC. Activation of the protein kinase A pathway in human endometrial stromal cells reveals sequential categorical gene regulation. Physiol Genomics. 2003;16:47–66. doi: 10.1152/physiolgenomics.00066.2003. [DOI] [PubMed] [Google Scholar]
- 23.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Shaw S, Shorter SC, Naish CE, Barlow DH, Starkey PM. Isolation and purification of human endometrial stromal and glandular cells using immunomagnetic microspheres. Hum Reprod. 1992;7:156–161. doi: 10.1093/oxfordjournals.humrep.a137609. [DOI] [PubMed] [Google Scholar]
- 25.Underhill-Day N, McGovern LA, Karpovich N, Mardon HJ, Barton VA, Heath JK. Functional characterization of W147A: a high-affinity inter-leukin-11 antagonist. Endocrinology. 2003;144:3406–3414. doi: 10.1210/en.2002-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacroix M, Siwek B, Marie PJ, Body JJ. Production and regulation of interleukin-11 by breast cancer cells. Cancer Lett. 1998;127:29–35. doi: 10.1016/s0304-3835(97)00542-9. [DOI] [PubMed] [Google Scholar]
- 27.Elias JA, Tang W, Horowitz MC. Cytokine and hormonal stimulation of human osteosarcoma interleukin-11 production. Endocrinology. 1995;136:489–498. doi: 10.1210/endo.136.2.7835281. [DOI] [PubMed] [Google Scholar]
- 28.Marie PJ. Cellular and molecular basis of fibrous dysplasia. Histol Histopathol. 2001;16:981–988. doi: 10.14670/HH-16.981. [DOI] [PubMed] [Google Scholar]
- 29.Telgmann R, Gellersen B. Marker genes of decidualization: activation of the decidual prolactin gene. Hum Reprod Update. 1998;4:472–9. doi: 10.1093/humupd/4.5.472. [DOI] [PubMed] [Google Scholar]
- 30.Tang B, Guller S, Gurpide E. Mechanisms involved in the decidualization of human endometrial stromal cells. Acta Eur Fertil. 1993;24:221–223. [PubMed] [Google Scholar]
- 31.Tierney EP, Giudice LC. Role of activin A as a mediator of in vitro endometrial stromal cell decidualization via the cyclic adenosine monophosphate pathway. Fertil Steril. 2004;81(Suppl 1):899–903. doi: 10.1016/j.fertnstert.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 32.Popovici RM, Kao LC, Giudice LC. Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology. 2000;141:3510–3513. doi: 10.1210/endo.141.9.7789. [DOI] [PubMed] [Google Scholar]


