Abstract
IL-11 is a member of the gp130 family of cytokines, which signal via assembly of multisubunit receptor complexes containing at least one molecule of the transmembrane signaling receptor gp130. IL-11 forms a high-affinity complex, thereby inducing gp130-dependent signaling. Previous studies have identified three distinct receptor binding sites, I, II, and III, crucial for the binding of murine IL-11 (mIL-11) to both the IL-11R and gp130. In this study, we have further characterized the role of the mIL-11 site III mutant W147A. We show that W147A is a high-affinity specific antagonist of mIL-11-mediated signaling in gp130/IL-11R-transfected Ba/F3 cells. The antagonistic action of W147A is due to its ability to competitively disrupt multimeric gp130/IL-11R signaling complex formation. We also show that W147A inhibits IL-11-mediated signaling in primary human endometrial cells, thus demonstrating the potential utility of W147A in suppressing IL-11 responses in vivo.
Abbreviations: GST, Glutathione-S-transferase; hIL, hIL, recombinant human IL; hLIF, human LIF; IL-11R, IL-11-specific receptor; LIF, leukemia inhibitory factor; mIL, murine IL; mLIFR, murine LIF receptor; MTT, 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide; SDS, sodium dodecyl sulfate; STAT, signal transducer and activator of transcription; vIL, viral IL
IL-11 IS A SECRETED polypeptide originally identified by its ability to stimulate the proliferation of the murine plasmacytoma cell line T1165 (1). Subsequent studies have revealed that IL-11 exhibits a diversity of biological functions in vitro. These include induction of acute phase response proteins in hepatocytes (2), induction of differentiation in immortalized hippocampal neurons (3), suppression of lipoprotein lipase activity in adipocytes (4), and inhibition of adipogenesis in cultured 3T3 L1 fibroblasts (5). In association with other cytokines, IL-11 exhibits a range of effects on both the proliferation and differentiation of a variety of hemopoietic cells (6). For example, administration of IL-11 in vivo results in stimulation of megakaryopoiesis and an increase in circulating platelet counts (7). Recombinant human IL-11 (hIL-11) is now in clinical use for the treatment of chemotherapy-induced thrombocytopenia (8). Further antiinflammatory clinical applications of IL-11 include chemotherapy-induced oral mucositis (9), Crohn’s disease, and rheumatoid arthritis (10). Finally, suppression of IL-11 function by targeted deletion of the gene encoding the IL-11-specific receptor (IL-11R) in mice has revealed that IL-11 plays an essential role in embryo implantation. Female mice deficient in IL-11R are infertile due to defective decidualization, following implantation of the embryo (11, 12). The expression of IL-11 and IL-11R in human uterine endometrium during the menstrual cycle indicates that IL-11 action may play an equally significant role in human female fertility (13, 14).
IL-11 is a member of the gp130 family of cytokines (15), characterized by the use of a common transmembrane signal transducing receptor gp130 (16–20). Other members of this family include IL-6, oncostatin M, leukemia inhibitory factor (LIF), cardiotrophin-1, ciliary neurotrophic factor, and a viral homolog of IL-6 encoded by the Kaposi’s sarcoma-associated herpes virus. The gp130 family of cytokines exhibit both shared and unique biological activities (15) dependent upon the exact composition of the receptor complex formed. The response to some gp130 cytokines, including IL-11, requires the expression of ligand-specific receptors, which, although not directly involved in signaling, promote the formation of a high-affinity complex between the ligand and the trans-membrane signaling receptor (15). The ligand-specific IL-11 receptor (IL-11R) (21) is required for the formation of a high-affinity complex between IL-11 and gp130 (22) and results in the activation of IL-11-dependent intracellular signals. These include phosphorylation of gp130 by the Janus kinase family, phosphorylation and activation of the STAT (signal transducer and activator of transcription) family of transcription factors, activation of the MAPK cascade and activation of Src family kinases (23–26). The presence of IL-11R is required for IL-11 action in vitro and in vivo.
Barton et al. (27) have shown that multiple copies of gp130, IL-11, and IL-11R are present in the ternary IL-11 receptor complex and that homodimerization of gp130 requires both IL-11 and IL-11R. This would indicate that IL-11 signals via formation of a hexameric signaling complex similar to that described for IL-6 (28). However, Neddermann et al. (28) concluded that the IL-11 receptor complex was a pentamer consisting of two IL-11 ligands, two IL-11 receptors and one gp130, suggesting that gp130 homodimerization is not involved in IL-11-mediated signaling but another, as yet unidentified, signaling receptor component is required. Finally Grotzinger et al. (29) have suggested that the IL-11/IL-11R/gp130 receptor complex may be a tetramer. In this instance, the ternary complex consists of one IL-11, one IL-11R, and two gp130 molecules. These different models of the IL-11R signaling complex may, in principle, be tested by the creation of IL-11 antagonists because they predict different usage of receptor recognition sites for IL-11 activity.
The gp130 cytokines share a common four α-helix bundle fold in an up-up-down-down topology (30, 31). Extensive structural analysis and mutagenesis studies have revealed receptor-binding epitopes conserved among the gp130 family of cytokines (32). IL-11 (33), as well as IL-6 (34, 35) and ciliary neurotrophic factor (36, 37) have three receptor binding sites termed I, II, and III. These sites have been characterized in murine IL-11 (mIL-11) (33). Site I enables IL-11 to bind to IL-11R, whereas sites II and III mediate binding to gp130 through two different epitopes. Site I is formed by residues in the carboxyl-terminal end of helix D and the helix A-helix B loop (38–40). Site II is formed by exposed residues on helices A and C (41), whereas site III is composed of residues in the helix C-helix D loop-NH2-terminal end of the D helix (35). Thus, the hexameric ternary receptor signaling complex is formed by the dimerization of two trimeric complexes containing one molecule of each component. Dimerization of these trimers involves the simultaneous interaction of gp130 with two molecules of IL-11 via sites II and III and the cytoplasmic domains of gp130 are brought into opposition leading to the activation of intracellular signaling processes. It would therefore be predicted from the hexameric model that a version of IL-11, which lacked the ability to dimerize gp130, would exhibit antagonistic activity.
Barton et al. (33) defined residues critical for the binding of mIL-11 to the IL-11R and gp130. In particular, alanine substitution of tryptophan 147 (W147) resulted in normal binding to IL-11R but abolished binding to gp130 via site III. This mutant proved to be biologically inactive because it was unable to form a complex capable of initiating signal transduction (33). In this study, we demonstrate that W147A is a high-affinity specific IL-11 antagonist. We show that W147A competitively disrupts the formation of a multimeric signaling complex in the presence of wild-type ligand. This shows that dimerization of gp130 requires site III interactions in the complex. We also show that this is accompanied by inhibition of IL-11 signaling functions in gp130/IL-11R transfected Ba/F3 cells. We go on to report that W147A also blocks IL-11-mediated activation of STAT3 in cultured primary human endometrial cells. This study shows that IL-11 signaling requires the formation of a multimeric signaling complex and that W147A is a powerful reagent for probing IL-11 activity in vitro and in vivo. W147A, therefore, has potential for the manipulation of IL-11 action in vivo including the regulation of endometrial cell function during the menstrual cycle.
Materials and Methods
Plasmid constructs
The design and construct of pIG/mIL-11R-Fc, pIG/mgp130-Fc, pGEX/human LIF (hLIF) and pGEX/mIL-11 (IL-11 wild-type and W147A mutant) plasmids have been previously described (22, 33, 42, 43). Briefly, fragments encoding the extracellular domain of murine gp130 (44) and for amino acids 1–363 murine IL-11R (i.e. the signal sequence and the extracellular region except for the three membrane proximal amino acids) were amplified by PCR and cloned into the pIG vector containing the human rhinoviral 3C protease (HRV3C) recognition site (pIG-1 vector provided by D. L. Simmons, Institute for Molecular Medicine, Oxford, UK). cDNA fragments encoding the mature form of hLIF and the mature form of mIL-11 (amino acids 1–178) were amplified by PCR and cloned into the bacterial expression vector, pGEX (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK) containing either the 2T recognition site for thrombin or the HRV3C recognition site (33, 42). The mutant mIL-11 DNA sequence (W147A) was created by PCR overlap using pGEX-mIL-11 as a template and specific oligonu-cleotide primers encoding the mutation (33). The mutant sequence was cloned into pGEX vector and the nucleotide sequence of the construct was confirmed by DNA sequencing using a sequencing reaction kit (Perkin-Elmer, Boston, MA). The pGEX-3 C-vIL-6 recombinant expression vector containing the Kaposi sarcoma herpes virus IL-6-like gene [encoding viral IL-6 (vIL-6)] fused to the glutathione-S-transferase (GST) sequence was prepared by Hudson, K. R. (Cancer Research U.K. Growth Factor Group, unpublished data).
Expression and purification of proteins
Both mIL-11R-Fc and mgp130-Fc were expressed in human embryonic kidney 293T cells (45) by transient expression (22). Cells were cultured in DMEM (Sigma, Poole, Dorset, UK) supplemented with fetal calf serum, 1 mm glutamine, 1 mm penicillin, 1 mm streptomycin, and 1 mm pyruvate at 37 C and 5% CO2. Cells were then transfected at between 50 and 70% confluence by calcium phosphate precipitation and incubated overnight at 37 C. Following a wash with fetal calf serum-free medium, 30 ml of UltraCho medium (BioWhittaker, Walkersville, MD) was added to the transfected cells. Five days post transfection, the conditioned media, containing the Fc-fusion receptors, was harvested and subjected to protein-A affinity chromatography as described by Karow et al. (22). Soluble forms of mIL-11R and mgp130 were cleaved from the Fc-portion using human rhinovirus 3c protease while bound to protein-A-Sepharose. The purified soluble proteins were examined using SDS-PAGE and, if necessary, purified by HPLC on a Superose 12 HR 10/30 gel filtration column attached to Ettan LC apparatus (Amersham Pharmacia Biotech). vIL-6, hLIF, mIL-11, and the site III mutant, W147A, were expressed as GST fusion proteins in the Escherichia coli strain JM109. Expression, purification, and cleavage of the proteins were carried out as previously described (42, 43). For protein induction, cultures were grown at 37 C in Luria-Bertani medium plus ampicillin (100 mg/ml) until they reached a mid-log phase (A600 = 0.6–0.8). Isopropyl-β-d-thiogalactopyranoside was then added to the culture to a final concentration of 0.1 mm and cultures incubated at 25 C for an additional 3 h. Intracellular fusion proteins were recovered from cell extracts by affinity binding to a slurry of glutathione-Sepharose (Pharmacia, Uppsala, Sweden). GST-fusion proteins were cleaved by either human rhinoviral 3C protease (mIL-11, W147A, and vIL6) or by human thrombin (hLIF). After digestion, the supernatant was combined with five washes of the gel matrix and dialyzed against PBS. Protein concentrations were determined using the Coomassie Blue Protein assay (Pierce, Rockford, IL).
Cell culture and ligand bioassays
Ba/F3 cells cultured in RPMI (Life Technologies Inc.) supplemented with 10% fetal calf serum, 1 mm glutamine, 1 mm penicillin, and 1 mm streptomycin were transfected with BCneo/mgp130, which encodes the entire open reading frame of murine gp130 (44). The cells were selected on 600 μg/ml G418 (Invitrogen, Paisley, UK). Stable Ba/F3-mgp130 transfectants were transfected with either pcDNA3/mIL-11R (33) or pcDNA3/murine LIF receptor (mLIFR) encoding the entire open reading frames of mIL-11R and mLIFR, respectively. Double transfectants were selected on 100 ng/ml mIL-11 or hLIF. Bioassays were carried out using transfected Ba/F3 cells. mIL-11 and the mIL-11 mutant, W147A, were tested for biological activity on Ba/F3-mgp130/mIL-11R cells and vIL-6 and hLIF on Ba/F3-mgp130/mLIFR cells using the 3-(4, 5-dimethyl thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) proliferation assay as previously described (22). Briefly, cells were washed in cytokine-free medium and cultured in 96-well plates at 1 × 104 cells/well in the presence of various concentrations of cytokines. After 72 h at 37 C/5% CO2, 10 μl of MTT was added and the plates incubated for a further 4 h. Fifty microliters of 10% sodium dodecyl sulfate (SDS)/0.01 m HCl were then added and the absorbance (A570) determined after incubating overnight at 37 C. Assays were performed at least three times.
Isolation of human endometrial stromal cells
Endometrial tissues at different stages of the menstrual cycle were obtained with approval from the Oxfordshire Research Ethics Committee (Oxford, Oxfordshire, UK) and informed consent from women aged 20–49 yr undergoing either hysterectomy for benign indications or sterilization. Patients had a regular 26–33 d menstrual cycle and had received no hormonal medication in the preceding three months. Endometrial stromal cells were isolated with a protocol based on a method described previously (46). Briefly, endometrial tissue was cut into small pieces and digested in 330 U/ml collagenase type I (Worthington Biochemical Corp., Lakewood, NJ) in DMEM for 1 h at 37 C. Stromal cells were separated from intact glands by filtration of the digested tissue through a 40-μm gauze (Lockertex, Warrington, UK). The stromal cells in the filtrate were purified by centrifugation through a 25–60% Percoll step gradient, diluted in PBS, pelleted by centrifugation, and resuspended in PBS. The cells were plated into 75-cm2 tissue culture flasks (106/flask) maintained in DMEM supplemented with 10% heat-inactivated fetal bovine serum and 50 IU/ml-50 μg/ml penicillin-streptomycin at 37 C in a humidified environment with 5% carbon dioxide. Stromal cells were used between passages 2 and 10.
Native PAGE assay
Equimolar concentrations (200 nm) of soluble mIL-11R and mgp130 were mixed together in the presence of various concentrations of wild-type mIL-11 and mutant mIL-11, W147A, in a total volume of 20 μl of PBS, 0.05% Tween 20. Complexes were allowed to form overnight at 18–20 C. Four microliters of native gel loading buffer [120 mm Tris (pH 6.8), 745 nm glycine, 50% glycerol, and 0.5% bromophenol blue] was added and each sample loaded onto a 4–20% pre-cast Tris-glycine gel (Novex, San Diego, CA). The gel was run in native gel running buffer (24 mm Tris and 149 mm glycine) at 10 mA for approximately 6 h. The presence of protein bands was detected using a standard silver-staining assay (47).
Western blot analysis
Following serum starvation for 4 h to reduce basal STAT3 phosphorylation, Ba/F3-mgp130/mIL-11R cells were stimulated with various concentrations of mIL-11 or with 0.5 ng/ml mIL-11 plus increasing concentrations of site III mutant, W147A, for 15 min at 37 C/5% CO2. Cells were washed with PBS and solubilized in ice-cold lysis buffer [50 mm Tris HCl, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 50 mm NaF, 1 mm sodium orthovanadate (Na3VO4), 1% Triton X-100 plus 1 complete protease inhibitor tablet (Roche Diagnostics GmbH, Mannheim, Germany) per 10 ml lysis buffer]. Even amounts of whole cell lysates were loaded onto 4–20% Tris-glycine gels and electrophoresed at 100 V for 1 h. Human endometrial stromal cells were plated at 5 × 106 cells per well into six-well plates and incubated at 37 C/5% CO2 for 48 h in DMEM supplemented with 0.5% charcoal-stripped fetal calf serum (Life Technologies, Paisley, UK), 100 IU/ml penicillin and 100 μg/ml streptomycin to reduce basal levels of activated STAT3. Following starvation in serum-free medium for 24 h, cells were stimulated with various concentrations of recombinant human IL-11 [hIL-11 (R&D Systems, Abingdon, Oxon, UK)] for 10 min, 50 ng/ml hIL-11 for various times (up to 60 min) or with 10 ng/ml hIL-11 plus increasing concentrations of W147A for 10 min (37 C/5% CO2). Cells were then washed with PBS, solubilized in ice-cold lysis buffer [62.5 mm Tris-HCl (pH 6.8)], 2% SDS, 10% glycerol, 50 mm dithiothreitol, and 0.1% bromophenol blue) and heated at 96 C for 5 min. Even amounts of whole cell lysates were loaded onto 7.5% acrylamide gels and separated by electrophoresis (Amersham Pharmacia Biotech). For Western blotting, proteins were electro-transferred onto polyvinylidene difluoride (Millipore, Watford, Hertfordshire, UK) or nitrocellulose membranes (Bio-Rad, Hemel Hempstead, Hertfordshire, UK) using a standard protocol.
Analysis of STAT3 tyrosine phosphorylation
Membranes were blocked overnight in TBS buffer [20 mm Tris (pH 7.5), 150 mm NaCl, and 0.1% Tween 20] plus 5% BSA and subjected to immunodetection using specific antibodies, polyclonal anti-phospho (Tyr705) STAT3 and polyclonal anti-STAT3 (New England Biolabs, Hitchin, Hertfordshire, UK), according to the manufacturer’s instructions. Membranes were then probed with secondary antirabbit horse-radish peroxidase-conjugated antibodies (Amersham Life Science, Little Chalfont, Buckinghamshire, UK) and blots developed using Super Signal West-Pico Enhanced Chemiluminescence (ECL) (Pierce). For den-sitometric analysis, the density of the bands representing phospho-STAT3 and STAT3 were measured using AlphaImager 1220 Analysis and Documentation System software (Alpha Innotech Inc., Cannock, Staffordshire, UK) and expressed as the ratio of phospho-STAT3 to STAT3.
Results
mIL-11 site III mutant, W147A, inhibits mgp130/mIL-11 receptor complex formation induced by mIL-11
To test the effect of the mIL-11 site III antagonist, W147A, on the ability of mIL-11 to form a multimeric ligand-receptor complex, nondenaturing PAGE was performed on various combinations of mIL-11 and W147A in the presence of soluble receptors, mgp130 and mIL-11R. The results show that wild-type mIL-11, but not the mutant W147A, can induce the formation of a stable, high-affinity complex in the presence of soluble mgp130 and mIL-11R (Figs. 1 and 2, lane 1). Previous studies (25) have confirmed that this complex contains two each of mIL-11, mIL-11R, and gp130 thus represents a hexameric ligand-receptor complex. However, W147A forms a dimeric complex with mIL-11R as shown by the presence of a smeared band (Fig. 1, lane 5) of similar molecular weight to mIL-11R (Fig. 1, lane 2). This smearing probably results from dissociation of W147A from mIL-11R during the experiment. Decreasing the amount of mutant mIL-11 while simultaneously increasing the level of wild-type mIL-11 (Fig. 1, lanes 6–9) resulted in an increase in the level of the stable, high-affinity complex and a concomitant decrease in the smeared mutant mIL-11-mIL-11R complex. In addition, the specific antagonistic effect of W147A on the mIL-11/mgp130/mIL-11R complex can be observed (Fig. 2). As the concentration of W147A is increased with respect to stable concentrations of mIL-11, mgp130 and mIL-11R the level of the high-affinity complex decreases (Fig. 2, lanes 2–5). These results show that W147A is able to compete with native IL-11, in a concentration-dependent fashion, in the formation of intermediate ligand-receptor species thus inhibits mIL-11/mgp130/mIL-11R complex formation.
Fig. 1.

Nondenaturing PAGE of mgp130/mIL-11 receptor complex formation mediated by mIL-11 and W147A. Equimolar concentrations of mgp130 and mIL-11R were mixed together with various combinations of mIL-11 and mIL-11 antagonist W147A, incubated overnight and subjected to nondenaturing PAGE. Detection of protein bands was carried out using silver staining. Numbers in the table (lanes 1–9) denote the final concentration (nanomolar) of construct in each sample. Lane 1, mgp130 and mIL-11R in the presence of mIL-11; lane 2, mIL-11R; lane 3, mgp130; lane 4, mgp130 and mIL-11R; lane 5, mgp130 and mIL-11R in the presence of W147A; lanes 6–9, mgp130 and mIL-11R in the presence of increasing amounts of mIL-11 and decreasing amounts of W147A.
Fig. 2.

mIL-11 site III mutant, W147A, inhibits gp130-IL-11 receptor complex formation induced by wild-type mIL-11. Stable concentrations of mgp130, mIL-11R, and mIL-11 were mixed together in the absence (lane 1) or presence (lanes 2–5) of increasing amounts of mIL-11 antagonist, W147A. Samples were incubated overnight and subjected to nondenaturing PAGE. Detection of protein bands was carried out using silver staining. Numbers (lanes 1–5) denote the final concentration (nanomolar) of construct in each sample.
W147A antagonizes the proliferative response of Ba/F3-mgp130/mIL-11R cells to wild-type mIL-11
The functional properties of mIL-11 and W147A were evaluated using a transfected Ba/F3 model system. Ba/F3 cells cotransfected with mgp130 and mIL-11R are able to proliferate in the presence of mIL-11 alone. We therefore measured the activity of wild-type mIL-11 or W147A on Ba/F3-mgp130/mIL-11R cell proliferation (Fig. 3). As expected, mIL-11 stimulated growth of Ba/F3-mgp130/mIL-11R cells in a concentration-dependent manner. In contrast, W147A alone displayed no activity on these cells. Addition of W147A (at 200 ng/ml) inhibited mIL-11-mediated cell proliferation to approximately one third of its level observed in the absence of the antagonist. These results confirm that mutation of tryptophan-147 abrogates mIL-11 agonistic activity and thus is critically involved in mIL-11-induced proliferation of Ba/F3-mgp130/mIL-11R cells. In addition, W147A competes with wild-type mIL-11 antagonizing its ability to mediate Ba/F3-mgp130/mIL-11R cell growth. Thus, only gp130 dimerization, as induced by wild-type mIL-11, is sufficient for the initiation of intracellular events that lead to cell proliferation.
Fig. 3.

mIL-11 site III mutant, W147A, inhibits the growth of Ba/F3-mgp130/mIL-11R cells in response to wild-type mIL-11. An increasing concentration of mIL-11 or W147A was applied to mgp130/mIL-11R transfected Ba/F3 cells plated at 1 × 105 cell/ml. mIL-11 was applied alone (▪) or in the presence (•) of 200 ng/ml mIL-11 antagonist, W147A. W147A was applied alone (▴). Results are expressed as the A570 value of cells assayed for proliferation by MTT. Values are the mean of triplicate experiments, and error bars represent the sem.
W147A has no effect on the proliferative activity of LIF or vIL-6 on Ba/F3-mgp130/mLIFR cells
The molecular basis of W147A antagonism is presumed to result from elimination of site III binding to gp130 and retention of site II binding to gp130. This latter interaction requires the presence of IL-11R bound through site I (33). This would suggest that the actions of W147A should be strictly dependent upon the presence of IL-11R because this interaction is required for Site II. This would in turn predict that W147A would be a specific antagonist for ligands, which bind the ligand-specific receptor IL-11R. We accordingly tested the ability of W147A to inhibit signaling via other gp130 ligands. hLIF induces the formation of a high-affinity heterodimeric signaling complex by association with the specific LIF receptor and gp130 shared receptor (15). vIL-6 is a pan-gp130 agonist, which dimerizes gp130 in the absence of a ligand-specific receptor (48). The functional properties of hLIF and vIL-6 in the presence and absence of W147A were evaluated using the transfected Ba/F3 system. Ba/F3 cells previously cotransfected with mgp130 and mLIFR were able to proliferate in the presence of either LIF (Fig. 4A) or vIL-6 (Fig. 4B). W147A, at a concentration of 200 ng/ml, had no effect upon the activity of either ligand in the stimulation of mgp130/mLIFR transfected Ba/F3 cell growth. Collectively, these results demonstrate that W147A is a specific antagonist for ligands, which interact only with the IL-11R.
Fig. 4.

mIL-11 site III mutant, W147A, does not block the growth promoting activities of hLIF or viral IL-6 on Ba/F3-mgp130/mLIFR cells. An increasing concentration of hLIF (A) or vIL-6 (B) was applied to mgp130/mLIFR transfected Ba/F3 cells in the presence (▴) or absence (▪) of 200 ng/ml mIL-11 antagonist, W147A. Results are expressed as the A570 value of cells assayed for proliferation by MTT. Values are the mean of triplicate experiments, and error bars represent the sem.
The growth-inhibitory activity of W147A is specific to mIL-11-mediated Ba/F3-mgp130/mIL-11R cell proliferation
To assess the effect of W147A on mIL-11 induced Ba/F3-mgp130/mIL-11R cell mitogenesis, cells were cocultured in the presence of 10 ng/ml mIL-11 with increasing concentrations of W147A (Fig. 5A). At concentrations above 50 ng/ml, W147A inhibits the proliferative response of Ba/F3-mgp130/mIL-11R cells to wild-type mIL-11. This effect was concentration dependent. A high level of W147A (500–1000 ng/ml) abolished mIL-11 induced mitogenic activity. W147A did not effect the ability of hLIF (Fig. 5B) or vIL-6 (Fig. 5C) to induce mitogenesis in mgp130/mLIFR transfected Ba/F3 cells. Together, these data suggest that inhibition of mIL-11/mgp130/mIL-11R multimeric complex formation by W147A is linked to a reduction in mIL-11-induced Ba/F3-mgp130/mIL-11R cell proliferation and therefore reduced cellular responses. This prompted an analysis of direct signaling events following receptor activation.
Fig. 5.
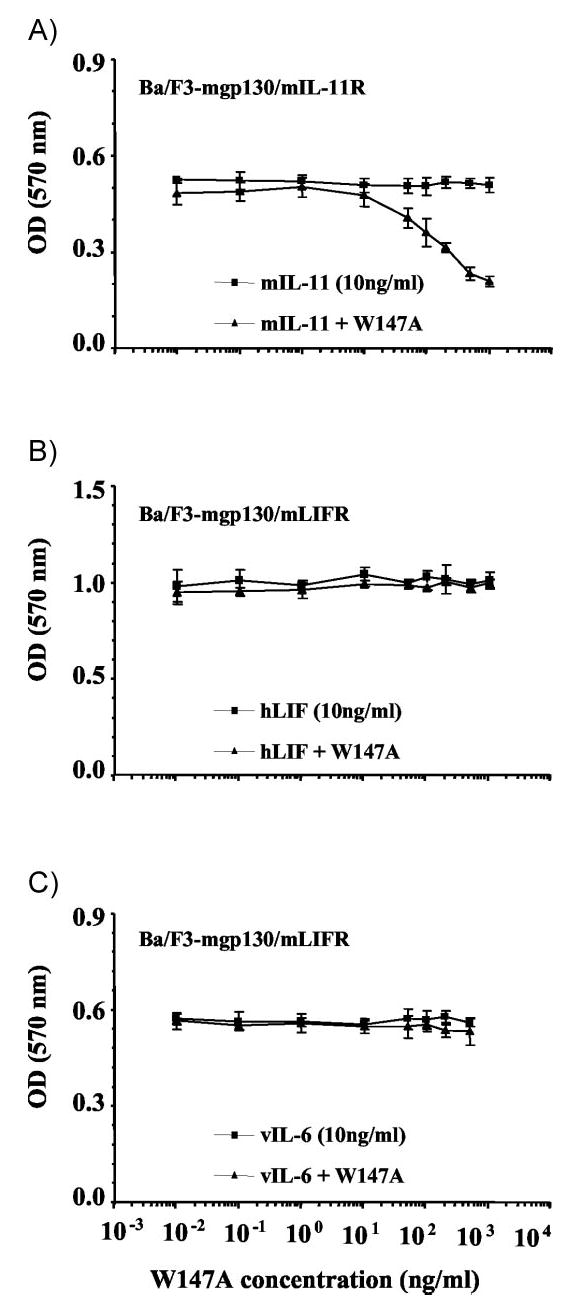
mIL-11 site III mutant, W147A, is a specific antagonist to mIL-11-mediated Ba/F3-gp130/mIL-11R cell growth. A constant concentration (10 ng/ml) of mIL-11, hLIF or vIL-6 or was applied to transfected Ba/F3 cells alone (▪) or in the presence (▴) of increasing concentrations of mIL-11 antagonist, W147A. Ba/F3-mgp130/mIL-11R cells were stimulated with mIL-11 ± W147A (A). Ba/F3-mgp130/mLIFR cells were stimulated with either hLIF ± W147A (B) or vIL-6 ± W147A (C). Results are expressed as the A570 value of cells assayed for proliferation by MTT. Values are the mean of triplicate experiments, and error bars represent the sem.
W147A antagonizes mIL-11 induced STAT3 tyrosine phosphorylation in Ba/F3-mgp130/mIL-11R cells
STAT monomers are tyrosine phosphorylated, allowing their homo- or hetero-dimerization and translocation to the nucleus where they regulate expression of various target genes (26, 49, 50). STAT3 is strongly activated by IL-6 cytokines (51), thus to test the effect of W147A, on the Jak/STAT pathway we first had to verify that wild-type mIL-11 was capable of inducing tyrosine phosphorylation of STAT3 in the Ba/F3-mgp130/mIL-11R cell line (Fig. 6). Phosphorylation of tyrosine-705, essential for dimerization and DNA binding, was detected after stimulation with 0.1 ng/ml mIL-11 (Fig. 6A, top). The level of STAT3 tyrosine phosphorylation increases with increasing mIL-11 concentration, with maximal phosphorylation observed at a dose of 5 ng/ml (which is a significantly lower concentration than that required for maximal induction of cell proliferation). STAT3 levels across the samples were constant (Fig. 6A, bottom). This confirmed that mIL-11 induces STAT3 tyrosine phosphorylation in Ba/F3-mgp130/mIL-11R cells in a dose-dependent manner. Stimulation of the Ba/F3-mgp130/mIL-11R cell line with 10 ng/ml mIL-11 resulted in activation of STAT3, within 5 min, which remains tyrosine-phosphorylated for over 4 h (data not shown). To determine the effect of W147A on mIL-11-induced STAT3 phosphorylation, Ba/F3-mgp130/mIL-11R cells were stimulated with 0.5 ng/ml mIL-11 in the presence of increasing concentrations of the mutant W147A (Fig. 6B). As antagonist levels increased, the level of STAT3 tyrosine phosphorylation decreased (Fig. 6B, top) despite a constant level of STAT3 protein (Fig. 6B, bottom). W147A (10 ng/ml) was enough to decrease the level of STAT3 phosphorylation. These data confirm that W147A antagonizes mIL-11 induced STAT3 pathway activation, a direct result of gp130 dimerization.
Fig. 6.

mIL-11-mediated tyrosine phosphorylation of STAT3 (Tyr 705) in Ba/F3-mgp130/mIL-11R cells is inhibited by W147A. Ba/F3-mgp130/mIL-11R cells were incubated for 15 min with increasing concentrations of mIL-11 (A) or a constant concentration of mIL-11 (0.5 ng/ml) and increasing concentrations of mIL-11 mutant, W147A (B). Cells were lysed with 1% Triton X-100 lysis buffer. Immunoblots were probed with the following antibodies: top row, antiphosphotyrosine (Tyr 705) STAT3 antibody; bottom row, parallel samples blotted with anti-STAT3 antibody.
W147A antagonizes IL-11 STAT3 signaling in primary human endometrial cells
The results above confirm that, in transfected cell systems, W147A is a potent and specific antagonist of signaling events, such as STAT3 phosphorylation, which are mediated by ligands that interact with IL-11R. However, the practical utility of W147A requires that it is able to suppress IL-11-mediated signaling in therapeutically significant IL-11-responsive cell types. Studies of IL-11R knockout mice (11, 12) and examination of the effects of IL-11 on human endometrial stromal cells (52–54) have shown that IL-11R-mediated signaling is required for endometrial decidualization and consequent embryo implantation. The human endometrium is a prime cellular target for blockade of IL-11 signaling because increased expression of IL-11 (and other IL-6 family cytokines) in endometrial tissues has been linked to the aberrant regulation of aromatase P450 expression in endometriosis (55). In addition, IL-11/IL-11R stimulation of the Janus kinase/STAT pathway has been implicated in the regulation of P450 expression (55). First, we tested the ability of hIL-11 to activate STAT3 in cultured human endometrial cells. Serum-starved early passage human primary endometrial cells were exposed to increasing concentrations of recombinant human IL-11 for 10 min (Fig. 7A) or to 50 ng/ml hIL-11 for increasing time periods (Fig. 7B) and examined for activation of STAT3. hIL-11 induced both dose- and time-dependent activation of STAT3 tyrosine phosphorylation. To our knowledge, this is the first report on IL-11-mediated STAT signaling in cultured human endometrial stromal cells. We accordingly tested the ability of W147A to suppress STAT3 activation in these cells. Serum-starved cells were exposed to 10 ng/ml hIL-11 in the presence of increasing concentrations of W147A. At high concentrations (1–2 μg/ml), W147A blocks the ability of hIL-11 to induce STAT3 phosphorylation (Fig. 8A) above basal levels. This result was confirmed by densitometric analysis of phosphorylated and nonphosphorylated STAT3 levels (Fig. 8B). The results of these experiments show that W147A blocks the ability of hIL-11 to activate STAT3 phosphorylation in these cells in dose-dependent fashion. It is also interesting to note that, in our hands, human endometrial cells exhibit detectable basal STAT3 phosphorylation, which is not suppressed by W147A (Fig. 8). This presumably reflects endogenous activation of STAT3 via a non-IL-11R-dependent route. These results confirm that W147A can block IL-11-dependent signaling in a therapeutically important target cell, which exhibits IL-11 responsiveness.
Fig. 7.

IL-11 signals through phosphorylation of STAT3 (Tyr 705) in cultured human endometrial stromal cells. Human endometrial stromal cells were incubated for 10 min with increasing concentrations of hIL-11 (A) or for increasing time periods with 50 ng/ml hIL-11 (B). C, Control lane, Commercial cell lysate from interferon-treated HeLa cells (New England Biolabs). Cells were lysed with 2% SDS lysis buffer. Immunoblots were probed with the following antibodies: top row, antiphosphotyrosine (Tyr 705) STAT3 antibody; bottom row, parallel samples blotted with anti-STAT3 antibody.
Fig. 8.
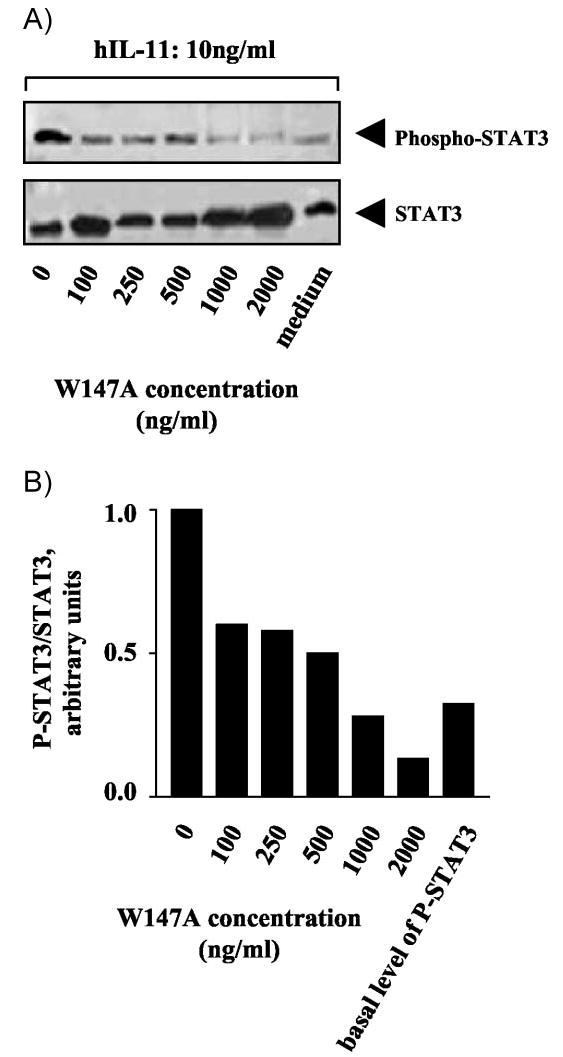
IL-11-mediated activation of STAT3 in cultured human endometrium stromal cells is inhibited by W147A. Human endometrial stromal cells were incubated for 10 min in the presence of 10 ng/ml hIL-11 and increasing concentrations of mIL-11 mutant, W147A (A). Cells were lysed with 2% SDS lysis buffer. Immunoblots were probed with the following antibodies: top row, antiphosphotyrosine (Tyr 705) STAT3 antibody; bottom row, parallel samples blotted with anti-STAT3 antibody. The densities of the bands corresponding to phosphorylated-STAT3 were expressed as the ratio to those corresponding to STAT3 (B).
Discussion
The four helical bundle family of cytokines elicit intracellular signaling via the sequential assembly of a high-affinity signaling complex by means of multiple ligand/receptor contacts (reviewed in Ref. 32). This mode of action provides a favorable situation for the creation of selective antagonists in which essential ligand/receptor contacts are abolished, whereas others are retained. The gp130 cytokines are an example of a situation in which both common and unique subunits are employed in the formation of a high-affinity receptor signaling complex (15). In principle, the selectivity of antagonists in such a system depends on the retention of a high-affinity interaction with a unique receptor subunit and abolition of a second high-affinity interaction with a common receptor subunit. Here we apply this concept to the characterization of a high-affinity selective antagonist for the clinically important gp130 cytokine IL-11.
We have previously shown that IL-11 exhibits three sites of interaction with cognate receptors (33): site I, which interacts with IL-11R; site II, which interacts with the cytokine homology domain of gp130; and site III, which interacts with the Ig domain of a second molecule of gp130. Here we show that the mIL-11 mutant W147A, which is unable to interact with gp130 via site III, is a high-affinity selective antagonist of mIL-11 function. This activity arises from the ability of W147A to disrupt the formation of the high-affinity signaling complex in the presence of wild-type mIL-11 and mIL11-R. The selectivity of W147A arises from retention of the unique high-affinity interaction between mIL-11 and mIL-11R.
Solution phase studies using soluble receptor ectodomains have shown that IL-11 (like IL-6; Ref. 56) forms a high-affinity, hexameric receptor complex comprising of two molecules of IL-11, two molecules of gp130 and two molecules of IL-11R (27). Here we show that the competitive addition of W147A in solution leads to the disruption of this hexameric receptor complex and induces the formation of a receptor complex containing just one molecule of the IL-11/IL-11R subunit. This activity is paralleled by the ability of W147A to competitively inhibit IL-11 signaling in transfected cells as measured by cell multiplication and activation of STAT3 phosphorylation. These effects on IL-11 signaling indicate that the antagonist action of W147A requires the concurrent presence of wild-type IL-11 in a signaling complex. This would indicate that, in live cells, the functional signaling unit is a hexamer. It has been argued on the basis of molecular modeling by Grotzinger et al. (29) that the hexamer represents an inert form of the receptor complex. They propose instead that the active complex is a tetramer with two gp130 molecules and one molecule of IL-11/IL-11R (29). In this model, the inactive hexameric complex is generated by addition of another subunit of IL-11/IL-11R. Our findings suggest that two molecules of IL-11/IL-11R are required to elicit signal transduction via dimerization of gp130.
Cytokine antagonists have proved to be powerful tools in the dissection of ligand-dependent signaling processes in vitro and in vivo. For example, LIF05 is a specific high-affinity antagonist of ligands that signal via the LIFR (57), which has been employed to dissect LIFR-mediated signaling in a number of cell systems (58, 59). A high-affinity and specific IL-11 antagonist should be valuable in studying and manipulating IL-11 responses in therapeutically significant cell types. Thus, disruption of IL-11R signaling in mice has revealed an essential role for IL-11 signaling in the endometrium for successful embryo implantation and we have shown in this study that W147A can suppress IL-11 signaling in endometrial cells cultured in vitro. This result indicates that W147A should be a potent agent for modifying uterine receptivity in vivo.
Acknowledgments
This work was supported by Cancer Research UK (to E.M., N.U., V.B., and J.H.) and the Medical Research Council (to N.K. and H.J.M.). We are grateful to Celine Jones for skilled technical support.
Footnotes
Present address for L.A.M.: Geneflow Limited, Fradley Business Park, Wood End Lane, Fradley, Staffordshire WS13 8NF, United Kingdom.
Present address for V.A.B.: KuDOS Pharmaceuticals, 327 Cambridge Science Park, Milton Road, Cambridge CB4 OWG, United Kingdom.
References
- 1.Paul SR, Bennett F, Calvetti JA, Kelleher K, Wood CR, O’Hara RM, Jr, Leary AC, Sibley B, Clark SC, Williams DA, Yang YC. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci USA. 1990;87:7512–7516. doi: 10.1073/pnas.87.19.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumann H, Schendel P. Interleukin-11 regulates the hepatic expression of the same plasma protein genes as interleukin-6. J Biol Chem. 1991;266:20424–20427. [PubMed] [Google Scholar]
- 3.Mehler MF, Rozental R, Dougherty M, Spray DC, Kessler JA. Cytokine regulation of neuronal differentiation of hippocampal progenitor cells. Nature. 1993;362:62–65. doi: 10.1038/362062a0. [DOI] [PubMed] [Google Scholar]
- 4.Ohsumi J, Miyadai K, Kawashima I, Sakakibara S, Yamaguchi J, Itoh Y. Regulation of lipoprotein lipase synthesis in 3T3-L1 adipocytes by interleukin-11/adipogenesis inhibitory factor. Biochem Mol Biol Int. 1994;32:705–712. [PubMed] [Google Scholar]
- 5.Kawashima I, Ohsumi J, Mita-Honjo K, Shimoda-Takano K, Ishikawa H, Sakakibara S, Miyadai K, Takiguchi Y. Molecular cloning of cDNA encoding adipogenesis inhibitory factor and identity with interleukin-11. FEBS Lett. 1991;283:199–202. doi: 10.1016/0014-5793(91)80587-s. [DOI] [PubMed] [Google Scholar]
- 6.Du XX, Williams DA. Interleukin-11: a multifunctional growth factor derived from the hematopoietic microenvironment. Blood. 1994;83:2023–2030. [PubMed] [Google Scholar]
- 7.Yonemura Y, Kawakita M, Masuda T, Fujimoto K, Takatsuki K. Effect of recombinant human interleukin-11 on rat megakaryopoiesis and thrombo-poiesis in vivo: comparative study with interleukin-6. Br J Haematol. 1993;84:16–23. doi: 10.1111/j.1365-2141.1993.tb03020.x. [DOI] [PubMed] [Google Scholar]
- 8.Schwertschlag US, Trepicchio WL, Dykstra KH, Keith JC, Turner KJ, Dorner AJ. Hematopoietic, immunomodulatory and epithelial effects of interleukin-11. Leukemia. 1999;13:1307–1315. doi: 10.1038/sj.leu.2401514. [DOI] [PubMed] [Google Scholar]
- 9.Sonis S, Muska A, O’Brien J, Van Vugt A, Langer-Safer P, Keith J. Alteration in the frequency, severity and duration of chemotherapy-induced mucositis in hamsters by interleukin-11. Eur J Cancer B Oral Oncol. 1995;31B:261–266. doi: 10.1016/0964-1955(95)00015-a. [DOI] [PubMed] [Google Scholar]
- 10.Hermann JA, Hall MA, Maini RN, Feldmann M, Brennan FM. Important immunoregulatory role of interleukin-11 in the inflammatory process in rheumatoid arthritis. Arthritis Rheum. 1998;41:1388–1397. doi: 10.1002/1529-0131(199808)41:8<1388::AID-ART7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 11.Bilinski P, Roopenian D, Gossler A. Maternal IL-11Rα function is required for normal decidua and fetoplacental development in mice. Genes Dev. 1998;12:2234–2243. doi: 10.1101/gad.12.14.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- 13.Dimitriadis E, Salamonsen LS, Robb L. Expression of interleukin-11 during the human menstrual cycle: coincidence with stromal cell decidualization and relationship to leukaemia inhibitory factor and prolactin. Mol Hum Reprod. 2000;6:907–914. doi: 10.1093/molehr/6.10.907. [DOI] [PubMed] [Google Scholar]
- 14.Karpovich N, Chobotova K, Carver J, Heath JK, Barlow DH, Mardon HJ. Expression and function of interleukin-11 and its receptor α in the human endometrium. Mol Hum Reprod. 2003;9:75–80. doi: 10.1093/molehr/gag012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taga T, Kishimoto T. Cytokine receptors and signal transduction. FASEB J. 1992;6:3387–3396. doi: 10.1096/fasebj.6.15.1334470. [DOI] [PubMed] [Google Scholar]
- 16.Yin T, Yang YC. Protein tyrosine phosphorylation and activation of primary response genes by interleukin 11 in B9-TY1 cells. Cell Growth Differ. 1993;4:603–609. [PubMed] [Google Scholar]
- 17.Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63:1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- 18.Gimble JM, Wanker F, Wang CS, Bass H, Wu X, Kelly K, Yancopoulos GD, Hill MR. Regulation of bone marrow stromal cell differentiation by cytokines whose receptors share the gp130 protein. J Cell Biochem. 1994;54:122–133. doi: 10.1002/jcb.240540113. [DOI] [PubMed] [Google Scholar]
- 19.Zhang XG, Gu JJ, Lu ZY, Yasukawa K, Yancopoulos GD, Turner K, Shoyab M, Taga T, Kishimoto T, Bataille R. Ciliary neurotropic factor, interleukin 11, leukemia inhibitory factor, and oncostatin M are growth factors for human myeloma cell lines using the interleukin 6 signal transducer gp130. J Exp Med. 1994;179:1337–1342. doi: 10.1084/jem.179.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fourcin M, Chevalier S, Lebrun JJ, Kelly P, Pouplard A, Wijdenes J, Gascan H. Involvement of gp130/interleukin-6 receptor transducing component in interleukin-11 receptor. Eur J Immunol. 1994;24:277–280. doi: 10.1002/eji.1830240143. [DOI] [PubMed] [Google Scholar]
- 21.Hilton DJ, Hilton AA, Raicevic A, Rakar S, Harrison-Smith M, Gough NM, Begley CG, Metcalf D, Nicola NA, Willson TA. Cloning of a murine IL-11 receptor α-chain; requirement for gp130 for high affinity binding and signal transduction. EMBO J. 1994;13:4765–4775. doi: 10.1002/j.1460-2075.1994.tb06802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karow J, Hudson KR, Hall MA, Vernallis AB, Taylor JA, Gossler A, Heath JK. Mediation of interleukin-11-dependent biological responses by a soluble form of the interleukin-11 receptor. Biochem J. 1996;318:489–495. doi: 10.1042/bj3180489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin T, Yang YC. Mitogen-activated protein kinases and ribosomal S6 protein kinases are involved in signaling pathways shared by interleukin-11, interleukin-6, leukemia inhibitory factor, and oncostatin M in mouse 3T3-L1 cells. J Biol Chem. 1994;269:3731–3738. [PubMed] [Google Scholar]
- 24.Wang XY, Fuhrer DK, Marshall MS, Yang YC. Interleukin-11 induces complex formation of Grb2, Fyn, and JAK2 in 3T3L1 cells. J Biol Chem. 1995;270:27999–28002. doi: 10.1074/jbc.270.47.27999. [DOI] [PubMed] [Google Scholar]
- 25.Fuhrer DK, Yang YC. Activation of Src-family protein tyrosine kinases and phosphatidylinositol 3-kinase in 3T3-L1 mouse preadipocytes by interleukin-11. Exp Hematol. 1996;24:195–203. [PubMed] [Google Scholar]
- 26.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barton VA, Hall MA, Hudson KR, Heath JK. Interleukin-11 signals through the formation of a hexameric receptor complex. J Biol Chem. 2000;275:36197–36203. doi: 10.1074/jbc.M004648200. [DOI] [PubMed] [Google Scholar]
- 28.Neddermann P, Graziani R, Ciliberto G, Paonessa G. Functional expression of soluble human interleukin-11 (IL-11) receptor α and stoichiometry of in vitro IL-11 receptor complexes with gp130. J Biol Chem. 1996;271:30986–30991. doi: 10.1074/jbc.271.48.30986. [DOI] [PubMed] [Google Scholar]
- 29.Grotzinger J, Kernebeck T, Kallen KJ, Rose-John S. IL-6 type cytokine receptor complexes: hexamer, tetramer or both? Biol Chem. 1999;380:803–813. doi: 10.1515/BC.1999.100. [DOI] [PubMed] [Google Scholar]
- 30.Czupryn M, Bennett F, Dube J, Grant K, Scoble H, Sookdeo H, McCoy JM. Alanine-scanning mutagenesis of human interleukin-11: identification of regions important for biological activity. Ann NY Acad Sci. 1995;762:152–164. doi: 10.1111/j.1749-6632.1995.tb32323.x. [DOI] [PubMed] [Google Scholar]
- 31.Bazan JF. Neuropoietic cytokines in the hematopoietic fold. Neuron. 1991;7:197–208. doi: 10.1016/0896-6273(91)90258-2. [DOI] [PubMed] [Google Scholar]
- 32.Bravo J, Heath JK. Receptor recognition by gp130 cytokines. EMBO J. 2000;19:2399–2411. doi: 10.1093/emboj/19.11.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barton VA, Hudson KR, Heath JK. Identification of three distinct receptor binding sites of murine interleukin-11. J Biol Chem. 1999;274:5755–5761. doi: 10.1074/jbc.274.9.5755. [DOI] [PubMed] [Google Scholar]
- 34.Brakenhoff JP, Hart M, De Groot ER, Di Padova F, Aarden LA. Structure-function analysis of human IL-6. Epitope mapping of neutralizing monoclonal antibodies with amino- and carboxyl-terminal deletion mutants. J Immunol. 1990;145:561–568. [PubMed] [Google Scholar]
- 35.Ciapponi L, Graziani R, Paonessa G, Lahm A, Ciliberto G, Savino R. Definition of a composite binding site for gp130 in human interleukin-6. J Biol Chem. 1995;270:31249–31254. doi: 10.1074/jbc.270.52.31249. [DOI] [PubMed] [Google Scholar]
- 36.Panayotatos N, Radziejewska E, Acheson A, Somogyi R, Thadani A, Hendrickson WA, McDonald NQ. Localization of functional receptor epitopes on the structure of ciliary neurotrophic factor indicates a conserved, function-related epitope topography among helical cytokines. J Biol Chem. 1995;270:14007–14014. doi: 10.1074/jbc.270.23.14007. [DOI] [PubMed] [Google Scholar]
- 37.Di Marco A, Gloaguen I, Graziani R, Paonessa G, Saggio I, Hudson KR, Laufer R. Identification of ciliary neurotrophic factor (CNTF) residues essential for leukemia inhibitory factor receptor binding and generation of CNTF receptor antagonists. Proc Natl Acad Sci USA. 1996;93:9247–9252. doi: 10.1073/pnas.93.17.9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fontaine V, Savino R, Arcone R, de Wit L, Brakenhoff JP, Content J, Ciliberto G. Involvement of the Arg179 in the active site of human IL-6. Eur J Biochem. 1993;211:749–755. doi: 10.1111/j.1432-1033.1993.tb17605.x. [DOI] [PubMed] [Google Scholar]
- 39.Ehlers M, Grotzinger J, deHon FD, Mullberg J, Brakenhoff JP, Liu J, Wollmer A, Rose-John S. Identification of two novel regions of human IL-6 responsible for receptor binding and signal transduction. J Immunol. 1994;153:1744–1753. [PubMed] [Google Scholar]
- 40.Savino R, Lahm A, Giorgio M, Cabibbo A, Tramontano A, Ciliberto G. Saturation mutagenesis of the human interleukin 6 receptor-binding site: implications for its three-dimensional structure. Proc Natl Acad Sci USA. 1993;90:4067–4071. doi: 10.1073/pnas.90.9.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savino R, Lahm A, Salvati AL, Ciapponi L, Sporeno E, Altamura S, Paonessa G, Toniatti C, Ciliberto G. Generation of interleukin-6 receptor antagonists by molecular-modeling guided mutagenesis of residues important for gp130 activation. EMBO J. 1994;13:1357–1367. doi: 10.1002/j.1460-2075.1994.tb06389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hudson KR, Vernallis AB, Heath JK. Characterization of the receptor binding sites of human leukemia inhibitory factor and creation of antagonists. J Biol Chem. 1996;271:11971–11978. doi: 10.1074/jbc.271.20.11971. [DOI] [PubMed] [Google Scholar]
- 43.Robinson RC, Grey LM, Staunton D, Vankelecom H, Vernalis AB, Moreau JF, Stuart DI, Heath JK, Jones EY. The crystal structure and biological function of leukaemia inhibitory factor: implications for receptor binding. Cell. 1994;77:1101–1116. doi: 10.1016/0092-8674(94)90449-9. [DOI] [PubMed] [Google Scholar]
- 44.Saito M, Yoshida K, Hibi M, Taga T, Kishimoto T. Molecular cloning of a murine IL-6 receptor-associated signal transducer, gp130, and its regulated expression in vivo. J Immunol. 1992;148:4066–4071. [PubMed] [Google Scholar]
- 45.DuBridge RB, Tang P, Hsia HC, Leong PM, Miller JH, Calos MP. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandez-Shaw S, Shorter SC, Naish CE, Barlow DH, Starkey PM. Isolation and purification of human endometrial stromal and glandular cells using immunomagnetic microspheres. Hum Reprod. 1992;7:156–161. doi: 10.1093/oxfordjournals.humrep.a137609. [DOI] [PubMed] [Google Scholar]
- 47.Ansorge W. Fast and sensitive detection of protein and DNA bands by treatment with potassium permanganate. J Biochem Biophys Meth. 1985;11:13–20. doi: 10.1016/0165-022x(85)90037-5. [DOI] [PubMed] [Google Scholar]
- 48.Aoki Y, Narazaki M, Kishimoto T, Tosato G. Receptor engagement by viral interleukin-6 encoded by Kaposi sarcoma-associated herpes virus. Blood. 2001;98:3042–3049. doi: 10.1182/blood.v98.10.3042. [DOI] [PubMed] [Google Scholar]
- 49.Ihle JN. Cytokine receptor signalling. Nature. 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 50.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 51.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka T, Sakamoto T, Miyama M, Ogita S, Umesaki N. Interleukin-11 enhances cell survival of decidualized normal human endometrial stromal cells. Gynecol Endocrinol. 2001;15:272–278. [PubMed] [Google Scholar]
- 53.Dimitriadis E, Robb L, Salamonsen LA. Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Mol Hum Reprod. 2002;8:636–643. doi: 10.1093/molehr/8.7.636. [DOI] [PubMed] [Google Scholar]
- 54.Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. J Clin Endocrinol Metab. 1996;81:174–179. doi: 10.1210/jcem.81.1.8550748. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Y, Nichols JE, Bulun SE, Mendelson CR, Simpson ER. Aromatase P450 gene expression in human adipose tissue. Role of a Jak/STAT pathway in regulation of the adipose-specific promoter. J Biol Chem. 1995;270:16449–16457. doi: 10.1074/jbc.270.27.16449. [DOI] [PubMed] [Google Scholar]
- 56.Yamasaki K, Taga T, Hirata Y, Yawata H, Kawanishi Y, Seed B, Taniguchi T, Hirano T, Kishimoto T. Cloning and expression of the human interleukin-6 (BSF-2/IFNβ2) receptor. Science. 1988;241:825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- 57.Vernallis AB, Hudson KR, Heath JK. An antagonist for the leukemia inhibitory factor receptor inhibits leukemia inhibitory factor, cardiotrophin-1, ciliary neurotrophic factor, and oncostatin M. J Biol Chem. 1997;272:26947. doi: 10.1074/jbc.272.43.26947. [DOI] [PubMed] [Google Scholar]
- 58.Aubert J, Dessolin S, Belmonte N, Li M, McKenzie FR, Staccini L, Villageois P, Barhanin B, Vernallis A, Smith AG, Ailhaud G, Dani C. Leukemia inhibitory factor and its receptor promote adipocyte differentiation via the mitogen-activated protein kinase cascade. J Biol Chem. 1999;274:24965–24972. doi: 10.1074/jbc.274.35.24965. [DOI] [PubMed] [Google Scholar]
- 59.Cafferty WB, Gardiner NJ, Gavazzi I, Powell J, McMahon SB, Heath JK, Munson J, Cohen J, Thompson SW. Leukemia inhibitory factor determines the growth status of injured adult sensory neurons. J Neurosci. 2001;21:7161–7170. doi: 10.1523/JNEUROSCI.21-18-07161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


