Abstract
The interleukin-11 (IL-11) receptor α has an important function in decidualization of mouse endometrial stroma but the function of IL-11 and its receptor in the human endometrium remains unknown. The mRNA for IL-11 and its receptor α in human endometrial tissue samples were analysed by semi-quantitative RT–PCR and RNase protection assays respectively. The proteins were detected in frozen endometrial tissue samples by immunofluorescence. The effect of heparin-binding epidermal growth factor (HB-EGF) on secretion of IL-11 by cultured endometrial stromal cells was assessed by enzyme-linked immunosorbent assay. The proliferative potential of IL-11 in endometrial stromal cells was assessed by [3H]thymidine uptake. IL-11 and its receptor α mRNAs and proteins were detected in the endometrium throughout the cycle. Distinct patterns of localization of the ligand and receptor were observed. HB-EGF induced IL-11 secretion by cultured stromal cells, and IL-11 induced [3H]thymidine uptake by these cells. Our data suggest that IL-11–receptor interactions may perform different functions in the human endometrium at different stages of the cycle, and that secretion of IL-11 is modulated by local growth factors.
Keywords: endometrium, heparin-binding epidermal growth factor, interleukin-11, interleukin-11 receptor α, proliferation
Introduction
Interleukin-11 (IL-11) is a secreted cytokine with pleiotropic functions in many tissues and cells (reviewed in Du and Williams, 1997). It belongs to the gp130 family of cytokines, which includes interleukin-6 (IL-6), leukaemia inhibitory factor (LIF), oncostatin M, cardiotrophin-1 and ciliary neurotrophic factor (CNTF). The gp130 receptor subunit forms a functional IL-11 receptor in complex with a ligand-binding specific receptor α. The human interleukin-11 receptor α chain (IL-11Rα) is a membrane-anchored glycoprotein that is also widely expressed in various tissues and cells (Chérel et al., 1995; Robb et al., 1996; Nandurkar et al., 1997). The IL-11/IL-11Rα/gp130 complex signals via activation of Janus kinases (JAKs) which subsequently phosphorylate tyrosine residues in the cytoplasmic domain of the gp130 subunit. This in turn triggers signalling cascades involving mitogen-activated protein kinases (MAPKs), ribosomal protein S6 kinase (pp90rsk) and signal transducer and activator of transcription (STAT) family, in particular STAT3 and STAT1 proteins, resulting in the activation of transcription of specific genes (Yin and Yang, 1994; Yin et al., 1994; Yang and Yin, 1995).
The complex cellular and molecular changes that take place during the menstrual cycle in response to steroid hormones are mediated by a number of cytokines. Recent evidence from targeted gene knockout experiments in mice revealed that IL-11Rα is a key regulator of the decidualization of the murine endometrial stroma (Bilinski et al., 1998; Robb et al., 1998). Female mice lacking the IL-11Rα gene are infertile due to defective differentiation of the stroma in response to an implanting blastocyst, leading to resorption of the embryo. The expression of IL-11 in the mouse endometrium is maximal during decidualization, suggesting that IL-11–receptor interactions in the decidua are important in this process (Robb et al., 1998).
Recent reports demonstrated that IL-11 mRNA and protein are expressed in the human endometrium throughout the menstrual cycle (Dimitriadis et al., 2000; Cork et al., 2001; Chen et al., 2002). Expression of IL-11 in the stroma was reported to be restricted to the pre-decidualized stromal cells in the late secretory phase (Dimitriadis et al., 2000; Cork et al., 2001), when spontaneous decidual transformation of the stroma occurs around spiral arteries and in a compact layer below the surface epithelium. However, Chen et al. (Chen et al., 2002) reported low levels of IL-11 only in late secretory phase endometrial epithelium and in the stroma and epithelium of first trimester decidual tissue. It was present in the stroma of first trimester decidual tissue. In addition, IL-11 production by human endometrial stromal and epithelial cells in vitro has been reported to be upregulated by tumour necrosis factor α (TNFα), transforming growth factor β (TGFβ) and interleukin-1α (IL-1α) (Cork et al., 2001). It has been shown by RT–PCR that both the common signal transducer gp130 and the specific IL-11Rα mRNAs are expressed in the human endometrium throughout the cycle (Dimitriadis et al., 2000; Chen et al., 2002). A recent report shows that IL-11 enhances progesterone-induced decidualization of endometrial stromal cells in vitro (Dimitriadis et al., 2002). However, given the pleiotropic functions of IL-11–receptor interactions in other tissues, it is possible that IL-11 exerts additional effects in the endometrium.
Here we report the detailed analyses of the expression and localization of IL-11 and IL-11Rα in human endometrium throughout the menstrual cycle as detected by RNA assays and immunohistochemistry. We demonstrate the proliferative potential of IL-11, and the effect of heparin-binding epidermal growth factor (HB-EGF), an important endometrial cytokine, on IL-11 expression in cultured human endometrial stromal cells.
Materials and methods
Tissue samples
Endometrial tissues were obtained from patients undergoing hysterectomy for benign conditions in accordance with the requirements of the Oxfordshire Clinical Research Ethics Committee. Patients were aged between 34 and 47 years, had a regular menstrual cycle of 26–31 days and were not using hormonal medication. The tissue was staged according to the last menstrual period and to standard histological criteria (Noyes et al., 1950). Tissue samples were snap-frozen in liquid nitrogen and stored at −80°C for subsequent RNA or immunohistochemical analyses.
RNA preparation
Total RNA from frozen tissue obtained at menstrual (days 1–5, n = 2), proliferative (days 6–14, n = 5), early secretory (days 15–18, n = 6), mid secretory (days 19–24, n = 9), and late secretory (days 25–31, n = 4) stages of the menstrual cycle was prepared using TRI-reagent (Helena BioSciences Ltd, UK) according to the manufacturer’s instructions.
Cloning of human IL-11Rα cDNA
A 462 bp human IL-11Rα cDNA, corresponding to IL-11Rα62–525 (Van Leuven et al., 1996), was obtained by amplification of 1 μg of placental RNA by RT–PCR using the following primers: 5′-AGTCGAATTCATGAGCAGCAGCTGCTCAGGG and 3′-AGTCCTCGAGTAGGACTGTCTTCTTCCTGTA containing EcoR1 and Xho1 restriction sites respectively. RT–PCR was performed using the one-step Access RT–PCR System (Promega Corporation, USA) according to the manufacturer’s instructions. The amplified cDNA fragment was cloned into the EcoR1/Xho1 site of pBluescript to generate pBS-IL11R. The sequence was confirmed by direct sequencing (DNA Sequencing Unit, Department of Biochemistry, University of Oxford, UK).
RNase protection assay
A 512 nucleotide IL-11Rα antisense riboprobe was generated by transcription of pBS-IL11R, digested with EcoR1, in the presence of [α-32P]rCTP (Amersham Pharmacia Biotech, UK) and T3 RNA polymerase using RiboProbe In Vitro Transcription Systems (Promega Corporation) according to the manufacturer’s instructions. A human glyceraldehyde-3-phosphatedehydrogenase (GAPDH) 226 nucleotide riboprobe was generated from pBS-hGAP linearized with HindIII as described previously (Yoo et al., 1997). Transcribed riboprobes were digested with 10 U DNase (Promega Corporation) at 37°C for 30 min and purified using a QIAquick PCR Purification Kit (Qiagen) according to the manufacturer’s instructions. RNA Century Marker Templates (Ambion Inc., USA) were transcribed and purified as described above using T7 RNA polymerase. RNase protection assays were performed by the use of the Hybspeed kit (Ambion Inc.) according to the manufacturer’s instructions with 20 μg of yeast or tissue RNA. Protected fragments were subjected to 6% polyacrylamide gel electrophoresis in denaturing conditions and subjected to autoradiography. The density of the bands representing the protected fragments were measured using AlphaImager 1220 Analysis and Documentation System software (Alpha Innotech Inc., USA).
RT–PCR
Total RNA samples (10 μg) were treated with DNA-free DNase (Ambion Inc.) and 1 μg of DNased RNA was subjected to reverse transcription using SuperScript™ II RNase H− Reverse Transcriptase (Life Technologies Inc., USA) according to the manufacturer’s instructions. Semi-quantitative RT–PCR was performed to detect and quantify IL-11 in endometrial tissues with the use of the Gene Specific Relative RT–PCR Kit (Ambion Inc.), FastStart Tag DNA Polymerase (Roche Diagnostics GmbH, Germany) and GC-RICH solution according to the manufacturer’s instructions. Optimal amplification of IL-11 cDNA within its linear range was achieved with 40 cycles of 30 s at 94°C, 1 min at 63°C and 1 min at 72°C with the final extension step of 7 min at 72°C. Amplification of 18S ribosomal RNA was performed as above but without GC-rich melting solution and with 30 amplification cycles. The sizes of the final products for IL-11 and 18S rRNA were 300 and 500 bp respectively. Negative controls were first strand reactions in which reverse transcriptase was substituted with water, and IL-11 cDNA, generously provided by the Genetics Institute (USA), served as a positive control. Final products were separated by 1.5% agarose gel electrophoresis. Band intensities were analysed on AlphaImager 1220 Analysis and Documentation System (Alpha Innotech Inc.).
Immunohistochemistry
Sections from menstrual (n = 2), proliferative (n = 4), early secretory (n = 3), mid secretory (n = 6) and late secretory (n = 2) human endometrial tissues were fixed in cold acetone for 10 min at 4°C and non-specific IgG-binding sites blocked by incubation in 20% donkey serum for 20 min at 20°C. Sections were incubated with 10 μg/ml of either goat anti-human IL-11Rα (Santa Cruz Biotechnology Inc., USA) recognizing the intracellular epitope of the receptor, or mouse anti-human IL-11 (R&D Systems, UK) diluted in 0.1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS-B) containing 10% normal human serum. Control sections were treated with goat or mouse IgGs at the same concentration as the primary antibody, or primary antibodies that had been pre-incubated with the corresponding antigen peptides (10-fold excess). Incubations were performed overnight at 4°C. Sections were washed in PBS-B and incubated with 8.3 μg/ml fluorescein isothiocyanate-conjugated donkey anti-mouse or anti-goat IgG (Jackson ImmunoResearch Laboratories Inc., USA) for 45–50 min at room temperature. Sections were washed as described above and mounted using Vectashield mountant (Vector Laboratories Ltd, UK). Staining was visualized on a Leitz DMRBE fluorescent microscope (Leica Wetzlar, Germany) and images were captured and analysed with the use of OpenLab software (Improvision Ltd, UK).
Detection of IL-11Rα in cultured human endometrial stromal cells
Cell culture media and reagents were purchased from Sigma (UK). Human endometrial stromal cells were prepared according to a protocol described previously (Fernandez-Shaw et al., 1992). In this study, cells were used between passages 1 and 2, and were at least 84 and 92% positive for Thy-1 and vimentin respectively. Cells were plated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS), 100 IU/ml penicillin and 100 μg/ml streptomycin, on glass coverslips (Chance Propper Ltd, UK) at 104 cells per coverslip and incubated for 2 days at 37°C in 5% CO2. Cells were fixed for 5 min with 4% paraformaldehyde in PBS and permeabilized with 10 mmol/l HEPES, pH 7.4, 200 mmol/l sucrose, 3 mmol/l MgCl2, 50 mmol/l NaCl, 0.5% Triton X-100, 0.2% NaN3 in PBS. Non-specific binding sites were blocked by incubating the cells with 3% BSA and 5% glucose in PBS for 10 min. Immunofluoresence was performed as described previously (Hotchin et al., 1999) with either 10 μg/ml goat anti-human IL-11Rα (Santa Cruz Biotechnology Inc.), 10 μg/ml mouse, goat IgG or primary antibody that had been pre-incubated with the corresponding antigen peptide (10-fold excess) overnight at 4°C for the controls, followed by 8.3 μg/ml FITC-conjugated donkey anti-goat IgG (Jackson ImmunoResearch Laboratories Inc.). Coverslips were mounted in Vectashield containing DAPI (Vector Laboratories Ltd) and observed and analysed as described above.
[3H]thymidine incorporation
The mitogenic potential of IL-11 in the human endometrium was assessed in DNA synthesis assays. Endometrial stromal cells prepared as above were plated (104 cells per well) in 96-well plates in DMEM supplemented with 10% FCS, 100 IU/ml penicillin and 100 μg/ml streptomycin, and incubated for 24 h at 37°C in 5% CO2. The medium was changed to serum-free and the cells incubated for 18 h. Cells were stimulated by the addition of 10 ng/ml rhIL-11 (R&D Systems) with or without IL-11 neutralizing antibody (1 or 10 μg/ml) (R&D Systems) or 125 or 250 ng/ml of W147, an IL-11 signalling inhibitor (Barton et al., 2000) to the serum-free medium and incubated for a further 24 h. The same concentrations of IL-11 neutralizing antibody or W147 were added to the control medium without IL-11. Methyl-[3H]thymidine was added at 1 μCi/well for the last 4 h of the incubation period. Cells were washed three times in PBS, harvested, and the amount of incorporated [3H]thymidine was determined using a β-plate counter (Wallac Ltd., Finland).
Enzyme-linked immunosorbent assay (ELISA)
The effect of HB-EGF on the production of IL-11 by human endometrial stromal cells was assessed by ELISA. Cells were plated as described above and grown to confluency, and incubated with or without 10 ng/ml rhHB-EGF (R&D Systems) in serum-free DMEM for 72 h. Supernatants were analysed for IL-11 using mouse anti-IL-11 monoclonal antibody and goat anti-IL-11 biotinylated polyclonal antibody (R&D Systems) in a sandwich ELISA format according to the manufacturer’s instructions. The minimum detection limit of IL-11 was 16 pg/ml. The inter-assay and intra-assay coefficient of variance were 6.7 and 2.5% respectively. The cells were subsequently lysed with 0.05 mol/l sodium hydroxide and the total protein concentration was measured using the Coomassie assay (Pierce Ltd, UK). The amount of IL-11 detected was normalized to the amount of total protein in each well.
Statistics
Data were analysed by the use of ANOVA one-way analysis of variance (DNA synthesis assays) or the unpaired t-test (IL-11 ELISA). Significance was assumed when P < 0.05.
Results
IL-11 and IL-11Rα mRNA are expressed in human endometrial tissues throughout the cycle
Levels of IL-11 and IL-11Rα mRNA in total RNA extracts from human endometrium throughout the menstrual cycle were measured in semi-quantitative RT–PCR (IL-11) and RNase protection assay (IL-11Rα) (Figure 1). IL-11Rα mRNA was expressed throughout the cycle (Figure 1a). There were no detectable differences in the levels of IL-11Rα mRNA in tissues obtained at different stages of the cycle (Figure 1b). Detection of IL-11 by RT–PCR revealed its presence in all the endometrial tissues analysed (Figure 1c) with similar levels throughout the cycle (Figure 1d). The levels of IL-11 were very low and barely detectable by RNase protection assay (data not shown).
Figure 1.
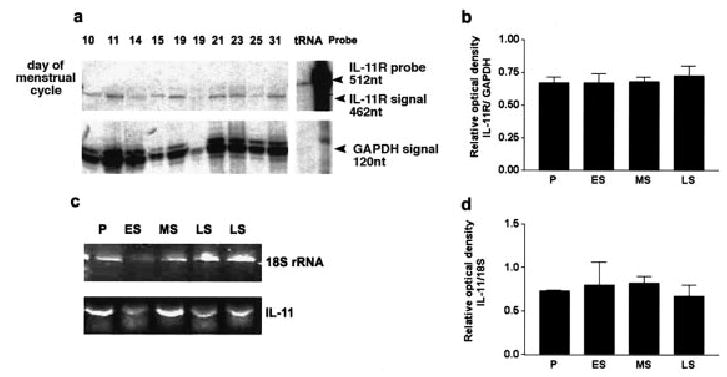
Expression of interleukin-11 (IL-11) and its receptor α chain (IL-11Rα) mRNAs in human endometrial tissues throughout the menstrual cycle. (a) Semi-quantitative RNase protection assay of IL-11Rα and glyceraldehyde-3-phosphatedehydrogenase (GAPDH) mRNA from endometrial samples obtained at different days of the cycle (indicated above the autoradiograph). The 462 and 120 nucleotide bands corresponded to the expected size of the IL-11Rα and GAPDH respectively, the 512 nucleotide bands corresponded to IL-11Rα undigested probe. The results shown are one of three representative independent experiments. (b) Densitometric analysis of autoradiographs from three independent experiments as shown in (a). The intensity of bands corresponding to IL-11Rα mRNA were expressed as the ratio of those corresponding to GAPDH. Values are means ± SEM (n = 4–9 per group). (c) RT–PCR with specific primers for IL-11 and 18S rRNA. P, proliferative (days 6–14); ES, early secretory (days 15–18); MS, mid secretory (days 19–24); LS, late secretory (days 25–31) phases of the menstrual cycle. (d) Densitometric analysis of the intensity of the bands corresponding to IL-11 mRNA expressed as the ratio of those corresponding to 18S rRNA. Values are means ± SEM (n = 2–4 per group).
IL-11 and IL-11Rα expression in the human endometrium
Representative sections of endometrium from the proliferative, early, mid and late secretory stages of the menstrual cycle stained with anti-IL-11 or anti-IL-11Rα are shown in Figure 2. Immunoreactive IL-11 (Figure 2a–c) and IL-11Rα (Figure 2h) were detected in both glandular and lumenal epithelium (data not shown) throughout the menstrual cycle. Staining for IL-11 in the glandular epithelium was most intense on the apical surface and distinctively punctate on the lateral and basal surfaces of the cells. The staining for IL-11 was also detected at low levels in the stroma at all stages of the cycle, with the occasional stromal cell exhibiting increased staining. Staining with haematoxylin and eosin confirmed the presence of pre-decidualized stromal cells in endometrium from the late secretory phase (Figure 2d). Background staining was negative, as assessed in control sections incubated with IL-11 antibodies pre-adsorbed with antigenic peptide and secondary antibodies (Figure 2e).
Figure 2.
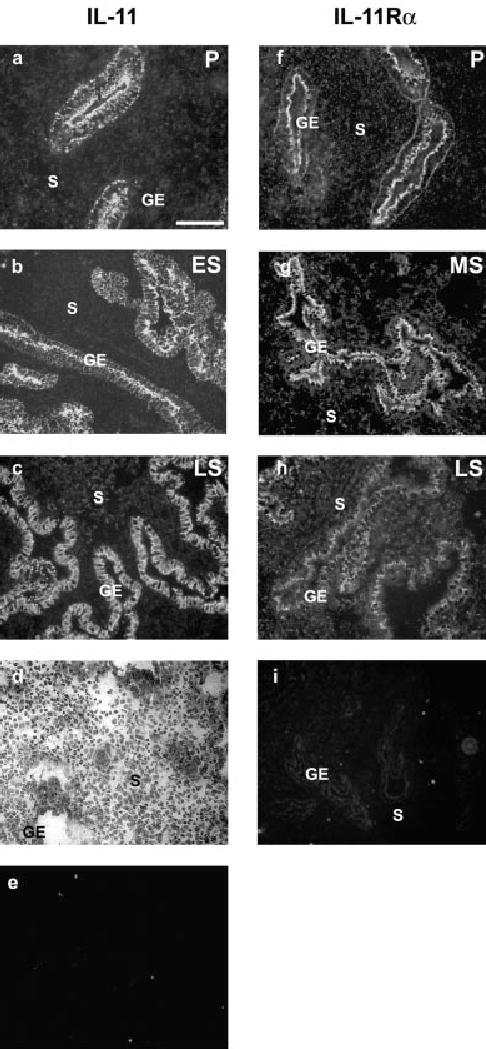
Expression of IL-11Rα and IL-11 in the human endometrium by fluorescent immunohistochemistry. Sections of endometrium obtained at different stages of the cycle were treated with (a–c) anti-IL-11 antibodies, (f–h) anti-IL-11Rα antibodies, (e) anti-IL-11 antibodies pre-incubated with IL-11 antigenic peptide, (i) anti-IL-11Rα antibody pre-incubated with IL-11Rα antigenic peptide (a, f) proliferative endometrium (P; day 8); (b) early secretory endometrium (ES; day 15); (g) mid secretory endometrium (MS; day 22); (c, d, h) late secretory endometrium (LS; day 28). Both the ligand and receptor were detected in the stroma (S) and glandular epithelium (GE) at all stages of the cycle. (d) Haematoxylin and eosin staining of endometrial tissue obtained from the late secretory phase (day 28) used for IL-11 and IL-11Rα immunodetection as seen in (c) and (h) showing decidualized stroma. Scale bar (a–c, e–i) = 200 mm; (d) = 100 mm.
Staining for IL-11Rα, which was achieved by using an antibody previously shown to detect IL-11Rα in sections of prostate tissue (Arap et al., 2002), was also detected in endometrium throughout the cycle. The intensity of staining for IL-11Rα was higher on the apical surface of the glandular epithelium than on the basal or lateral surfaces, and in this respect was similar to IL-11. In contrast to IL-11, the relative levels of IL-11Rα in the stroma were higher. The background staining in a section treated with goat IgG instead of primary antibody is shown in Figure 2i.
IL-11Rα is expressed in cultured human endometrial stromal cells
In order to further investigate the function of IL-11 in cultured endometrial stromal cells, we first confirmed the presence of IL-11Rα in these cells by immunocytochemistry (Figure 3). The staining for IL-11-Rα was punctate and evenly distributed across the cell membranes (Figure 3a). Cells incubated with pre-adsorbed anti-IL-11-Rα (Figure 3b) and goat IgG instead of primary antibody (data not shown) were negative.
Figure 3.
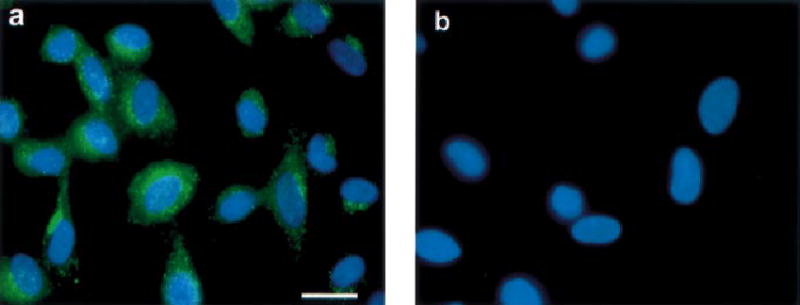
Immunofluorescent staining of cultured human endometrial stromal cells with (a) anti-IL-11Rα antibodies, or (b) anti-IL-11Rα antibodies pre-incubated with IL-11Rα antigenic peptide. Scale bar = 15 μm.
IL-11 induces DNA synthesis in human endometrial stromal cells
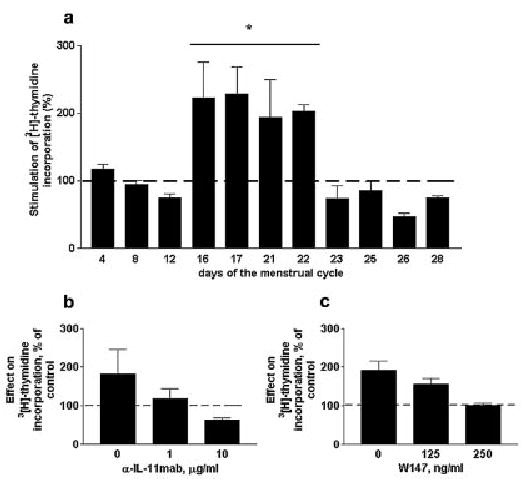
Having established that IL-11Rα is expressed in cultured endometrial stromal cells, we examined the mitogenic potential of rhIL-11 on endometrial stromal cells (Figure 4a). Four of the 11 stromal cells lines tested demonstrated induction of DNA synthesis as determined by [3H]thymidine uptake in response to IL-11. The response was atleast 2-fold higher than the control in these four cell lines (P < 0.05). These four cell lines were derived from tissues obtained at the early–mid secretory stages of the cycle (between days 16 and 22).
Figure 4.
[3H]thymidine incorporation by cultured human endometrial stromal cells treated with rhIL-11 (10 ng/ml). (a) Each bar represents data from an individual cell line derived from endometrium obtained at different days of the cycle. Values are means ± SEM of six replicates of each cell line normalized to values obtained for control cultures incubated in the absence of IL-11 (100%). Dose-dependent inhibitory effect of (b) anti-IL-11 neutralizing antibodies or (c) IL-11 signalling inhibitor W147 on [3H]thymidine incorporation by cultured human endometrial stromal cells treated with rhIL-11 (10 ng/ml). Values are means ± SEM of four replicates normalized to values obtained for control cultures incubated in the absence of IL-11 (100%). *P < 0.05.
We then tested the specificity of this response. In the first experiment, an IL-11 neutralizing anti-IL-11 antibody reduced [3H]thymidine uptake in a dose-dependent manner (Figure 4b). However, the antibody is an order of magnitude larger than the ligand itself, and may thus exert steric hindrance that could have additional affects that do not specifically result from the interaction of IL-11 with its receptor. In addition, the antibody was effective at high concentrations (1 μg/ml). We therefore tested the specificity further by the use of the specific IL-11 signalling inhibitor W147. In the presence of IL-11, proliferation was dose-dependently inhibited by the addition of 125 and 250 ng/ml W147 to the culture medium (Figure 4c).
HB-EGF induces production of secreted IL-11 in human endometrial stromal cells
We examined the effect of rhHB-EGF on the secretion of IL-11 by cultured endometrial stromal cells using ELISA. Cultured stromal cells derived from tissues obtained at both proliferative and secretory stages of the cycle secreted similar levels of IL-11, ranging between 1.2 and 125 pg/μg protein, prior to stimulation with rhHB-EGF (data not shown). However, in the presence of rhHB-EGF, levels of secreted IL-11 were elevated 1.5-fold (P < 0.05) in stromal cells derived from the secretory, but not the proliferative, stages of the cycle (Figure 5).
Figure 5.
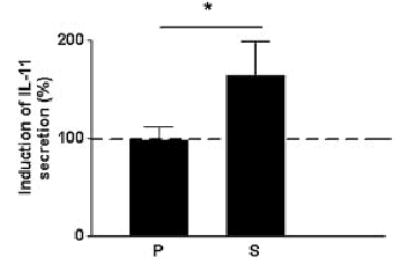
The effect of rhHB-EGF on IL-11 secretion by cultured human endometrial stromal cells isolated from the (P) proliferative (n = 4) and the (S) secretory (n = 6) phases of the menstrual cycle. The graph shows results from an enzyme-linked immunosorbent assay of IL-11 levels in culture supernatants normalized to total cell protein of each culture. Values are means ± SEM. *P < 0.05.
Discussion
Data from previous genetic analyses of IL-11Rα function in knockout mice suggest an important function for IL-11–receptor interactions in decidualization of the mouse endometrium (Bilinski et al., 1998; Robb et al., 1998). However, the function of IL-11 in the human endometrium remains unclear. Here we show that: (i) IL-11 and IL-11Rα mRNAs are co-expressed in the human endometrium throughout the menstrual cycle; (ii) IL-11 protein is present at high levels in the epithelium and relatively low levels in the stroma whereas the levels of IL-11Rα are similar in the epithelium and stroma throughout the menstrual cycle; (iii) IL-11 has a potential mitogenic function in human endometrial stromal cells; and (iv) secretion of IL-11 by cultured endometrial stromal cells is modulated by HB-EGF.
The semi-quantitative analyses we report here show that endometrial IL-11 and IL-11Rα mRNA levels are constant throughout the menstrual cycle. These data are consistent with our findings for the immunohistochemical studies. We demonstrate high levels of IL-11 on both the apical, lateral and, to a lesser extent, basal surfaces of the epithelium, and lower levels of IL-11 in the stroma. Our results are in agreement with others who have previously reported high levels of IL-11 in glandular epithelium (Dimitriadis et al., 2000; Cork et al., 2001). However, the pattern of IL-11 staining we observe in the stroma is different from previous observations. In previous studies, elevated levels of IL-11 in late secretory stage stroma (in cells identified as pre-decidual cells) were reported (Dimitriadis et al., 2000; Cork et al., 2001). In another report (Chen et al., 2002) IL-11 protein was found to be present only in secretory stage endometrial glands at very low levels and was absent in stroma, but IL-11 was found to be elevated in first trimester decidua. The reason for the apparent discrepancies in IL-11 expression is not clear, although the different detection methods utilized may offer one possible explanation.
The IL-11Rα co-localizes with the ligand in the epithelium, largely on the apical surface, and is present at relatively high levels in the stroma throughout the cycle. Detection of IL-11Rα by RT–PCR (Dimitriadis et al., 2000; Chen et al., 2002) and in situ hybridization (Chen et al., 2002) suggest that it is present at constant levels throughout the cycle, which is in agreement with the data we present here. It has been shown previously that the gp130 signalling subunit that forms a functional IL-11 receptor with IL-11Rα is, like IL-11Rα, also expressed in human endometrium throughout the cycle at constant levels (Tabibzadeh et al., 1995; Cullinan et al., 1996; Dimitriadis et al., 2000). Taken together, these data suggest a paracrine and autocrine function for IL-11 in the human endometrium.
Studies in the mouse (Blinski et al., 1998; Robb et al., 1998) also suggest that the expression of IL-11 and IL-11Rα is different from the human. IL-11 is transiently expressed at the initiation of decidualization in response to the implanting embryo, and is expressed at low levels in the non-pregnant endometrium. In contrast, IL-11Rα is expressed in both the non-pregnant and pregnant mouse endometrium, but is concentrated in the decidualized areas of the pregnant endometrium during the period of maximal decidual transformation. These data are thus different from those obtained for the human, which probably reflects differences in the decidualization process in the two species.
Endometrial stroma undergoes a dramatic increase in mass in preparation for embryo receptivity. Here we investigated the possibility that IL-11 is mitogenic for endometrial stromal cells. We demonstrated the presence of IL-11Rα in cultured endometrial stromal cells and have shown that in these cells IL-11 activates STAT3 in a dose-dependent manner (our unpublished observations) thus confirming the suitability of these cell lines as a model system to study the function of IL-11 and the mechanisms of its regulation. We found that IL-11 induced [3H]thymidine incorporation into stromal cells, suggesting that IL-11 has a mitogenic function in the human endometrium. This mitogenic activity was specifically inhibited by the addition of the IL-11Rα inhibitor W147. There is evidence that IL-11–IL-11Rα interactions have a function in proliferation during endometrial decidualization in the mouse (Bilinski et al., 1998). Our observation that the elevated response to IL-11 occurs in cells derived from early to mid secretory stage endometrial tissues, and not those from other stages of the cycle, further raises the possibility that these cell lines retain different degrees of responsiveness to IL-11, according to the stage of the cycle from which they are derived.
A number of cytokines are expressed in endometrium in an apparent coordinated cascade of events leading to implantation (Rice and Chard, 1998; Tabibzadeh, 1998; Salamonsen et al., 2000). Levels of HB-EGF mRNA increase prior to the implantation window in human endometrium, HB-EGF protein is expressed in human mid secretory endometrium (Yoo et al., 1997; Leach et al., 1999), suggesting that HB-EGF may act at a relatively early control point in the events leading up to the acquisition of endometrial receptivity. It has been demonstrated that HB-EGF plays an important role in facilitating endometrial receptivity and implantation and that it is mitogenic for human endometrial stromal cells (Chobotova et al., 2002a,b). Our data demonstrate that HB-EGF induces secretion of IL-11 by endometrial stromal cell lines derived from the early to mid secretory stages of the cycle. We propose that HB-EGF and IL-11 are thus sequentially linked in the cascade of signalling events that occurs during endometrial receptivity and implantation, and that HB-EGF may have a local function in the induction of IL-11 prior to, or during, implantation. However, we do not exclude the possibility that other factors may act to modulate the secretion of IL-11 in the endometrium. Indeed cultured human endometrial stromal cells have been shown to secrete high levels of IL-11 in response to growth factors compared to epithelial cells (Cork et al., 2001).
The function of IL-11 in decidualization in the mouse has been determined in vivo (Bilinski et al., 1998; Robb et al., 1998). A recent report suggests that IL-11 has a function in decidualization of human endometrial stromal cells in vitro (Dimitriadis et al., 2002). It has also been reported that in in vitro cAMP-induced decidualized human endometrial stromal cells, IL-11 enhances cell viability in a dose-dependent manner and therefore possibly has a role in protecting these cells from apoptosis during embryo implantation and invasion of the trophobast (Tanaka et al., 2001).
In conclusion, we have demonstrated the cellular localization of IL-11 receptor in human endometrium and suggested a mechanism whereby the levels of IL-11 may be modulated according to temporo-spatially regulated local cues, for example local signals generated during implantation. The endometrial expression profiles and functional data obtained so far suggest that the functions of IL-11 and its receptor in the human endometrium may be diverse, and that it may induce discrete downstream effects in different cellular compartments at different stages of the cycle.
Acknowledgments
This work was funded by the Wellcome Trust, The Medical Research Council, Cancer Research UK and the Oxford IVF Unit.
References
- Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardó-Vila M, Giordano RJ, Mintz PJ, Ardelt PU, Yao VJ, Vidal CI, et al. Steps towards mapping the human vasculature by phage display. Nat Med. 2002;8:121–127. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- Barton VA, Hall MA, Hudson KR, Heath JK. Interleukin-11 signals through the formation of a hexameric receptor complex. J Biol Chem. 2000;275:36197–36203. doi: 10.1074/jbc.M004648200. [DOI] [PubMed] [Google Scholar]
- Bilinski P, Roopenian D, Gossler A. Maternal IL-11Rα function is required for normal decidua and fetoplacental development in mice. Genes Dev. 1998;12:2234–2243. doi: 10.1101/gad.12.14.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HF, Lin CY, Chao KH, Wu MY, Yang YS, Ho HN. Defective production of interleukin-11 by decidua and chorionic villi in human anembryonic pregnancy. J Clin Endocrinol Metab. 2002;87:2320–2328. doi: 10.1210/jcem.87.5.8478. [DOI] [PubMed] [Google Scholar]
- Chérel M, Sorel M, Lebeau B, Dubois S, Moreau JF, Bataille R, Minvielle S, Jacques Y. Molecular cloning of two isoforms of a receptor for the human hematopoietic cytokine interleukin-11. Blood. 1995;86:2534–2540. [PubMed] [Google Scholar]
- Chobotova K, Muchmore ME, Carver J, Yoo HY, Manek S, Gullick WJ, Barlow DH, Mardon HJ. The mitogenic potential of heparin-binding epidermal growth factor in the human endometrium is mediated by the epidermal growth factor receptor and modulated by tumor necrosis factor a. J Clin Endocrinol Metab. 2002a;87:5769–5777. doi: 10.1210/jc.2002-020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobotova K, Spyropoulou I, Carver J, Manek S, Gullick WJ, Barlow DH, Sargent I, Mardon HJ. A function for heparin binding epidermal growth factor and its receptors in implantation of the human blastocyst. Mech Dev. 2002b;119:137–144. doi: 10.1016/s0925-4773(02)00342-8. [DOI] [PubMed] [Google Scholar]
- Cork BA, Li TC, Warren MA, Laird SM. Interleukin-11 (IL-11) in human endometrium: expression throughout the menstrual cycle and the effects of cytokines on endometrial IL-11 production in vitro. J Reprod Immunol. 2001;50:3–17. doi: 10.1016/s0165-0378(00)00089-9. [DOI] [PubMed] [Google Scholar]
- Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc Natl Acad Sci USA. 1996;93:3115–3120. doi: 10.1073/pnas.93.7.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis E, Salamonsen LA, Robb L. Expression of interleukin-11 during the human menstrual cycle: coincidence with stromal cell decidualization and relationship to leukaemia inhibitory factor and prolactin. Mol Hum Reprod. 2000;6:907–914. doi: 10.1093/molehr/6.10.907. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, Robb L, Salamonsen LA. Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Mol Hum Reprod. 2002;8:636–643. doi: 10.1093/molehr/8.7.636. [DOI] [PubMed] [Google Scholar]
- Du X, Williams DA. Interleukin-11: review of molecular, cell biology and clinical use. Blood. 1997;89:3897–3908. [PubMed] [Google Scholar]
- Fernandez-Shaw S, Shorter SC, Naish CE, Barlow DH, Starkey PM. Isolation and purification of human endometrial and glandular cells using immunomagnetic microspheres. Hum Reprod. 1992;7:156–161. doi: 10.1093/oxfordjournals.humrep.a137609. [DOI] [PubMed] [Google Scholar]
- Hotchin NA, Kidd AG, Altroff H, Mardon HJ. Differential activation of focal adhesion kinase, Rho and Rac by the ninth and tenth FIII domains of fibronectin. J Cell Sci. 1999;112:2937–2946. doi: 10.1242/jcs.112.17.2937. [DOI] [PubMed] [Google Scholar]
- Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab. 1999;84:3355–3363. doi: 10.1210/jcem.84.9.5980. [DOI] [PubMed] [Google Scholar]
- Nandurkar HH, Robb L, Nicholl JK, Hilton DJ, Sutherland GR, Begley CG. The gene for the human interleukin-11 receptor alpha chain locus is highly homologous to the murine gene and contains alternatively spliced first exons. Int J Biochem Cell Biol. 1997;29:753–766. doi: 10.1016/s1357-2725(97)00011-3. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- Rice A, Chard T. Cytokines in implantation. Cytokine Growth Factor Rev. 1998;9:287–296. doi: 10.1016/s1359-6101(98)00020-3. [DOI] [PubMed] [Google Scholar]
- Robb L, Hilton DJ, Willson TA, Begley CG. Structural analysis of the gene encoding the murine interleukin-11 receptor α-chain and a related locus. J Biol Chem. 1996;271:13754–13761. doi: 10.1074/jbc.271.23.13754. [DOI] [PubMed] [Google Scholar]
- Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin-11 is due to a defective uterine response to implantation. Nat Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- Salamonsen LA, Dimitriadis E, Robb L. Cytokines in implanatation. Semin Reprod Med. 2000;18:299–310. doi: 10.1055/s-2000-12567. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S. Molecular control of the implantation window. Hum Reprod Update. 1998;4:465–471. doi: 10.1093/humupd/4.5.465. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S, Kong QF, Babaknia A, May LT. Progressive rise in the expression of interleukin-6 in human endometrium during menstrual cycle is initiated during the implantation window. Hum Reprod. 1995;10:2793–2799. doi: 10.1093/oxfordjournals.humrep.a135793. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Sakamoto T, Miyama M, Ogita S, Umesaki N. Interleukin-11 enhances cell survival of decidualized normal human endometrial stromal cells. Gynaecol Endocrinol. 2001;15:272–278. [PubMed] [Google Scholar]
- Van Leuven F, Stas L, Hilliker C, Miyake Y, Bilinski P, Gossler A. Molecular cloning and characterization of the human interleukin-11 receptor a-chain gene, IL11RA, located on chromosome 9p13. Genomics. 1996;31:65–70. doi: 10.1006/geno.1996.0010. [DOI] [PubMed] [Google Scholar]
- Yang YC, Yin T. Interleukin (IL)-11 mediated signal transduction. Ann N Y Acad Sci. 1995;762:31–40. doi: 10.1111/j.1749-6632.1995.tb32312.x. [DOI] [PubMed] [Google Scholar]
- Yin T, Yang YC. Mitogen-activated protein kinases and ribosomal S6 protein kinases are involved in signalling pathways shared by interleukin-11, interleukin-6, leukemia inhibitory factor and oncostatin M in mouse 3T3-L1 cells. J Biol Chem. 1994;269:3731–3738. [PubMed] [Google Scholar]
- Yin T, Yasukava K, Taga T, Kishimoto T, Yang YC. Identification of a 130-kilodalton tyrosine-phosphorylated protein induced by interleukin-11 as JAK2 tyrosine kinase, which associates with gp130 signal transducer. Exp Hematol. 1994;22:467–472. [PubMed] [Google Scholar]
- Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst implantation. Dev Genet. 1997;21:102–108. doi: 10.1002/(SICI)1520-6408(1997)21:1<102::AID-DVG12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]