Abstract
Background
African-Americans are more likely be seen by physicians with less clinical training or treated at hospitals with deficient times to acute reperfusion therapies. Less is known about differences in health outcomes. This paper compares risk-adjusted mortality following Acute Myocardial Infarction (AMI) between U.S. hospitals with high and low fractions of elderly black AMI patients.
Methods and Results
A prospective cohort study was performed for fee-for-service Medicare patients hospitalized for AMI during 1997–2001 (N = 1,136,736). Hospitals (N =4289) were classified into approximate deciles depending on the extent to which the hospital served the African-American population. The lowest category (12.5 percent of AMI patients) included hospitals without any African-American AMI admissions during 1997–2001. Decile 10 (10 percent of AMI patients) included hospitals with the highest fraction of black AMI patients (33.6 percent). The main outcome measures were 90-day and 30-day mortality following AMI.
Patients admitted to hospitals disproportionately serving African-Americans experienced no greater level of morbidities or severity of the infarction. Yet hospitals in Decile 10 experienced risk-adjusted 90-day mortality rate of 23.7 percent (95% CI: 23.2–24.2) compared to 20.1 percent (95% CI: 19.7–20.4) in Decile 1 hospitals. Differences in outcomes between hospitals were not explained by income, hospital ownership status, hospital volume, Census region, urban status, or hospital surgical treatment intensity.
Conclusions
Risk-adjusted mortality following AMI is significantly higher in U.S. hospitals that disproportionately serve African-Americans. A reduction in overall mortality at these hospitals could reduce dramatically black-white disparities in health care outcomes.
The Institute of Medicine study on racial disparities in health and health care has documented the sharp differences in the treatment of diseases for African-Americans, particularly for cardiovascular diseases.1–24 Less well understood is the mechanism generating disparities in health outcome. Do physicians or hospitals provide poorer quality care to their black patients compared to their white patients? Or are African-American patients more likely to be treated by a physician or hospital where all patients receive lower quality care, regardless of race?25–27 The importance of the latter hypothesis has been demonstrated recently in studies showing African-Americans are more likely to be seen by physicians with less clinical training than those treating whites,28 and treated at hospitals with higher risk-adjusted surgical mortality and lower rates of evidence-based treatments and protocols.29–33 However, the association between the racial composition of hospitals and health outcomes such as mortality is not known.
This study compares outcomes, measured by 90-day and 30-day adjusted mortality rates after Acute Myocardial Infarction (AMI), for hospitals that disproportionately treat African-American patients relative to those that do not. To address this question, we draw on a nearly 100 percent sample of fee-for-service Medicare patients with an index AMI between January 1997 and September 2001, comprising 1.14 million individuals. We measure the percentage of all AMI patients in a hospital who were African-American and categorize hospitals into 10 approximate deciles ranked by the extent to which a hospital serves the African-American community. Risk-adjusted mortality is examined across these deciles, under the hypothesis that hospitals with a large share of African-American patients are different from hospitals with a smaller (or zero) share. The importance of factors such as income, hospital ownership, surgical treatment intensity, racial composition, region, and unmeasured health status are considered separately as potential confounders.
Methods
Data
The primary data set is a longitudinal sample from the 100 percent Medicare fee-for-service population hospitalized for AMI between January 1997 and September 2001. The criterion for determining the presence of AMI from the claims is a primary diagnosis code of AMI (41000–41091) without evidence of an old MI. Federal hospitals are excluded. The initial sample with valid provider and location identification was 1,254,786 individuals. Patients were assigned to their hospital of initial admission for heart disease treatment, even if the patient was later treated at another hospital. Using information from the Medicare Denominator File, the race of each patient was determined as black, other, which includes Hispanic enrollees, and the residual group. Because of concerns about the statistical power required for discerning outcome differences, and the low sensitivity of Hispanic responses34 we exclude respondents in the “other” category (N = 42,200), leaving two groups, African-American, and the residual group which we denote as “white.” There is a very strong correlation between African-American racial measures in the Medicare claims data and self-reported racial identity.34
Observations were excluded if there was evidence from claims data of a previous MI (N = 54,357), or if patients enrolled in an HMO during the calendar year following the AMI index event (N = 17,160). (Patients enrolled in a risk-bearing HMO at the time of the AMI were not in this sample, because there is no record of the AMI on the claims data.) Additional criterion for exclusion was inability to match the patient’s zip code to region of residence (N = 114), lack of valid income data for that zip code (N = 2,819), and hospitals with fewer than 10 AMIs over the entire period of analysis (N = 1,400) leaving a sample of 1,136,736.
This sample was used to calculate the percent of all AMI patients in a hospital who were African-American. We then created approximate deciles of this measure to provide a summary measure of the extent to which a hospital serves the African-American community. The lowest “decile” comprised the 12.5 percent of patients admitted to hospitals without any black AMI patient during the period 1997–2001. Using this slightly larger grouping avoided splitting the sample in an arbitrary way. Decile 2 is attenuated as a result, so that the bottom two groups comprise one-fifth of the sample. The remaining deciles are defined conventionally. Patient counts in each of these higher deciles were not exactly 10 percent since patients in a given hospital were retained in the same decile category.
Measuring Health Care Outcomes
The primary measure of outcomes is risk adjusted 90-day mortality rates. While risk adjusted 30-day mortality rates are also presented, we favor 90-day rates because they are less likely to penalize hospitals with high rates of revascularization and subsequent operative mortality. Previous uses of these outcome data have been described elsewhere.35–38 As noted by previous studies, measures of hospital performance using patient outcome data can be biased by differences across hospitals in the average severity of disease.39 However, measures of risk-adjusted AMI mortality have been shown to be valid indicators of hospital quality, and have been incorporated into hospital profiling efforts, for example those developed by the Agency for Healthcare Research and Quality.40
Flexible quadratic age and gender interaction terms (age, age2, sex, age × sex, and age2 × sex) were included in all analyses. As well, the following disease categories were entered separately as categorical variables: vascular disease, dementia, renal, pulmonary, diabetes (with and without complications), liver (with and without complications), and cancer (non-metastatic and metastatic). Also included were year categorical variables (with the year 2001 the reference year) and categorical variables indicating the severity of the AMI: whether anterior, inferior, sub-endocardial, or a reference “other” category.
Analysis
Multivariable logistic regression models were estimated for risk-adjusted 90-day (and 30-day) mortality across the deciles of percent African-American in each hospital. In each model reported in the paper, standard-errors are clustered at the hospital level and all statistical analysis was performed in STATA v8.0.41 We wish to facilitate exposition and to avoid misinterpreting odds-ratios as relative-risks when the underlying event is not rare,42 and so report expected probabilities rather than odds-ratios. We use the ADJUST command in STATA which sets all covariates to their mean values, and then “turns on” each of the decile categorical variables in turn. For each decile, an estimate and confidence interval is calculated in log-odds ratios; these are then converted into probability units. This is the expected mortality rate (and confidence interval) for a representative patient, one with average risk characteristics.
Potential Explanations for Differences in Risk-Adjusted Mortality Outcome Measures
We examine the role of six observable factors that might explain differences in hospital-level mortality outcomes. The first is that the different racial composition of the deciles could lead to unmeasured confounding if African-American patients exhibited higher rates of mortality even after adjusting for risk factors. We address this hypothesis in two ways. The first is to estimate the logistic model with race-decile interaction terms, which allows for two separate mortality gradients, one for black and the other for white AMI patients. The disadvantage of this approach is that there are very few African-American AMI patients in the lower deciles, with a corresponding deficiency in statistical power. We therefore combined deciles 2–6 into one group comprising 11.4 percent of black patients and 39.8 percent of white patients. The second approach is to estimate a logistic model separately for black and white patients, but with a single variable, the percentage of black AMI patients in the hospital (a hospital-level variable), to test for a linear race-specific gradient in the logistic regression.
Second, hospitals admitting African-American AMI patients have been shown to be less likely to perform surgical interventions.3–19,43 To capture these effects, hospital-specific rates of coronary artery bypass grafts (CABG) and percutaneous coronary interventions (PCI) in the sample are included in one specification of the regression. Third, hospitals may differ with respect to average volume of treatment.30,44–46 The hospital-specific AMI volume is therefore included as an additional explanatory variable. Fourth, the ownership status of hospitals could confound racial effects if African-American patients are more likely to be admitted to government hospitals. We therefore use indicator variables to adjust for the teaching and ownership status of the hospital (government (non-federal), not-for-profit, for-profit). As noted by others, these variables are markers for multiple competing factors,47 and should not be interpreted as measuring the effect of ownership, volume, or treatment intensity per se.
Fifth, there may be systematic differences in income levels across regions, and so we adjust by median household income by zip code from the 2000 Census. Finally, we adjust for location of residence using the four U.S. Census regions and whether the individual lives in an urban area. We note that adjusting for geography can lead to underestimates (or overestimates) of true racial disparities.27 If a large fraction of African-Americans live in the South, then adjusting for Southern residence automatically removes one factor – average mortality differences between Southern and Non-Southern hospitals – that can explain overall racial disparities in outcomes.
Unmeasured confounding factors could bias estimates. Specifically, if patients seen in hospitals that disproportionately treated African-Americans experienced a higher prevalence of comorbidities not observed in the data, we would spuriously attribute elevated mortality rates to such hospitals. To examine this hypothesis we constructed an index of disease severity, as proxied by observed comorbidities and the location of the infarct. This index is estimated using a logistic regression that predicts 90-day mortality, and includes race, age, sex, all measured comorbidities, and the severity of the AMI. Differences in the severity of the comorbidities and location of the AMI generate variation in the index of predicted mortality, and are presented by hospital racial decile in terms of predicted 90-day mortality. When there are no differences across hospital deciles in the comorbidity index, the role for unmeasured confounding variables to bias estimates is circumscribed. The reason is that confounding variables tend to be correlated with one another; smokers for example (an unmeasured variable) tend to have lower incomes and are more likely to present with COPD or cancer (measured variables). Finding that the measured variables are unassociated with hospital deciles therefore reduces the likelihood that the unmeasured (correlated) covariates are positively associated with hospital deciles.48
Results
Table 1 presents summary statistics for fee-for-service Medicare beneficiaries who were treated for AMI between January 1st 1997 and September 30th 2001. The table illustrates the construction of the deciles used in the analysis. The average Medicare AMI patient was treated in a hospital where 6.9 percent of the patients were African-American. The bottom “decile” accounted for 12.5 percent of the population who were admitted to 1369 hospitals (comprising 32 percent of all hospitals) that saw no African-American AMI patients over the duration of the study period. These hospitals constitute Decile 1 (the lowest decile) of percent African-American patients in the hospital. On the other end of the spectrum, 33.6 percent of patients in Decile 10 hospitals were African-American. Patients admitted to hospitals with the highest fraction of African-American patients were more likely to live in the South, and less likely to live in an urban setting. Differences across the deciles are significant statistically (p < .001).
Table 1.
Characteristics of AMI Patients and Hospitals, by the Average Percentage of African-American AMI Patients in the Admitting Hospital
| Deciles of Percent African American in Hospital | Average Percentage of patients who are African American | Range (Min-Max) | Number of Hospitals | Percent of Patients in an Urban Area | Percent of Patients in the South | Number of Patients |
|---|---|---|---|---|---|---|
| Lowest Decile | 0.0% | 0-0% | 1,369 | 24.0% | 12.5% | 142,666 |
| 2 | 0.3% | 0.0–0.4% | 162 | 40.1% | 9.7% | 84,971 |
| 3 | 0.7% | 0.4–0.9% | 276 | 47.8% | 21.3% | 113,853 |
| 4 | 1.4% | 0.9–1.8% | 342 | 43.9% | 29.8% | 113,526 |
| 5 | 2.2% | 1.8–2.8% | 287 | 48.4% | 36.9% | 113,769 |
| 6 | 3.4% | 2.8–4.2% | 291 | 43.9% | 38.3% | 112,265 |
| 7 | 5.2% | 4.2–6.4% | 326 | 41.2% | 39.9% | 114,056 |
| 8 | 8.2% | 6.4–10.4% | 337 | 43.6% | 51.2% | 114,348 |
| 9 | 14.2% | 10.4–19.4% | 358 | 43.8% | 71.6% | 114,686 |
| Highest Decile | 33.6% | 19.5–98.6% | 541 | 35.2% | 68.9% | 115,596 |
| Total | 6.9 % | 0.0–98.6% | 4,289 | 40.8% | 38.0% | 1,136,736 |
Notes: AMI index events from January 1, 1997 to September 30, 2001 for beneficiaries enrolled in fee-for-service Medicare. In calculating averages, each hospital is weighted by the number of patients treated over the study period. Urban areas are defined as counties which are classified being part of a Metropolitan Statistical Area (MSA). States are classified as being in the South or West using United States Census Bureau definitions for these regions. All differences across deciles are jointly significant at the p < .001 level.
There was large variation in ownership status and treatment intensity between hospitals based on the extent to which they treat the African-American population (Table 2). Relative to the hospital that the average AMI patient was treated at, hospitals that disproportionately treat African-Americans are more likely to be teaching hospitals, more likely to be government (non-federal), and less likely to be not for profit. These hospitals are similar in terms of CABG and PTCA intensity, but have lower AMI volume. All differences across the deciles are highly significant statistically (p < .001).
Table 2.
Hospital Ownership Characteristics and Hospital Treatment Characteristics, by the Average Percentage of African-American AMI Patients in the Admitting Hospital
| Hospital Teaching and Ownership Status | Hospital Treatment Characteristics | ||||||
|---|---|---|---|---|---|---|---|
| Percent African American in Hospital (Deciles) | Teaching
|
Govt. Non-Federal
|
Not for Profit
|
For Profit
|
Average CABG Rate after AMI
|
Average PTCA Rate after AMI
|
Annual AMI Volume
|
| Lowest | 3% | 18% | 76% | 7% | 9% | 16% | 48 |
| 2 | 7% | 6% | 91% | 3% | 12% | 25% | 143 |
| 3 | 8% | 7% | 77% | 15% | 12% | 22% | 139 |
| 4 | 8% | 9% | 82% | 9% | 11% | 21% | 117 |
| 5 | 16% | 8% | 81% | 11% | 13% | 23% | 154 |
| 6 | 15% | 7% | 83% | 11% | 13% | 24% | 154 |
| 7 | 23% | 8% | 80% | 13% | 12% | 23% | 152 |
| 8 | 28% | 14% | 74% | 12% | 12% | 23% | 143 |
| 9 | 20% | 17% | 70% | 13% | 12% | 23% | 129 |
| Highest | 30% | 17% | 70% | 13% | 11% | 20% | 107 |
| Average | 16% | 11% | 78% | 11% | 12% | 22% | 126 |
Source: AMI index events from January 1, 1997 to September 30, 2001 for beneficiaries enrolled in fee-for-service Medicare. Each hospital is weighted by the number of patients treated over the study period. Annual AMI Volume is the average number of patients aged 65 and over in the Medicare program admitted to the hospital for AMI. All differences across deciles are jointly significant at the p < .001 level
With the exception of hospitals that treat no African-Americans, the distribution of comorbidities and severity of the AMI across hospitals (adjusted for age, race and sex) is similar (Figure 1). In decile 2 hospitals (where only 0.3 percent of patients were African-American), the index of predicted 90-day mortality based solely on comorbidities and severity of the AMI was 22.2 percent (95% CI: 22.1–22.3%). It was 22.1 percent (95% CI: 22.0–22.2%) and 22.0 (95% CI: 21.9–22.1%) for hospitals in deciles 9 and 10 respectively. The noticeable exception to the similarity in comorbidities across deciles of percent African-American is seen for patients in Decile 1 hospitals. These patients, all of whom are white, have predicted 90-day mortality of 23.7% (95% CI: 23.6–23.8%), 7 percent higher than the expected mortality in the other deciles. While not reported in the table, the elevated mortality in decile 1 is attributable largely to the elevated prevalence of renal failure (2.9 percent in this decile compared to 2.2 percent for other deciles; p<0.001), and a lower likelihood of being diagnosed with a subendocardical infarction (39.0 versus 49.0 percent for other deciles; p<0.001).
Figure 1. Index of Comorbidity and AMI Severity by the Average Percentage of African-American AMI Patients Admitted to the Hospital.
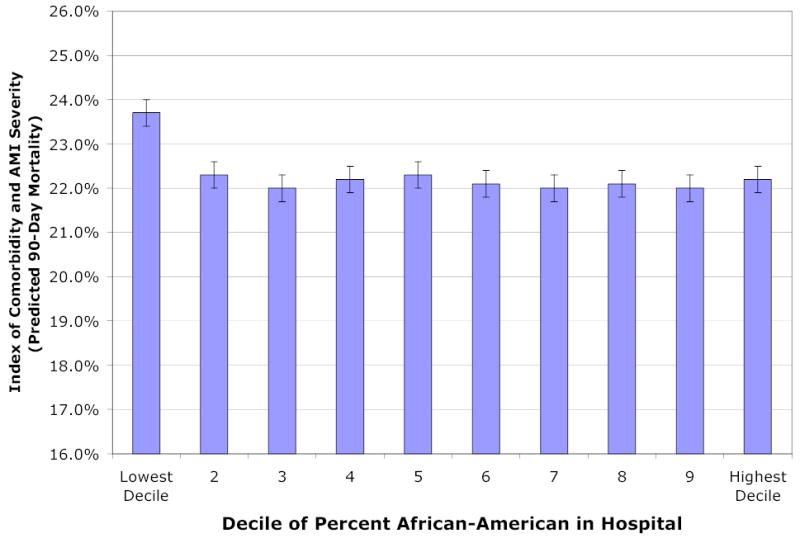
Legend: The graph reports the average index of Comorbidity and AMI Severity by hospital decile according to the average percentage of African-American AMI patients admitted to that hospital. Multiple indicators for severity are used: the presence of vascular disease, pulmonary disease, dementia, diabetes, renal failure, cancer, and the location of the infarct, anterior, inferior, sub-endocardial, or other. These indicators are combined into one index using as weights the coefficients from a prediction model for 90-day mortality. Thus the index predicts 90-day mortality based on comorbidities and severity of the AMI, after adjusting for age, gender, and race. This index is intended to test the hypothesis that AMI patients are sicker in hospitals that disproportionately admit African-Americans. The graph indicates that this hypothesis is rejected; indeed those patients admitted to the lowest decile (no African-American admissions) experience elevated risk factors.
Figure 2 illustrates risk-adjusted 90-day mortality across hospital deciles. Hospitals that have a greater share of African-American AMI patients have substantially higher risk-adjusted mortality. Even though patients in decile 1 hospitals are the sickest (as measured by the index of comorbidities) they experience the lowest risk-adjusted mortality following AMI. Figure 2 presents results from two models. In the first, outcomes are adjusted for age, race, sex and comorbidities. In the second, we further adjusted for income, hospital ownership, region, and treatment characteristics. The two models yield similar results suggesting that the hospital characteristics and income are not significant explanatory variables once comorbidities have been adjusted for. The area under the Receiver Operator Curve (ROC) is 0.679 for the first model, and 0.681 for the second.
Figure 2. Risk-Adjusted 90-Day Mortality after AMI, by the Average Percentage of African-American AMI Patients Admitted to the Hospital.
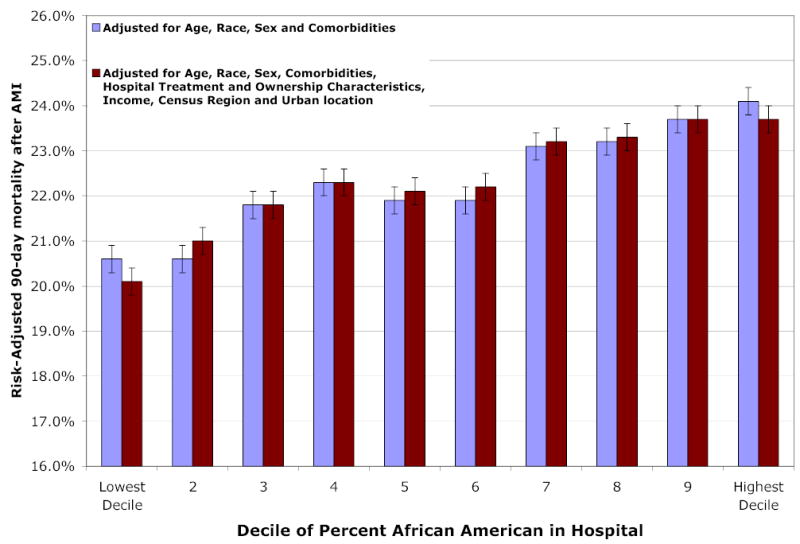
Legend: The graph reports 90 day mortality after adjusting for age, gender, race, comorbidities, and location of the infarct (anterior, inferior, sub-endocardial, other). Comorbidities include presence of vascular disease, pulmonary disease, dementia, diabetes, renal failure, and cancer. Hospital ownership and treatment characteristics are listed in Table 2, and include teaching hospital, government non-federal ownership, non-government not for profit, investor owned (for profit), hospital PTCA and CABG rates and annual AMI volume. Income refers to beneficiary’s zip-code income. Region refers to the 4 Census regions. A joint test of the importance of hospitals deciles is significant at the p < .001 level.
Figure 3 presents estimated adjusted mortality separately by race. Because of the small number of African-American AMI patients in deciles 2–6, these deciles were combined to improve statistical power in estimating race-specific adjusted mortality. Estimated mortality for African-Americans in decile 10 hospitals are significantly higher than for decile 2–6 hospitals (p = .04). The difference between black and white adjusted mortality rates is not significant within each hospital decile, but a joint test of significance rejects the null hypothesis of equality (p < 0.001). For the logistic regressions estimated separately for white and black AMI patients, the mortality gradient (by fraction of African-American admissions to the hospital) was significant for both white (p < .001) and black (p = .007) patients.
Figure 3. Risk-Adjusted 90-Day Mortality after AMI, by Race and Average Percentage of African-American AMI Patients Admitted to the Hospital.
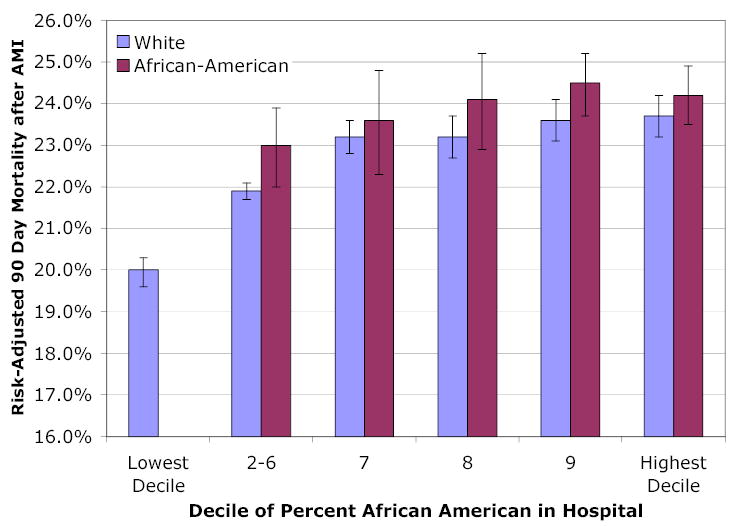
Legend: This regression includes all covariates described in the Legend for Figure 2, but with black and white hospital decile effects allowed to differ. To improve statistical power, Deciles 2–6, which together comprise 11 percent of the black AMI sample, are combined.
We obtained similar results regarding the association between hospital deciles and mortality using 30-day mortality. In these models, which also adjusted age, race, gender, comorbidities, hospital teaching status, region, ownership and treatment intensity, 30-day mortality in Decile 1 hospitals is 14.9% (95% CI: 14.6–15.2%), in Decile 2 15.6% (95% CI: 15.2–16.1%), and in Decile 10 17.6% (95% CI: 17.2–18.0%). Using these estimates, hospitals in decile 10 experienced 18 percent higher mortality relative to decile 1 hospitals. However, 30-day mortality within hospitals was not significantly higher among African-American patients.
Any potential burdens of higher mortality risk in hospitals that serve African-Americans is borne disproportionately by African-American patients, since a large fraction of this population is seen in the hospitals that comprise decile 9 and decile 10 hospitals. As Figure 4 shows, nearly half of African-Americans are seen in decile 10 hospitals, those with among the highest risk-adjusted mortality. Sixty-nine percent of African-American patients are seen in the 21 percent of hospitals that constitute decile 9 and 10 hospitals.
Figure 4. Distribution of African-American and White Patients, by the Average Percentage of African-American AMI Patients Admitted to the Hospital.
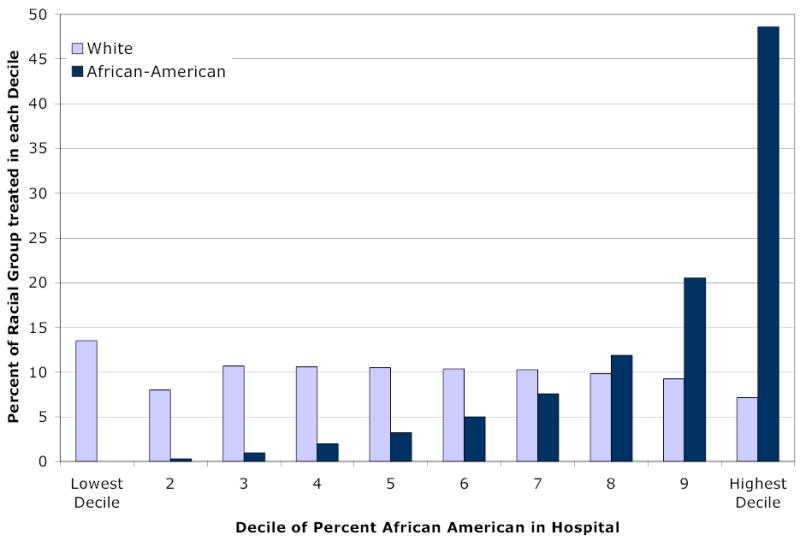
Legend: The graph reports the share of each racial group (relative to all African-American or white AMI patients in the Medicare fee-for-service population) treated in hospitals within each decile category. A joint test of the importance of hospitals deciles is significant at the p < .001 level.
Discussion
Risk-adjusted mortality following AMI is significantly higher in hospitals that disproportionately serve African-Americans, and this result holds even after adjusting for a variety of potential confounding factors. Our results may appear inconsistent with those in Kahn et al., who found African-Americans with a variety of clinical conditions were more likely to be admitted to higher-quality urban teaching hospitals.49 However, their study used a different time period (1981–86), and their sample was limited to 5 states. More recently, several studies have noted that African-American patients are treated by physicians with less clinical training,28 referred to lower quality cardiac surgeons,50 and treated at hospitals with higher risk-adjusted surgical mortality.30 Other studies have also found a negative association between the fraction African-American admitted to the hospital and the use of emerging medical technologies33 and favorable birth outcomes.51,52
Within hospitals, 90-day mortality rates for African-Americans were somewhat higher than for whites. These results contrast with most studies using data from earlier periods that have not generally found elevated mortality risks among African-American AMI patients.6,12,13,20,49,53,54 More recent studies, however, have found higher rates of mortality and functional disability among African-American AMI patients.18,19
The most important limitation of this paper is the possibility that unobservable health status of AMI patients in neighborhoods served by hospitals with a disproportionate number of African-American AMI admissions is systematically different from the average. If so, the higher mortality rates observed in these hospitals could be the result of unmeasured confounding factors, rather than hospital performance per se. One obvious difference across hospital deciles is simply that there are more African-American patients in the higher deciles, and if they are systematically sicker –conditional on covariates – then the estimates could be biased. However, even if outcomes are measured using white mortality rates or African-American mortality rates separately a significant mortality gradient is obtained.
Another limitation arises if risk adjustment does not adjust adequately for underlying illness. If the categorical comorbidity variables do not measure the severity of the disease, for example if diabetes is more severe among African-American AMI patients in decile 2 hospitals than among African-American AMI patients in decile 10 hospitals, then the results could be biased. It could also be the case that unmeasured confounding factors play a role in the elevated rates of mortality in the high-decile hospitals, for example smoking or exercise behavior. But the role for unmeasured confounding factors is constrained. For unmeasured confounding to bias the results, they would need to be unassociated with the measured confounders, which, as shown in Figure 1, explain none of the observed mortality gradient.
As well, hospitals serving a disproportionate number of African-American AMI patients tend to be located in low income neighborhoods, so the race variable could reflect socioeconomic status. A parallel analysis (not reported) assigning hospitals to deciles of zip code income (rather than deciles of the proportion of African-American AMI patients) failed to show any consistent patterns: risk-adjusted mortality in decile 10 (high income) hospitals was not significantly different from risk-adjusted mortality in decile 1 (low income) hospitals. Similarly, accounting for the broad region of residence or urban status did not alter our results.
Why is risk-adjusted mortality – for both African-Americans and whites -- associated with a higher fraction of African-American hospital admissions? One hypothesis is that African-American AMI patients are more likely to be admitted to hospitals with lower volume, and lower volume has predicted worse outcomes in other studies.44–46 Similarly, it could be the case that overall revascularization rates in hospitals with a large fraction of African-American patients could be lower, as suggested by previous research documenting racial gaps in surgical treatment of cardiovascular disease.3–19,43 These explanations alone cannot explain the gradient, since the regression analysis adjusts for such factors. A plausible explanation is differences in hospital-level quality not adequately adjusted for in our analysis but highlighted in recent studies, such as time to reperfusion, the prescription of beta-blockers, post-surgical mortality, or the quality of physicians, could explain observed differences in outcomes.28,30–32,55,56
Another limitation of the study is that it does not address racial disparities that take place within the hospital because of differences in the use of effective treatments or lack of communication between African-American patients and a largely white clinical staff. For example, Barnato et. al. documented substantially lower rates of PTCA and CABG for African-American AMI patients even after adjusting for the hospital to which they were admitted.32 Statistical analysis that distinguishes between these two explanations – disparities within hospitals, and disparities occurring because blacks go to different hospitals than whites – are therefore critical for future research on disparities.57
The potential benefits that come from increasing quality of care are well understood.58–61 One implication of this study is that reducing mortality rates in high-mortality hospitals can have implications for reducing racial disparities in health outcomes. Because 21 percent of hospitals treat 69 percent of elderly African-American AMI patients, targeting quality improvements at hospitals that disproportionately serve African-Americans could dramatically reduce black-white disparities in care. As well, since many African-American Medicare beneficiaries live in urban areas with more than one hospital, efforts to better direct patients towards high quality hospitals may also be an effective means of reducing disparities.
Acknowledgments
This research was funded through the National Institute on Aging Grant NIA PO1 AG19783 and the Robert Wood Johnson Foundation. The assistance of Weiping Zhou and Debra Reeves is gratefully acknowledged. Dr. Lee’s contributions were completed while she was a Research Analyst at the National Bureau of Economic Research. The views expressed in the paper are those of the authors and should not be interpreted as those of the Congressional Budget Office.
Footnotes
Conflict of Interest Disclosures
None.
References
- 1.Smedley B, Stith A, Nelson A, editors. Unequal Treatment: Contronting Racial and Ethnic Disparities in Health Care. Washington DC: Institute of Medicine; 2002. [PubMed] [Google Scholar]
- 2.Borzak S, Joseph C, Havstad S, Tilley B, Smith ST, Housholder SD, Gheorghiade M. Lower thrombolytic use for African Americans with myocardial infarction: an influence of clinical presentation? American Heart Journal. 1999;137:338–45. doi: 10.1053/hj.1999.v137.92523. [DOI] [PubMed] [Google Scholar]
- 3.Ayanian J, Udvarhelyi I, Gatsonis C, Pashos C, Epstein A. Racial Differences in the Use of Revascularization Procedures After Coronary Angiography. Journal of the American Medical Association. 1993;269:2642–6. [PubMed] [Google Scholar]
- 4.Allison JJ, Kiefe CI, Centor RM, Box JB, Farmer RM. Racial differences in the medical treatment of elderly Medicare patients with acute myocardial infarction. Journal of General Internal Medicine. 1996;11:736–43. doi: 10.1007/BF02598987. [DOI] [PubMed] [Google Scholar]
- 5.Carlisle D, Leake B, Shapiro M. Racial and Ethnic Disparities in the Use of Cardiovascular Procedures: Associations with Types of Health Insurance. American Journal of Public Health. 1997;87:263–267. doi: 10.2105/ajph.87.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Rathore S, Radford M, Wang Y, Krumholz H. Racial differences in the use of cardiac catheterization after acute myocardial infarction. New England Journal of Medicine. 2001;344:1443–1449. doi: 10.1056/NEJM200105103441906. [DOI] [PubMed] [Google Scholar]
- 7.Conigliaro J, Whittle J, Good CB, Hanusa BH, Passman LJ, Lofgren RP, Allman R, Ubel PA, O'Connor M, Macpherson DS. Understanding racial variation in the use of coronary revascularization procedures: the role of clinical factors. Archives of Internal Medicine. 2000;160:1329–35. doi: 10.1001/archinte.160.9.1329. [DOI] [PubMed] [Google Scholar]
- 8.Einbinder LC, Schulman KA. The effect of race on the referral process for invasive cardiac procedures. Medical Care Research & Review. 2000;57:162–80. doi: 10.1177/1077558700057001S08. [DOI] [PubMed] [Google Scholar]
- 9.Ford E, Newman J, Deosaransingh K. Racial and ethnic differences in the use of cardiovascular procedures: findings from the California Cooperative Cardiovascular Project. American Journal of Public Health. 2000;90:1128–34. doi: 10.2105/ajph.90.7.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillum RF, Gillum BS, Francis CK. Coronary revascularization and cardiac catheterization in the United States: trends in racial differences. Journal of the American College of Cardiology. 1997;29:1557–62. doi: 10.1016/s0735-1097(97)00089-2. [DOI] [PubMed] [Google Scholar]
- 11.Kressin NR, Peterson LA. Racial Differences in the Use of Invasive Cardiovascular Procedures: Review of the Literature and Prescription for Future Research. Annals of Internal Medicine. 2001;135:352–366. doi: 10.7326/0003-4819-135-5-200109040-00012. [DOI] [PubMed] [Google Scholar]
- 12.Petersen L, Sright S, Peterson E, Daley J. Impact of race on cardiac care and outcomes in veterans with acute myocardial infarction. Medical Care. 2002;40:I86–I96. doi: 10.1097/00005650-200201001-00010. [DOI] [PubMed] [Google Scholar]
- 13.Peterson E, Shaw L, DeLong E, Pryor D, Califf R, Mark D. Racial Variation in the Use of Coronary-Revascularization Procedures: Are the Differences Real? Do they Matter? NEJM. 1997;336:480–486. doi: 10.1056/NEJM199702133360706. [DOI] [PubMed] [Google Scholar]
- 14.Schneider E, Leape L, Weissman J, Piana R, Gatsonis C, Epstein A. Racial Differences in Cardiac Revascularization Rates: Does "Overuse" Explain Higher Rates Among White Patients? Annals of Internal Medicine. 2001;135:328–337. doi: 10.7326/0003-4819-135-5-200109040-00009. [DOI] [PubMed] [Google Scholar]
- 15.Schulman K, Berlin J, Harless W, Kerner J, Sistrunk S, Gersh B, Dube R, Taleghani C, Burke J, Williams S, Eisenberg J, Escarce J, Ayers W. The Effect of Race and Sex on Physicians' Recommendations for Cardiac Catheterization. NEJM. 1999;340:618–626. doi: 10.1056/NEJM199902253400806. [DOI] [PubMed] [Google Scholar]
- 16.Whittle J, Conigliaro J, Good C, Lofgren R. Racial Differences in the Use of Invasive Cardiovascular Procedures in the Department of Veterans Affairs Medical System. NEJM. 1993;329:621–627. doi: 10.1056/NEJM199308263290907. [DOI] [PubMed] [Google Scholar]
- 17.Epstein A, Weissman J, Schneider E, Gatsonis C, Leape L, Piana R. Race and gender disparities in rates of cardiac revascularization: do they reflect appropriate use of procedures or problems in the quality of care? Medical Care. 2003;41:1240–55. doi: 10.1097/01.MLR.0000093423.38746.8C. [DOI] [PubMed] [Google Scholar]
- 18.Sabatine M, Blake G, Drazner M, Morrow D, Scirica B, Murphy S, McCabe C, Weintraub W, Gibson C, Cannon C. Influence of race on death and ischemic complications in patients with non-ST-elevation acute coronary syndromes despite modern, protocol-guided treatment. Circulation. 2005;111:1217–1224. doi: 10.1161/01.CIR.0000157733.50479.B9. [DOI] [PubMed] [Google Scholar]
- 19.Kaul P, Lytle B, Spertus J, DeLong E, Peterson E. Influence of racial disparities in procedure use on functional status outcomes among patients with coronary artery disease. Circulation. 2005;111:1284–1290. doi: 10.1161/01.CIR.0000157731.66268.E1. [DOI] [PubMed] [Google Scholar]
- 20.Gordon HS, Harper DL, Rosenthal GE. Racial variation in predicted and observed inhospital death. A regional analysis. Jama. 1996;276:1639–44. [PubMed] [Google Scholar]
- 21.Gornick M, Eggers P, Reilley T, Mentnech R, Fitterman L, Kucken L, Vladeck B. Effects of Race and Income on Mortality and Use of Services Among Medicare Beneficiaries. NEJM. 1996;335:791–799. doi: 10.1056/NEJM199609123351106. [DOI] [PubMed] [Google Scholar]
- 22.Rathore SS, Berger AK, Weinfurt KP, Feinleib M, Oetgen WJ, Gersh BJ, Schulman KA. Race, sex, poverty, and the medical treatment of acute myocardial infarction in the elderly. Circulation. 2000;102:642–8. doi: 10.1161/01.cir.102.6.642. [DOI] [PubMed] [Google Scholar]
- 23.Sedlis SP, Fisher VJ, Tice D, Esposito R, Madmon L, Steinberg EH. Racial differences in performance of invasive cardiac procedures in a Department of Veterans Affairs Medical Center. Journal of Clinical Epidemiology. 1997;50:899–901. doi: 10.1016/s0895-4356(97)00089-9. [DOI] [PubMed] [Google Scholar]
- 24.Schneider E, Zaslavsky A, Epstein A. Racial disparities in the quality of care for enrollees in Medicare managed care. JAMA. 2002;287:1288–1294. doi: 10.1001/jama.287.10.1288. [DOI] [PubMed] [Google Scholar]
- 25.Epstein A. Health care in America -- Still too separate, not yet equal. New England Journal of Medicine. 2004;351:603–605. doi: 10.1056/NEJMe048181. [DOI] [PubMed] [Google Scholar]
- 26.Baicker K, Chandra A, Skinner JS, Wennberg JE. Who You Are And Where You Live: How Race And Geography Affect The Treatment Of Medicare Beneficiaries. Health Aff (Millwood) 2004 doi: 10.1377/hlthaff.var.33. [DOI] [PubMed] [Google Scholar]
- 27.Chandra A, Skinner J. Geography and Health Disparities. In: Anderson NB, Bulatao R, Cohen B, editors. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington D.C.: National Research Council of the National Academies; 2004. [PubMed] [Google Scholar]
- 28.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575–84. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 29.Rothenberg B, Pearson T, Zwanziger J, Mukamel D. Explaining Disparities in Access to High-Quality Cardiac Surgeons. Annals of Thoracic Surgery. 2004;78:18–24. doi: 10.1016/j.athoracsur.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Konety S, Zarrazin M, Rosenthal G. Patient and hospital differences underlying racial variations in outcomes after coronary artery bypass graft surgery. Circulation. 2005;111:1210–1216. doi: 10.1161/01.CIR.0000157728.49918.9F. [DOI] [PubMed] [Google Scholar]
- 31.Bradley EH, Herrin J, Wang Y, McNamara RL, Webster TR, Magid DJ, Blaney M, Peterson ED, Canto JG, Pollack CV, Jr, Krumholz HM. Racial and ethnic differences in time to acute reperfusion therapy for patients hospitalized with myocardial infarction. Jama. 2004;292:1563–72. doi: 10.1001/jama.292.13.1563. [DOI] [PubMed] [Google Scholar]
- 32.Barnato A, Lucas F, Staiger D, Wennberg D, Chandra A. Hospital-Level Racial Disparities in Acute Myocardial Infarction Treatment and Outcomes. Medical Care. 2005;43:308–319. doi: 10.1097/01.mlr.0000156848.62086.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groeneveld P, Laufer S, Garber A. Technology Diffusion, Hospital Variation, and Racial Disparities Among Elderly Medicare Beneficiaries 1989–2000. Medical Care. 2005;43:320–329. doi: 10.1097/01.mlr.0000156849.15166.ec. [DOI] [PubMed] [Google Scholar]
- 34.Arday S, Arday D, Monroe S, Zhang J. HCFA's Racial and Ethnic Data: Current Accuracy and Recent Improvements. Health Care Financing Review. 2000;21:107–116. [PMC free article] [PubMed] [Google Scholar]
- 35.McClellan M, McNeil B, Newhouse J. Does More Intensive Treatment of Acute Myocardial Infarction Reduce Mortality? JAMA. 1994;272:859–866. [PubMed] [Google Scholar]
- 36.McClellan M, Noguchi H. Technological Change in Heart-Disease Treatment: Does High Tech Mean Low Value? American Economic Review. 1998;88:90–96. [Google Scholar]
- 37.Kessler D, McClellan M. Is Hospital Competition Socially Wasteful? Quarterly Journal of Economics. 2000;115:577–615. [Google Scholar]
- 38.Skinner J, Weinstein J, Sporer S, Wennberg J. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. New England Journal of Medicine. 2003;349:1350–1359. doi: 10.1056/NEJMsa021569. [DOI] [PubMed] [Google Scholar]
- 39.Park R, Brook R, Kosecoff J, Keesey J, Rubenstein L, Keeler E, Kahn K, Rogers W, Chassin M. Explaining variations in hospital death rates. JAMA. 1990;264:484–490. [PubMed] [Google Scholar]
- 40.AHRQ. Refinement of the HCUP Quality Indicators. Rockville, MD: Agency for Healthcare Research and Quality; 2001. [PubMed] [Google Scholar]
- 41.StataCorp. Stata Statistical Software: Release 8.0. College Station, TX: Stata Corporation; 2003. [Google Scholar]
- 42.Schwartz LM, Woloshin S, Welch HG. Misunderstandings about the effects of race and sex on physicians' referrals for cardiac catheterization. N Engl J Med. 1999;341:279–83. doi: 10.1056/NEJM199907223410411. discussion 286–7. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz L, Wolosin S, Welch H. Misunderstandings About the Effects of Race and Sex on Physician's Referrals for Cardiac Catheterization. NEJM. 1999;341:279–283. doi: 10.1056/NEJM199907223410411. [DOI] [PubMed] [Google Scholar]
- 44.Flood A, Scott W. Hospital Structure and Performance. Baltimore: Johns Hopkins Press; 1987. [Google Scholar]
- 45.Hannon E, O'Donnell J, Kilburn H, Bernard H, Yaziei A. Investigation of the relationship between volume and mortality for surgical procedures performed in New York State hospitals. JAMA. 1989;262:503–510. [PubMed] [Google Scholar]
- 46.Birkmeyer J, Siewers A, Finlayson E, Stukel T, Lucas F, Batista I, Welch H, Wennberg D. Hospital volume and surgical mortality in the United States. New England Journal of Medicine. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 47.Alter DA, Naylor CD, Austin PC, Tu JV. Long-term MI outcomes at hospitals with or without on-site revascularization. Jama. 2001;285:2101–8. doi: 10.1001/jama.285.16.2101. [DOI] [PubMed] [Google Scholar]
- 48.Altonji J, Elder T, Taber C. Selection on observed and unobserved variables: Assessing the effectiveness of Catholic schools. Journal of Political Economy. 2005 [Google Scholar]
- 49.Kahn K, Pearson M, Harrison L, Desmond K, Rogers W, Rubenstein L, Brook R, Keeler E. Health care for Black and poor hospitalized Medicare patients. JAMA. 1994;271:1169–1174. [PubMed] [Google Scholar]
- 50.Mukamel D, Murthy A, Weimer D. Racial differences in access to high-quality cardiac surgeons. American Journal of Public Health. 2000;90:1174–1177. doi: 10.2105/ajph.90.11.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morales L, Staiger D, Horbar J, Carpenter J, Kenny M, Geppert J, Rogowski J. Mortality Among Very Low Birthweight Infants in Hospitals Serving Minority Populations. American Journal of Public Health. 2005 doi: 10.2105/AJPH.2004.046730. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aizer A, Lleras-Muney A, Stabile M. Working Papers. Cambridge, MA: National Bureau of Economic Research; 2004. Access to care, provider choice, and racial disparities. [Google Scholar]
- 53.Marks D, Mensah G, Kennard E, Detre K, Holmes D. Race, baseline characteristics, and clinical outcomes after coronary intervention: The New Approaches in Coronary Interventions (NACI) registry. American Heart Journal. 2000;140:162–169. doi: 10.1067/mhj.2000.106645. [DOI] [PubMed] [Google Scholar]
- 54.Taylor H, Canto J, Sanderson B, Rogers W, Hilbe W. Management and outcomes for Black patients with Acute Myocardial Infarction in the Reperfusion Era. American Journal of Cardiology. 1998;82:1019–1023. doi: 10.1016/s0002-9149(98)00547-5. [DOI] [PubMed] [Google Scholar]
- 55.Baicker K, Chandra A. Medicare Spending, The Physician Workforce, And Beneficiaries' Quality Of Care. Health Aff (Millwood) 2004 doi: 10.1377/hlthaff.w4.184. [DOI] [PubMed] [Google Scholar]
- 56.Lucas F, Stukel T, Morris A, Siewers A, Birkmeyer J. Race and surgical mortality in the United States. Annals of Surgery. 2005 doi: 10.1097/01.sla.0000197560.92456.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zaslavsky AM, Ayanian JZ. Integrating Research on Racial and Ethnic Disparities in Health Care Over Place and Time. Medical Care. 2005;43:303–307. doi: 10.1097/01.mlr.0000159975.43573.8d. [DOI] [PubMed] [Google Scholar]
- 58.Chassen M, Galvin R. The urgent need to improve health care quality. JAMA. 1998;280:1000–1005. doi: 10.1001/jama.280.11.1000. [DOI] [PubMed] [Google Scholar]
- 59.Committee on Quality of Health Care in America IoM. Crossing the Quality Chasm: A New Heatlh System for the 21st Century. Washington DC: National Academy Press; 2001. [Google Scholar]
- 60.O'Connor G, Plume S, Olmstead E, Morton J, Maloney C, Nugent W, Hernandez F, Jr, Clough R, Leavitt B, Coffin L, Marrin C, Wennberg D, Birkmeyer J, Charlesworth D, Malenka D, Quinton H, Kasper J. A regional intervention to improve the hospital mortality associated with coronary artery bypass graft surgery. The Northern New England Cardiovascular Disease Study Group. JAMA. 1996;275:841–6. [PubMed] [Google Scholar]
- 61.Kiefe C, Allison J, Williams O, Person D, Weaver M, Weissman N. Improving quality improvement using achievable benchmarks for physician feedback: A randomized controlled trial. JAMA. 2001;285:2871–2879. doi: 10.1001/jama.285.22.2871. [DOI] [PubMed] [Google Scholar]


