Abstract
Vascular endothelial growth factor (VEGF) plays an important role in normal and pathological angiogenesis. VEGF receptors (VEGFRs, including VEGFR-1, VEGFR-2, and VEGFR-3) and neuropilins (NRPs, including NRP-1 and NRP-2) are high-affinity receptors for VEGF and are typically considered to be specific for endothelial cells. Here we showed expression of VEGFRs and NRPs on cultured epidermal keratinocytes at both mRNA and protein levels. We further localized these receptors by immunofluorescence (IF) staining in the epidermis of surgical skin specimens. We found positive staining for VEGFRs and NRPs in all layers of the epidermis except for the stratum corneum. VEGFR-1 and VEGFR-2 are primarily expressed on the cytoplasmic membrane of basal cells and the adjacent spinosum keratinocytes. All layers of the epidermis except for the horny cell layer demonstrated a uniform pattern of VEGFR-3, NRP-1, and NRP-2. Sections staining for NRP-1 and NRP-2 also showed diffuse intense fluorescence and were localized to the cell membrane and cytoplasm of keratinocytes. In another panel of experiments, keratinocytes were treated with different concentrations of VEGF, with or without VEGFR-2 neutralizing antibody in culture. VEGF enhanced the proliferation and migration of keratinocytes, and these effects were partially inhibited by pretreatment with VEGFR-2 neutralizing antibody. Adhesion of keratinocytes to type IV collagen–coated culture plates was decreased by VEGF treatment, but this reduction could be completely reversed by pretreatment with VEGFR-2 neutralizing antibody. Taken together, our results suggest that the expression of VEGFRs and NRPs on keratinocytes may constitute important regulators for its activity and may possibly be responsible for the autocrine signaling in the epidermis.
INTRODUCTION
Vascular endothelial growth factor (VEGF) is a family of growth factors, including VEGF-A, -B, -C, -D, and -E, and placental growth factor (PlGF) (1). VEGF has been studied extensively for its angiogenic behavior in physiological and pathological conditions. Recent evidence indicates that VEGF also has direct effects on neural cells (2). The biological effects of VEGF are mediated by 5 known receptors, VEGFR-1 (flt-1), VEGFR-2 (KDR/flk-1), VEGFR-3 (flt-4), neuropilin-1 (NRP-1), and neuropilin-2 (NRP-2) (3–7).
VEGFR-1 and VEGFR-2 are expressed predominantly on endothelial cells, but a few additional types of cells express one or both of these receptors (1,5,7). VEGFR-3 is initially expressed throughout the embryonic vasculature and is limited to lymphatic endothelial cells in later stages of development (8). VEGFRs belong to the receptor tyrosine kinase (RTK) family and have a characteristic structure with 7 Ig-like domains in the extracellular domain and a cytoplasmic tyrosine kinase domain with a long kinase insert region (1,5,6,9). VEGF also interacts with neuropilins (NRPs), a family of non–tyrosine kinase transmembrane receptors with a small cytoplasmic domain and multiple extracellular domains (1,5,10). NRPs play active roles in immunology, neuronal development, and angiogenesis (11). Precise control of NRP-1 and NRP-2 expression appears to be critical for normal vascular and neuronal development (10,11). Both VEGFR-1 and VEGFR-2 can form complexes with NRP-1 and -2 (12,13). NRP-2 can bind VEGF-C and coexpress with VEGFR-3, suggesting a possible association between these 2 receptors (14).
Until recently, VEGFRs were thought to be absent in epidermis. In normal human skin, VEGF is expressed and secreted by epidermal keratinocytes (15,16). VEGF is overexpressed in skin disorders such as psoriasis (17). It is possible that VEGF may have some autocrine effects on the behavior of epidermal cells. In this study, we systematically screened the expression of VEGF receptors and coreceptors in the epidermis. While this work was going on, Wilgus et al. (18) reported that VEGFR-1, but not VEGFR-2, was detected in murine keratinocytes during wound repair and in normal human epidermal keratinocytes. Based on other functional assays, they claimed a novel role of VEGF and VEGFR-1 interaction in keratinocytes during wound healing (18). Most recently, the expression of NRP-1 was also characterized in keratinocytes in vitro and in vivo (19). Both reports showed the absence of VEGFR-2 expression on keratinocytes (18,19). Kurschat and colleagues (19) also failed to detect VEGFR-1 and VEGFR-3 in a keratinocyte cell line, HaCaT cells, by RT-PCR. In our data, we found expression of all 5 known VEGFRs and NRPs. The discrepancies between our study and others were investigated in this study.
MATERIALS AND METHODS
Chemicals and Reagents
Dispase, trypsin, defined keratinocyte serum free medium (KSFM) supplemented with keratinocyte growth factor (KGF), human endothelial-SFM, fetal bovine serum (FBS), and Trizol were obtained from Gibco and Invitrogen. Monoclonal mouse anti-human VEGFR-1, VEGFR-2 (MAB3571), VEGFR-3, and NRP-2 were purchased from R&D Systems (Minneapolis, MN, USA); mouse anti-human NRP-1 and another mouse anti-human VEGFR-2 antibody (SC-6251) from Santa Cruz Biotechnology (Santa Cruz, CA, USA); mouse anti-human GAPDH from Acris Antibodies (Hiddenhausen, Germany); horseradish peroxidase–linked anti-mouse IgG from Jackson ImmunoResearch Laboratories (West Grove, PA, USA); polyclonal rabbit anti-mouse immunoglobulins/FITC from DakoCytomation (Denmark); VEGF165 from Chemicon International Inc. (Temecula, CA, USA); PVDF membrane, Whattman filter, and ECL plus reagent from Amersham Biosciences (Piscataway, NJ, USA); Moloney murine leukemia virus (MMLV) reverse transcriptase and RNase inhibitor from Fermantas (Amherst, NY, USA); cocktail protease inhibitors from Roche Diagnostics (Indianapolis, IN, USA); and poly-l-lysine, propidium iodide (PI), and mouse IgG from serum from Sigma-Aldrich (St. Louis, MO, USA). Primers were synthesized by Sangon (Shanghai, China), and purified PCR products were directly sequenced by the same company.
Human Subjects
Three healthy skin specimens, from 3 donors aged 19, 36, and 54 years, were obtained from cosmetic surgery with informed consent and handled aseptically. After removing subcutaneous elements, the specimens were cut into 2 parts. One half was snap-frozen and embedded in OCT compound (Miles, Naperville, IL, USA) and then stored in liquid nitrogen until processed. The other part of the specimen was put into 0.5% dispase (Gibco, Invitrogen, Auckland, USA) immediately.
Isolation and Culture of Epidermal Keratinocytes from Human Skin
Following overnight incubation at 4 °C in 0.5% dispase, the epidermis was peeled off from the dermis. The epidermis was further incubated in 0.25% trypsin for 10 min at 37 °C. Trypsin activity was neutralized by adding FBS. The keratinocyte suspension was filtered through nylon gauze (200 μm mesh) and cells were washed twice at 500g for 5 min prior to resuspension in defined serum-free keratinocyte growth medium supplemented with KGF. Cells were plated into 25-cm2 tissue culture dishes (Fluka, UK), maintained at 37 °C in a humidified atmosphere containing 5% CO2. Passages 2 to 5 were used in all experiments. HUVECs, expressing all 5 VEGF receptors (5) and serving as a positive control, were obtained from isolated umbilical veins by a standard method (20).
Histological Analyses
Coverslips were incubated in 50% H2SO4 overnight and then washed for 30 min in running tap water. The coverslips were incubated in 1 μg/mL poly-l-lysine for 10 min at room temperature and dried on filter paper. Normal skin tissue specimens embedded in OCT compound were processed into 4-μm sections using a Leica CM 1850 cryostat (Meyer Instruments, Houston, TX, USA) and placed on the surface of coverslips. After being fixed with 4% paraformaldehyde buffer for 20 min at room temperature, the coverslips were rinsed 3 times with PBS, placed into preheated sodium citrate buffer (10 mM, pH 8.5), and maintained at 95 °C for 20 min, then rinsed 3 times in PBS. The sections were permeabilized at room temperature with PBS containing 0.1% Triton-100 for 15 min and incubated with 10% normal rabbit serum for 1 h at room temperature to avoid non-specific binding. The sections were then incubated with 20 μg/mL primary monoclonal antibody diluted with 10% rabbit serum in PBS against each of the antibodies (VEGFR-1, VEGFR-2, VEGFR-3, NRP-1, and NRP-2) overnight at 4 °C. After 3 washes with 1% rabbit serum in PBS, the sections were incubated with FITC-conjugated rabbit anti-mouse secondary antibody that was diluted 1:40 with 10% rabbit serum in PBS for 2 h at room temperature in the dark. After 3 washes with 1% rabbit serum in PBS, the sections were counterstained with PI mounting medium to visualize the nuclei and pictured under fluorescence microscopy (Olympus, Japan). Negative controls that were stained with mouse IgG were included in all experiments. Sections from human umbilical vein were used as a positive control.
RT-PCR
Total RNA was isolated from 80% to 90% confluent keratinocytes in a 25-cm2 dish using 1 mL Trizol according to the manufacturer’s instructions and stored at −80 °C until use. After denaturation in DEPC-treated water for 10 min at 70 °C, 2 μg total RNA was reverse-transcribed into cDNA in a reaction volume of 20 μL, which contained 1× RT buffer (50 mM Tris-HCl, pH 8.3, 75 mM KCl, and 3 mM MgCl2), 20 units RNase inhibitor, 50 mM dNTPs, 200 units MMLV reverse transcriptase, and 0.5 μg oligo(dT)18 as primer. The reactions was incubated at 42 °C for 60 min and inactivated at 95 °C for 10 min and stored at −80 °C or used immediately. Each experiment included samples containing no reverse transcriptase (as negative controls) in the following RT-PCR, to exclude amplification from contaminated genomic DNA. Specific primers for VEGFR-1, VEGFR-2, and VEGFR-3, which were located at C-terminal of the kinase domain of each receptor, were designed by using PrimerSelect program in DNAstar package (DNAStar, Madison, WI, USA). Primers for NRP-1 and NRP-2 were located at N-terminal of both receptors and were selected by using the same program (Table 1). RT-PCR amplification was performed with a PTC 225 thermal cycler (MJ Research) in a volume of 50 μL, which included 1 μL of reaction cDNA mixture, 10 pmol each primer, 200 mM each dNTP, 2 mM MgCl2, 10 mM Tris-HCl, pH 8.3, 50 mM KCl, and 2 units of Taq DNA polymerase (Sangon, Shanghai, China). The amplification was composed of 1 cycle of 96 °C for 3 min, 35 cycles of 96 °C for 1 min, 57 °C (for VEGFR-1, VEGFR-2, and NRP-1) or 63 °C (for VEGFR-3 and NRP-2) for 70 s, and 72 °C for 80 s, and ending with a full extension cycle of 72 °C for 7 min. In each experiment, a negative control was included. To exclude potential contamination of endothelial cells from dermis in cultured keratinocytes, an endothelial-specific marker, CD31, was amplified using the same amplification conditions except for annealing at 55 °C (Table 1). The PCR products were then electrophoresed on 1.5% agarose gels, stained with ethidium bromide, and photographed on a UV transilluminator. Results were expressed for each sample as band intensity relative to that of GAPDH. Sequence identity of PCR product for each VEGFR and NRP was confirmed by direct sequencing.
Table 1.
PCR primers used in this study.
| Gene | GenBank accession no. | Primer sequence | Location | Product length (bp) |
|---|---|---|---|---|
| VEGFR-1 | NM_002019 | 5′-ATGGCTCCCGAATCTATCTTTGAC-3′ | 3445-3468 | 655 |
| 5′-GCCCCGACTCCTTACTTTTACTGG-3′ | 4099-4076 | |||
| VEGFR-2 | NM_002253 | 5′-CTGGCGGCACGAAATATCCTCTTA-3′ | 3388-3411 | 614 |
| 5′-GGCCGGCTCTTTCGCTTACTGTTC-3′ | 4001-3978 | |||
| VEGFR-3 | NM_182925 | 5′-AGGCCGGCCCACGCAGACATC-3′ | 2424-2444 | 346 |
| 5′-TGCACGCCCCGAGGAGGTTGA C-3′ | 2769-2748 | |||
| NRP-1 | NM_001024629 | 5′-AGCCCCTCCTCCTGTTGTGTCTTC-3′ | 461-484 | 441 |
| 5′-GCTATCGCGCTGTCGGTGTAAAAA-3′ | 901-878 | |||
| NRP-2 | NM_201264 | 5′-CAGCCGGCCCCCAGCACAT-3′ | 29-47 | 436 |
| 5′-TTCCCGAAAGCCAAGCGTAACAAT-3′ | 464-441 | |||
| CD31 | BC051822 | 5′-CTTCGCGGATGTCAGCACCAC-3′ | 384-404 | 419 |
| 5′-CCTCAACGGGGAATTCCAGTATCA-3′ | 802-779 | |||
| GAPDH | NM_002046 | 5′-TGAAGGTCGGAGTCAACGG-3′ | 113-131 | 223 |
| 5′-TGGAAGATGGTGATGGGAT-3′ | 335-317 |
Western Blot Analysis
Keratinocytes grown to 80% to 90% confluence were washed twice in ice-cold PBS, scraped, and centrifuged (1000g, 5 min at 4 °C). The pellet was incubated for 30 min in modified RIPA lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.2 mM EDTA, 1% Triton X-114, 50 mM Hepes, 50 mM sodium fluoride, 1 mM sodium orthovanadate) and 1 tablet/10 mL of protease inhibitor cocktail tablets then centrifuged (16,000g, 15 min at 4 °C). Protein concentrations were measured using the QuantiPro BCA assay kit (Sigma-Aldrich). Protein (100 μg) was boiled in 2× Laemmli buffer (0.125 M Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, and 10% β-mercaptoethanol, 1% bromophenol blue) for 10 min, and 40 μg of protein was loaded for each lane on 6% SDS polyacrylamide gels. Proteins were transferred to PVDF membrane (Hybond-P, Amersham), and blots were blocked for 30 min at room temperature in PBS containing 1% Tween-20. After rinsing 3 times for 10 min with PBS containing 0.05% Tween-20, the membrane was probed with the respective primary antibody (0.5 μg/mL) overnight at 4 °C in PBS containing 1% Tween-20. Blots were then washed 4 times (5 min each) in PBS containing 0.05% Tween-20 and incubated for 1 h with horseradish peroxidase–conjugated sheep polyclonal anti-mouse IgG antibody (1:5000; Jackson). The membrane was washed 5 times (5 min each) with 0.05% Tween-20-PBS, and the immunoreactive bands were detected using enhanced chemiluminescent (ECL) plus reagent kit before exposure for at least 3 min to Kodak film (Kodak, Rochester, NJ, USA). Mouse monoclonal anti-GAPDH (diluted 1:5000) was used as a loading control in the same panel. The results of densitometric scanning of immunoreactivity on each lane were corrected for GAPDH immunoreactivity. HUVECs were used as a positive control in all panels.
Effect of VEGF165 on Keratinocyte Proliferation
To determine if VEGF165 has effects on proliferation of keratinocytes, normal human epidermal keratinocytes were plated in 96-well plates at 5000 cells/well in defined KSFM and cultured for 24 h. Cells were then treated with various concentrations of VEGF165 at 0, 1, 5, 10, 25, 50, and 100 ng/mL in defined KSFM and incubated for 48 h. To test whether VEGFR-2 could mediate the enhanced proliferation in response to VEGF165 treatment, cells were treated with 5 μg/mL VEGFR-2 neutralizing antibody MAB3571 for 1 h and were seeded into wells with or without VEGF165 (10 ng/mL) for 48 h. At the end of the incubation, freshly prepared and filtered MTT (Sigma, St Louis, MO, USA) was added to a final concentration of 5 mg/mL in PBS and the mixture was further incubated for 3 h. After the medium was removed, cells in each well were dissolved with 100 μL DMSO, and optical density was read at 570 nm with a 96-well plate ELISA reader (Bio-Tek Instruments, Winooski, VT, USA). All MTT assays were conducted at least in triplicate.
Cell Migration Assays
Cell migration assays were performed as previously described (21,22) with some modifications. In brief, modified Boyden chambers (Neuroprobe, Bethesda, MD, USA) with 8-μm porosity polyvinylpyrrolidone-free polycarbonate filters were precoated with 20 μg/mL type IV collagen (Sigma). In the lower chamber, 27 μL defined KSFM with different VEGF concentrations was added. Cell suspension (5 × 104/mL) was prepared and incubated with or without 5 μg/mL VEGFR-2 neutralizing antibody MAB3571 for 30 min at 37 °C. An aliquot of 50 μL cell suspension was added to the upper chamber, and cells were allowed to migrate for 12 h at 37°C in 5% CO2. Cells on the upper surface of the membrane were carefully wiped off. Cells on the undersurface of the membrane were fixed with 4% paraformaldehyde for 10 min and stained with 2% crystal violet blue for 5 min, then rinsed off gently with running water. We quantified the number of migrated cells in each well by counting 5 randomly chosen fields at 400× magnification. Each determination represents the mean of 3 individual wells ± SD. For all experiments reported, assays were repeated at least 3 times with similar results.
Cell Adhesion Assays
Keratinocytes pre-incubated with or without VEGF165 at different concentrations were plated into 96-well plates pre-coated with 20 μg/mL type IV collagen (Sigma,) at 1 × 105 cells/well with defined KSFM and cultured for 4 h. Cells were rinsed gently 3 times with 0.1% BSA in prewarmed culture medium to remove unattached cells. Adherent cells were then incubated with 5 mg/mL MTT for 3 h. The following assay was similar to the above proliferation assay. For VEGFR-2 neutralizing assay, cells were incubated with VEGFR-2 neutralizing antibody MAB3571 at 5 μg/mL for 30 min prior to seeding. The data were expressed as the mean of triplicate wells ± SD. Experiments were repeated at least triplicate with similar results.
Statistical analyses
For in vitro data, results are expressed as mean ± SD. All determinations were performed in triplicate, and experiments were repeated at least 3 times. One-way ANOVA was used to evaluate significant differences. Statistical analyses were performed by SPSS Software (V13.0, SPSS, USA), with a P value < 0.05 considered to be statistically significant.
RESULTS
Expression of VEGFRs and NRPs in Cultured Keratinocytes
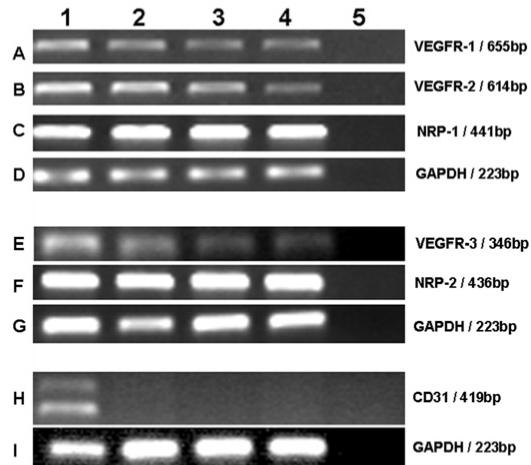
As shown in Figure 1, expression of VEGFR and NRP mRNA was detected in cultured keratinocytes from all 3 normal donors. Direct sequencing for these PCR products was identical to published sequences of human VEGFR-1, VEGFR-2, VEGFR-3, NRP-1, and NRP-2 (Table 1). The expression of CD31 gene was detected in HUVECs but not in any sample of cultured keratinocytes (Figure 1H), suggesting no contamination of endothelial cells in keratinocytes.
Figure 1.
Expression of mRNA of VEGFRs and NRPs in cultured keratinocytes. RT-PCR analysis of VEGFR-1 (A), VEGFR-2 (B), VGFR-3 (E), NRP-1 (C), NRP-2 (F) and CD31 gene (H) in HUVECs (lane 1) and cultured keratinocytes from 3 different healthy samples (lanes 2–4). Lane 5 is a negative control showing no amplification from potential genomic DNA contamination. GAPDH served as an internal control for different amplification performed for respective genes (D, G, I).
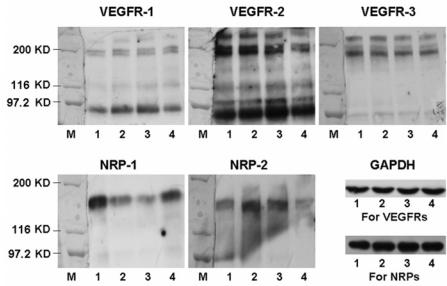
The protein levels of VEGFRs and NRPs in cultured keratinocytes are shown in Figure 2. We observed main bands with approximate sizes of 180 and 200 kDa for all 3 VEGFRs, but the intensity of bands for each VEGFR varied. Blots for NRP-1 and NRP-2 both detected a strong band at approximately 140 kDa. Based on the relative intensity of VEGFRs and NRPs (by comparing with GAPDH), it seems that VEGFR-2, NRP-1, and NRP-2 were highly expressed in keratinocytes from the 3 donors, whereas VEGFR-1 and VEGFR-3 were modestly expressed.
Figure 2.
Immunoblot analysis of VEGFRs and NRPs in cultured keratinocytes. The anti–VEGFR-1, anti–VEGFR-2, and anti–VEGFR-3 antibodies show bands at about 180 and 200 kDa. The anti–NRP-1 and anti–NRP-2 antibodies show bands at approximate 140 kDa. The anti-GAPDH antibody was used as loading control for normalization. M, marker; lane 1, HUVECs; lanes 2–4, cultured keratinocytes from 3 different healthy samples.
Localization of VEGFRs and NRPs in Normal Human Epidermis
Cryostat sections of skin specimens from all 3 healthy donors were examined by histological immunofluorescence staining. Positive staining for VEGFRs and NRPs were observed in epidermis of all 3 donors. The representative data presented in Figure 3 were from the skin of a 19-year-old male donor. The distribution pattern of these receptors varied in epidermis: VEGFRs showed a membranous staining pattern along the inter-cellular junctions in the basal and suprabasal layers of the epidermis except for the horny cell layer (Figures 3A1, 3B1, and 3C1). The staining pattern of NRPs differed from that of VEGFRs. NRPs are stained in the membrane and in the cytoplasm of keratinocytes in the whole epidermis except for the stratum corneum (Figures 3D1 and 3E1). Specifically, VEGFR-1 and VEGFR-2 (both MAB3571 and SC-6251) strongly labeled keratinocytes in stratum basal and spinosum adjacent to basal, whereas all layers of the epidermis except the horny cell layer demonstrated a uniform expression pattern of VEGFR-3, NRP-1, and NRP-2. Moreover, NRP-1 and NRP-2 showed diffuse intense fluorescence compared with VEGFRs. HUVECs (as apositive control) (column 2 in Figure 3) show strong staining for endothelial cells and vessel smooth muscle cells by all antibodies, whereas negative control sections incubated with mouse IgG were unstained (column 3 in Figure 3).
Figure 3.
Representative immunofluorescent staining of VEGFRs and NRPs in human healthy skin epidermis. The presence of VEGFRs and NRPs is indicated by green fluorescence staining. Red indicates PI nuclear staining. Column 1, normal human skin from a 19-year-old male donor; column 2, human umbilical cord vein; column 3, negative staining by mouse IgG. The letters in the figures refer to epidermis (e), dermis (d), and basal layer of epidermis (b) in skin sections. The luminal side of the human umbilical cord vein is indicated by capital letter L. Scale bar, 50 μm. VEGFR-1 (A1) and VEGFR-2 (B1) are strongly detected at the stratum basal and spinosum adjacent to the basal of epidermis (arrows). VEGFR-3 (C1) is detected uniformly at all layers of the epidermis except for the horny cell layer (arrow). NRP-1 (D1) and NRP-2 (E1) are distributed uniformly among all layers of the epidermis except for the horny cell layer (arrows). Positive controls of human umbilical cord vein showed strong staining for HUVECs and smooth muscle cells for all 5 antibodies for VEGFRs and NRPs (arrows). Negative staining controls of human skin by using mouse IgG are unstained in all experiments (arrowheads refer to the outer edge of skin).
Partial Blockage of VEGF Induced Proliferation and Migration of Keratinocytes by VEGFR-2 Neutralizing Antibody
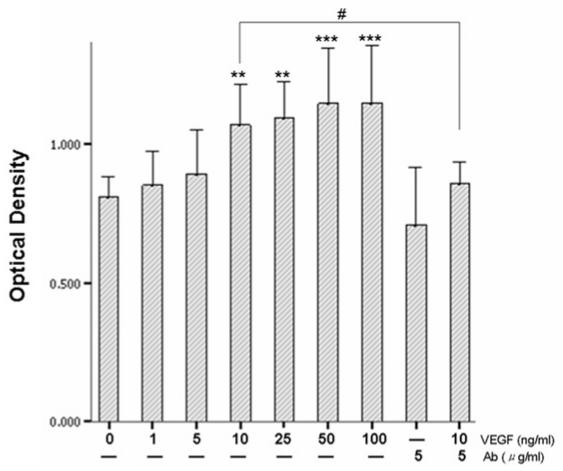
We first examined the effect of VEGF165 on keratinocyte proliferation and migration. As shown in Figure 4, VEGF165 increased the proliferation of keratinocytes in a dose-dependent manner, and this induction reached a plateau at a concentration of 10 ng/mL. VEGFR-2 neutralizing antibody (MAB3571) could partially block this induction (P < 0.05 vs. control).
Figure 4.
Effect of VEGF165 on keratinocyte proliferation. MTT-based assays were performed to determine the effects of VEGF alone or in combination with VEGFR-2 neutralizing antibody for 48-h treatment. Data represent the mean values of optical density measured in 6 wells for each treatment in a representative experiment. This experiment was repeated at least 3 times with similar results. **P ≤ 0.01; ***P < 0.001, significant differences between VEGF-treated and untreated samples. #P < 0.05, significant difference between VEGF-treated cells and cells treated with both VEGF and VEGFR-2 neutralizing antibody.
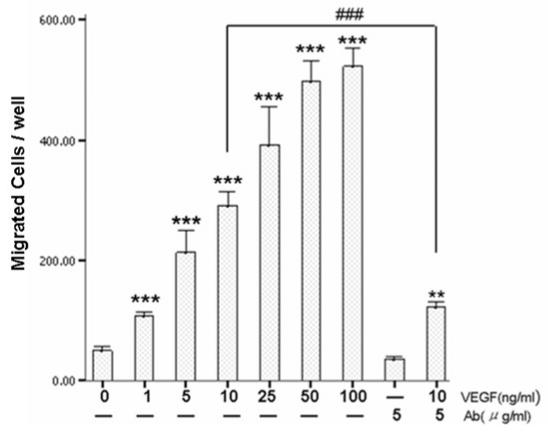
We next determined the effect of VEGF165 on chemotactic motility of keratinocytes by employing a modified Boyden chamber assay. The motility of keratinocytes was greatly affected by elevated concentrations of VEGF165 in a dose-dependent manner (Figure 5). VEGF165 at 100 ng/mL could induce about 10-fold migration over the control. Pretreatment with the anti–VEGFR-2 antibody significantly suppressed the induction of migration by VEGF165 at 10 ng/mL (P < 0.001 vs. control) (Figure 5); the migration of keratinocytes was reduced more than 2-fold in the presence of 5 μg/mL MAB3571 and 10 ng/mL VEGF165 compared with 10 ng/mL VEGF165 treatment alone. No significant difference was observed between cells treated with VEGFR-2 neutralizing antibody and cells without any treatment.
Figure 5.
Effect of VEGF165 on keratinocyte migration. Incubation with VEGF165 significantly increases keratinocyte migration, and this induction is partially abrogated by pre-treatment with VEGFR-2 neutralizing antibody. Data are expressed as mean values ± SD for the total number of cells observed in 5 randomly selected fields from 3 separate wells. **P ≤ 0.01; ***P < 0.001, significant differences between VEGF-treated and untreated cells. ###P < 0.001, significant difference between VEGF-treated cells and cells treated with both VEGF and VEGFR-2 neutralizing antibody.
VEGF Reduced Adhesion of Keratinocytes, Which Could Be Completely Reversed by VEGFR-2 Neutralizing Antibody
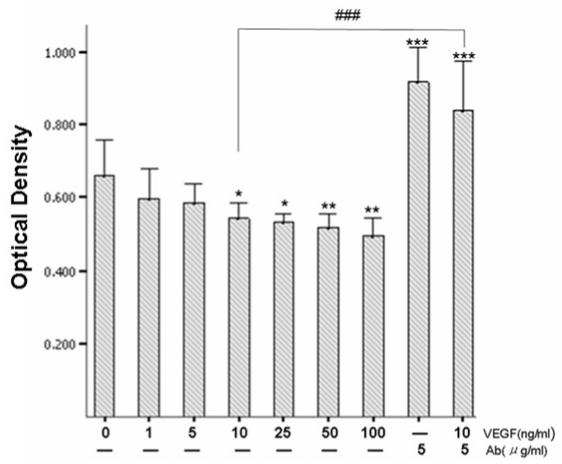
Adhesion of keratinocytes to type IV collagen–coated culture plates after 4 h of culture did not change dramatically under basal or slightly stimulated conditions. VEGF165 at a concentration of 10 ng/mL decreased keratinocyte adhesion by approximately 20%. This decrease was found to be more prominent when VEGF165 concentration was further increased (Figure 6). The binding of keratinocytes to type IV collagen–coated plates could be completely reversed by a pretreatment with VEGFR-2 neutralizing antibody in the presence of VEGF (Figure 6).
Figure 6.
Effect of VEGF165 on keratinocyte adhesion. *P < 0.05; **P ≤ 0.01; ***P < 0.001, significant differences between VEGF-treated and untreated cells. Cells pretreated with VEGFR-2 neutralizing antibody had significantly increased keratinocyte adhesion compared with untreated cells (***P < 0.001). The reduction of adhesion of keratinocytes by VEGF treatment at 10 ng/mL was significantly restored by pretreatment with VEGFR-2 neutralizing antibody (###P < 0.001).
DISCUSSION
Although VEGFRs and NRPs were initially thought to be expressed exclusively in endothelial cells (1,3,5,7,23,24), recent studies have found expression of VEGF receptors and coreceptors in other cell types and tissues (25–34). Our data demonstrate that keratinocytes in epidermis express VEGFR-1, VEGFR-2, VEGFR-3, and the coreceptors NRP-1 and NRP-2 at both mRNA and protein levels. To our knowledge, this is the first report that examined all 5 known VEGF receptors and coreceptors in keratinocytes of the epidermis.
The presence of VEGFRs and NRPs in different tissues varied, and each of these receptors might have specific biological activities ranging from enhancing proliferation to increasing cellular migration. Besides the presence in endothelial cells and endothelial cell progenitors (35,36), VEGFR-1 mRNA and protein are expressed in some immune cells, such as macrophage-osteoclast lineages (33,34, 37), natural killer cells (38), and neutrophils (32), as well as in other normal cells, including trophoblast cells, renal mesangial cells (5), melanocytes (25), vascular smooth muscle cells (27,39), pericytes (28), and neurons (29). Other studies have shown VEGFR-1 to be expressed on tumor cells such as human prostate and breast carcinoma cells (40,41) and pancreatic cancer cells (42), and to be implicated in tumor growth and progression (42–44). Fan et al. (26) found that VEGFR-1 was present in colorectal cancer cells of epithelial origin and was involved in tumor progression and metastasis. Recently, the expression of functional VEGFR-1 on keratinocytes was first reported by Wilgus et al. (18), who suggested a novel autocrine mechanism in keratinocytes by which VEGF may act to stimulate wound healing. We detected expression of VEGFR-1 in cultured keratinocytes (Figures 1 and 2) and observed positive staining for this receptor in all layers of epidermis except for stratum corneum (Figure 3). Our results are thus consistent with the observation of Wilgus et al. (18) but differ from those of Kurschat et al. (19), who failed to identify all 3 VEGFRs in cultured HaCaT keratinocytes.
VEGFR-2 is the major receptor for endothelial cell function (1,2,5) and was reported to be expressed in hematopoietic stem cells, megakaryocytes, neuronal cells, osteoblasts, pancreatic duct cells, and retinal progenitor cells (5,30), as well as in some tumor cells, such as malignant melanoma cells (5), papillary thyroid cancer, non–small-cell lung cancer, mesothelioma, and breast cancer (14). Neutrophils (32), monocytes (33,34), and stromal cells (5) are also found to express VEGFR-2 (5). Both Wilgus et al. (18) and Kurschat et al. (19) failed to detect the expression of VEGFR-2 in keratinocytes. In our study, however, we observed strong expression of this receptor in cultured keratinocytes and positive staining for VEGFR-2 in all layers of epidermis with the exception of stratum corneum. This is in accordance with what we have found—that VEGFR-2 was also expressed in HaCaT cells (45). Furthermore, the expression pattern of VEGFR-2 and VEGFR-1 are similar and show strong expression in the stratum basal layer and the adjacent spinosum layer of the epidermis (Figures 3A1 and 3A2). This distribution pattern may be associated with the proliferation of keratinocytes, as the basal cells and the adjacent spinosum cells are main sources for keratinocytes undergoing active proliferation (44). The expression of VEGFR-2 in epidermis was confirmed by 2 different antibodies used in this study. We speculate that the discrepancies between our study and those of Wilgus et al. (18) and Kurschat et al. (19) might be attributed to specificity and sensitivity of primers used in RT-PCR and antibodies in Western blot and immunostaining, as well as other technical reasons. For instance, we failed to obtain amplification using the primer pair for VEGFR-2 in Wilgus et al.’s paper (18) even in HUVECs; Kurschat et al. (19) did not show the primer sequences in their paper.
The presence of VEGFRs in keratinocytes and their potential role in cell proliferation and migration, as induced by VEGF treatment, could be compensated by pretreatment with a neutralizing antibody for VEGFR-2 (Figures 4 and 5), lending further support to our observations based on immunostaining and RT-PCR. As described above, pretreatment with VEGFR-2 neutralizing antibody could partially abolish the induced proliferation and migration of cultured keratinocytes by VEGF treatment (Figures 4 and 5). This indicates that VEGFR-2 is required for VEGF-induced phenotypic changes and the existence of an autocrine loop of VEGF in keratinocytes. Moreover, neutralizing VEGFR-2 reversed the downregulation effects of exogenous VEGF on adhesion (Figure 6). These results suggest that VEGFR-2 plays an important biological role in keratinocyte function. Together with a previous report on the function of VEGFR-1 on keratinocytes (18), it seems that both VEGFR-2 and VEGFR-1 are responsible for the autocrine signaling in epidermis.
VEGFR-3 is involved in angiogenesis in both the middle stage of embryogenesis and the entire process of lymphangiogenesis (8,44). Stimulation of the VEGFR-3 signal transduction pathway is sufficient to specifically induce lymphangiogenesis in vivo (8,44). VEGFR-3 was once considered to be almost exclusively expressed in lymphatic endothelium in normal adults (46,47). Recently, VEGFR-3 has been shown to be expressed in blood capillaries of normal breast tissue, neuroendocrine organs, and chronic wounds (48). In addition, monocytes, macrophages, and some dendritic cells express this receptor (48).
In our study, VEGFR-3 was expressed in keratinocytes in all layers of epidermis except for the horny cell layer, and presented a uniform distribution pattern that was not observed for VEGFR-1 and -2. The potential function of VEGFR-3 in epidermis is unknown based on this study.
The expression of the 2 coreceptors of VEGFRs, NRP-1 and NRP-2, has been well characterized in endothelial cells and in a variety of neural and nonneural tissues, and they are required for normal development (49). NRPs are also expressed by several types of cancer cell, such as lung tumor cell lines (50,51) and melanoma cell lines (14,52). Mice lacking a functional NRP-1 gene died at the embryo stage because of a failure to develop a proper cardiovascular system (30,53), whereas loss of NRP-2 gene was found to be accompanied with tumor progression in carcinoid tumors (54). Most recently, Kurschat et al. (19) provided in vivo and in vitro evidence for NRP-1 expression in keratinocytes and found that the neuron restrictive silencer factor downregulated NRP-1, but not NRP-2, expression in HaCaT keratinocyte cell line. In our study, we observed both NRP-1 and NRP-2 expression in all layers of keratinocytes in the epidermis excluding the horny layer. Also, the distribution patterns of NRP-1 and NRP-2 are different from those of VEGFRs (Figure 3). Our data showed that the expression of NRPs were not completely overlapped with VEGFRs in the epidermis, which suggested that NRP-1 and NRP-2 might have some function in epidermis independent of VEGFRs. Based on the fact that NRP-1 has been found to mediate heterophilic cell adhesion (54–56) and keratinocyte migration (19), it would be rewarding for future investigations to target the potential mechanism of NRPs on proliferation and migration of keratinocytes.
In summary, through a systematic examination of the expression of all 5 known VEGF receptors and coreceptors in vivo and in vitro, our results provided some important inferences regarding the autocrine role for VEGF in keratinocytes. The capacity of keratinocytes to express these receptors may constitute a novel regulation for keratinocyte activity in the epidermis during wound healing and skin development and maintenance. The observation that VEGF could induce keratinocyte proliferation and migration but reduce its adhesion, and that all these effects can be inhibited by pretreatment with a neutralizing antibody for VEGFR-2, give direct evidence for an active role of VEGF-VEGFR interaction in the epidermis. Combined with other published data (18,19) and the fact that VEGFR-1 and VEGFR-2 were overexpressed in epidermis of psoriasis vulgaris (57), it is reasonable to believe that VEGFRs and NRPs may play important roles in epidermis that are different from their traditional contribution in angiogenesis.
ACKNOWLEDGMENTS
We thank Drs. Jian-Ping Cai (Dianon/LabCopr in Tampa, Florida, USA) and Mark R Pitterlkow (Mayo Clinic College of Medicine, Rochester, Minnesota, USA) for helpful discussion. We are grateful to Ms. Aleah Smith for her help in editing the language. This work was supported by a grant (no. 30471565) from the National Natural Science Foundation of China (NSFC).
Footnotes
Online address: http://www.molmed.org
X.-Y.M. and X.-H.Y. contributed equally to this work.
REFERENCES
- 1.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 3.Stacker SA, Achen MA. The vascular endothelial growth factor family: signalling for vascular development. Growth Factors. 1999;17:1–11. doi: 10.3109/08977199909001058. [DOI] [PubMed] [Google Scholar]
- 4.Veikkola T, Alitalo K. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999;9:211–20. doi: 10.1006/scbi.1998.0091. [DOI] [PubMed] [Google Scholar]
- 5.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 6.Gu C, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 8.Veikkola T, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223–31. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibuya M. Vascular endothelial growth factor receptor family genes: when did the three genes phylogenetically segregate? Biol Chem. 2002;383:1573–9. doi: 10.1515/BC.2002.177. [DOI] [PubMed] [Google Scholar]
- 10.Yang H, Li M, Chai H, Yan S, Zhang R, Yao Q, Chen C. Expression and regulation of neuropilins and VEGF receptors by TNF-alpha in human endothelial cells. J Surg Res. 2004;122:249–55. doi: 10.1016/j.jss.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Li M, Chai H, Yang H, Fisher W, Yao Q. Roles of neuropilins in neuronal development, angiogenesis, and cancers. World J Surg. 2005;29:271–5. doi: 10.1007/s00268-004-7818-1. [DOI] [PubMed] [Google Scholar]
- 12.Fuh G, Garcia KC, DeVos AM. The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor Flt-1. J Biol Chem. 2000;275:690–5. doi: 10.1074/jbc.M003955200. [DOI] [PubMed] [Google Scholar]
- 13.Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203–12. [PubMed] [Google Scholar]
- 14.Wey JS, Stoeltzing O, Ellis LM. Vascular endothelial growth factor receptors: expression and function in solid tumors. Clin Adv Hematol Oncol. 2005;3:37–45. [PubMed] [Google Scholar]
- 15.Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–9. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz BV, Lenoir MC, Ladoux A, Frelin C, Demarchez M, Michel S. Regulation of vascular endothelial growth factor expression in human keratinocytes by retinoids. J Biol Chem. 2000;275:642–50. doi: 10.1074/jbc.275.1.642. [DOI] [PubMed] [Google Scholar]
- 17.Detmar M, et al. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994;180:1141–6. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilgus TA, et al. Novel function for vascular endothelial growth factor receptor-1 on epidermal keratinocytes. Am J Pathol. 2005;167:1257–66. doi: 10.1016/S0002-9440(10)61213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurschat P, Bielenberg D, Rossignol-Tallandier M, Stahl A, Klagsbrun M. Neuron restrictive silencer factor NRSF/REST is a transcriptional repressor of neuropilin-1 and diminishes the ability of semaphorin 3A to inhibit keratinocyte migration. J Biol Chem. 2006;281:2721–9. doi: 10.1074/jbc.M507860200. [DOI] [PubMed] [Google Scholar]
- 20.Larrivee B, Karsan A. Isolation and culture of primary endothelial cells. Methods Mol Biol. 2004;290:315–29. doi: 10.1385/1-59259-838-2:315. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto K, Matsumoto K, Nakamura T, Kramer RH. Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of focal adhesion kinase (p125FAK) and promotes migration and invasion by oral squamous cell carcinoma cells. J Biol Chem. 1994;269:31807–13. [PubMed] [Google Scholar]
- 22.Zhang K, Kramer RH. Laminin 5 deposition promotes keratinocyte motility. Exp Cell Res. 1996;227:309–22. doi: 10.1006/excr.1996.0280. [DOI] [PubMed] [Google Scholar]
- 23.Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Dvorak HF, Senger DR. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 1993;143:1255–62. [PMC free article] [PubMed] [Google Scholar]
- 24.Brown LF, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727–35. [PubMed] [Google Scholar]
- 25.Kim EJ, Park HY, Yaar M, Gilchrest BA. Modulation of vascular endothelial growth factor receptors in melanocytes. Exp Dermatol. 2005;14:625–33. doi: 10.1111/j.0906-6705.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 26.Fan F, et al. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene. 2005;24:2647–53. doi: 10.1038/sj.onc.1208246. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Keiser JA. Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: role of flt-1. Circ Res. 1998;83:832–40. doi: 10.1161/01.res.83.8.832. [DOI] [PubMed] [Google Scholar]
- 28.Takagi H, King GL, Aiello LP. Identification and characterization of vascular endothelial growth factor receptor (Flt) in bovine retinal pericytes. Diabetes. 1996;45:1016–23. doi: 10.2337/diab.45.8.1016. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y, Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor promotes proliferation of cortical neuron precursors by regulating E2F expression. FASEB J. 2003;17:186–93. doi: 10.1096/fj.02-0515com. [DOI] [PubMed] [Google Scholar]
- 30.Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–63. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Wey JS, et al. Vascular endothelial growth factor receptor-1 promotes migration and invasion in pancreatic carcinoma cell lines. Cancer. 2005;104:427–38. doi: 10.1002/cncr.21145. [DOI] [PubMed] [Google Scholar]
- 32.Ancelin M, Chollet-Martin S, Herve MA, Legrand C, El Benna J, Perrot-Applanat M. Vascular endothelial growth factor VEGF189 induces human neutrophil chemotaxis in extravascular tissue via an autocrine amplification mechanism. Lab Invest. 2004;84:502–12. doi: 10.1038/labinvest.3700053. [DOI] [PubMed] [Google Scholar]
- 33.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–43. [PubMed] [Google Scholar]
- 34.Sawano A, Iwai S, Sakurai Y, Ito M, Shitara K, Nakahata T, Shibuya M. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood. 2001;97:785–91. doi: 10.1182/blood.v97.3.785. [DOI] [PubMed] [Google Scholar]
- 35.Lyden D, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 36.LeCouter J, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–3. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 37.Clauss M, et al. The vascular endothelial growth factor receptor Flt-1 mediates biological activities: implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996;271:17629–34. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 38.Chen WS, Kitson RP, Goldfarb RH. Modulation of human NK cell lines by vascular endothelial growth factor and receptor VEGFR-1 (FLT-1) In Vivo. 2002;16:439–45. [PubMed] [Google Scholar]
- 39.Ishida A, Murray J, Saito Y, Kanthou C, Benzakour O, Shibuya M, Wijelath ES. Expression of vascular endothelial growth factor receptors in smooth muscle cells. J Cell Physiol. 2001;188:359–68. doi: 10.1002/jcp.1121. [DOI] [PubMed] [Google Scholar]
- 40.Hahn D, Simak R, Steiner GE, Handisurya A, Susani M, Marberger M. Expression of the VEGF-receptor Flt-1 in benign, premalignant and malignant prostate tissues. J Urol. 2000;164:506–10. [PubMed] [Google Scholar]
- 41.Zhukova LG, Zhukov NV, Lichinitser MR. Expression of Flt-1 and Flk-1 receptors for vascular endothelial growth factor on tumor cells as a new prognostic criterion for locally advanced breast cancer. Bull Exp Biol Med. 2003;135:478–81. doi: 10.1023/a:1024975627843. [DOI] [PubMed] [Google Scholar]
- 42.von Marschall Z, et al. De novo expression of vascular endothelial growth factor in human pancreatic cancer: evidence for an autocrine mitogenic loop. Gastroenterology. 2000;119:1358–72. doi: 10.1053/gast.2000.19578. [DOI] [PubMed] [Google Scholar]
- 43.Masood R, Cai J, Zheng T, Smith DL, Hinton DR, Gill PS. Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF receptor-positive human tumors. Blood. 2001;98:1904–13. doi: 10.1182/blood.v98.6.1904. [DOI] [PubMed] [Google Scholar]
- 44.La Rosa S, Uccella S, Finzi G, Albarello L, Sessa F, Capella C. Localization of vascular endothelial growth factor and its receptors in digestive endocrine tumors: correlation with micro-vessel density and clinicopathologic features. Hum Pathol. 2003;34:18–27. doi: 10.1053/hupa.2003.56. [DOI] [PubMed] [Google Scholar]
- 45.Yang XH, Man XY, Cai SQ, Yao YG, Bu ZY, Zheng M. Expression of VEGFR-2 on HaCaT cells is regulated by VEGF and plays an active role in mediating VEGF induced effects. Biochem Biophy Res Commun. 2006;349:31–8. doi: 10.1016/j.bbrc.2006.07.213. [DOI] [PubMed] [Google Scholar]
- 46.Witmer AN, van Blijswijk BC, Dai J, Hofman P, Partanen TA, Vrensen GF, Schlingemann RO. VEGFR-3 in adult angiogenesis. J Pathol. 2001;195:490–7. doi: 10.1002/path.969. [DOI] [PubMed] [Google Scholar]
- 47.Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004;25:387–95. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Aoki Y, Tosato G. Lymphatic regeneration: new insights from VEGFR-3 blockade. J Natl Cancer Inst. 2005;97:2–3. doi: 10.1093/jnci/dji015. [DOI] [PubMed] [Google Scholar]
- 49.Roche J, Drabkin H, Brambilla E. Neuropilin and its ligands in normal lung and cancer. Adv Exp Med Biol. 2002;515:103–14. doi: 10.1007/978-1-4615-0119-0_9. [DOI] [PubMed] [Google Scholar]
- 50.Kawakami T, Tokunaga T, Hatanaka H, et al. Neuropilin 1 and neuropilin 2 co-expression is significantly correlated with increased vascularity and poor prognosis in nonsmall cell lung carcinoma. Cancer. 2002;95:2196–201. doi: 10.1002/cncr.10936. [DOI] [PubMed] [Google Scholar]
- 51.Lantuejoul S, Constantin B, Drabkin H, Brambilla C, Roche J, Brambilla E. Expression of VEGF, semaphorin SEMA3F, and their common receptors neuropilins NP1 and NP2 in preinvasive bronchial lesions, lung tumours, and cell lines. J Pathol. 2003;200:336–47. doi: 10.1002/path.1367. [DOI] [PubMed] [Google Scholar]
- 52.Straume O, Akslen LA. Increased expression of VEGF-receptors (FLT-1, KDR, NRP-1) and thrombospondin-1 is associated with glomeruloid microvascular proliferation, an aggressive angiogenic phenotype, in malignant melanoma. Angiogenesis. 2003;6:295–301. doi: 10.1023/B:AGEN.0000029408.08638.aa. [DOI] [PubMed] [Google Scholar]
- 53.Kitsukawa T, Shimizu M, Sanbo M, et al. Neuropilin-semaphorin III/D-mediated chemo-repulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- 54.Fujisawa H, Kitsukawa T, Kawakami A, Takagi S, Shimizu M, Hirata T. Roles of a neuronal cell-surface molecule, neuropilin, in nerve fiber fasciculation and guidance. Cell Tissue Res. 1997;290:465–70. doi: 10.1007/s004410050954. [DOI] [PubMed] [Google Scholar]
- 55.Fujisawa H. From the discovery of neuropilin to the determination of its adhesion sites. Adv Exp Med Biol. 2002;515:1–12. doi: 10.1007/978-1-4615-0119-0_1. [DOI] [PubMed] [Google Scholar]
- 56.Shimizu M, Murakami Y, Suto F, Fujisawa H. Determination of cell adhesion sites of neuropilin-1. J Cell Biol. 2000;148:1283–93. doi: 10.1083/jcb.148.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng M, Zhu F. Overexpression of vascular endothelial growth factor receptors in epidermis of psoriasis vulgaris. J Invest Dermatol. 2004;122862 A1. [Google Scholar]