Abstract
Advanced glycation end products (AGEs) are senescent macroprotein derivatives that are formed at an accelerated rate in patients with chronic renal failure (CRF). AGE formation and accumulation in plasma and vascular tissues contribute to accelerated atherosclerosis in this devastating disorder. AST–120 is an oral adsorbent that attenuates the progression of CRF by removing uremic toxins. Recently, AST–120 has been reported to reduce the progression of atherosclerosis as well. However, whether AST–120 decreases serum levels of AGEs and subsequently exerts atheroprotective properties remains to be elucidated. Ten nondiabetic CRF patients were enrolled in this study. All patients were kept on regular therapeutic diet and medications throughout the study. Serum AGE levels before and after AST–120 treatments were measured using enzyme–linked immunosorbent assay. Effects of patient–derived serum on atherosclerosis–related gene expression in cultured human umbilical vein endothelial cells (HUVECs) were analyzed by semiquantitative RT–PCR. Administration of AST–120 (6 g/day) for 3 months significantly decreased serum levels of AGEs in nondiabetic CRF patients, whereas AGE levels remained unchanged in age– and renal function-matched CRF patients without AST–120 treatment (n = 6). Patient serum after AST–120 treatment significantly reduced mRNA levels of receptor for AGEs, monocyte chemoattractant protein–1, and vascular adhesion molecule–1 in HUVECs compared with serum before treatment. Moreover, in vitro, AST–120 was found to adsorb carboxymethyllysine (CML), one of the well–characterized, digested food-derived AGEs. This study suggests that atheroprotective properties of AST–120 can be ascribed, at least in part, to its AGE–lowering ability via absorption of CML.
INTRODUCTION
Reducing sugars can react nonenzymatically with amino groups of protein to form Amadori products. These early glycation products undergo further complex reaction such as rearrangement, dehydration, and condensation to become irreversibly cross–linked, heterogeneous fluorescent derivatives, termed advanced glycation end products (AGEs) (1). The formation and accumulation of AGEs have been known to progress at an accelerated rate in diabetes and/or chronic renal failure (CRF) (2). Recent understanding of this process has confirmed that AGEs are implicated in the pathogenesis of accelerated atherosclerosis in these devastating disorders (3,4). Furthermore, there is a growing body of evidence that receptor for AGEs (RAGE) is a signal–transducing receptor for AGEs and that engagement of RAGE by AGEs elicits oxidative stress generation and inflammatory responses in vascular wall cells (5,6).
AST–120 (Kremezine) is an oral adsorbent that attenuates the progression of CRF by removing uremic toxins, resulting in the delay of dialysis (7,8). AST–120 is also reported to reduce carotid intima media thickness (IMT) and arterial stiffness, one of the surrogate markers for atherosclerosis, in CRF patients before dialysis (9). However, the precise molecular mechanism for the anti–atherosclerotic effects of AST–120 remains to be elucidated.
AGEs represent an important class of uremic toxins as well (2,10). Further, recently, diet–derived AGEs were found to play an important role in the pathogenesis of atherosclerosis (10–12). These observations led us to speculate that AST–120 could exert atheroprotective properties by adsorbing diet–derived AGEs and subsequently decreasing serum AGE levels. In this study, we investigated whether AST–120 treatment (6 g/day) for 3 months could decrease serum levels of AGEs in nondiabetic CRF patients. We next examined the effects of patient–derived serum before and after AST–120 treatments on atherosclerosis–related gene expression in cultured human umbilical vein endothelial cells (HUVECs). To elucidate the molecular mechanism underlying the AGE–lowering effects of AST–120, we further studied here whether AST–120 could adsorb carboxymethyllysine (CML), one of the well–characterized, digested food-derived AGEs in vitro (13).
METHODS
Subjects
The study involved 10 CRF patients with chronic glomerulonephritis (n = 8) and nephrosclerosis (n = 2) (5 men and 5 women, mean age 59 ± 10 years, mean serum creatinine 4.3 ± 1.7 mg/dL). Six age– and renal function-matched nondiabetic CRF patients (4 men and 2 women, mean age 63 ± 9.7 years, mean serum creatinine 4.5 ± 2.0 mg/dL) who did not receive AST–120 treatment served as control subjects. None of the patients in this study received immunosuppressive therapy. We limited our analysis to nondiabetic subjects because serum AGEs levels are influenced by blood glucose levels, which could confound the effects of AST–120 on circulating AGE levels. After informed consent was obtained from all the subjects, the patients were treated with 2 g AST–120 (Kureha–Chemical Co., Tokyo, Japan) 3 times a day for 3 months. Blood pressure (BP) and biochemical markers were monitored before and after treatments of AST–120. BP was measured with a standard sphygmomanometer in the sitting position after a 5–min rest. Blood samples were obtained after a 12–h overnight fast. Serum levels of AGEs were measured using ELISA as described previously (14). CML was generously provided by Fushimi Pharmaceutical Co. (Kagawa, Japan). Serum creatinine, blood urea nitrogen (BUN), and urinary protein excretion were measured with commercial kits (Mizuho Medy Co., Tosu, Japan; Daiichi Pure Chemicals Co., Tokyo, Japan; and Wako Chemicals, Osaka, Japan). Plasma glucose was measured using the glucose oxidase method (A&T Co., Yokohama, Japan). Although our patients received antihypertensive and/or antihyperlipidemic agents such as angiotensin II receptor blockers and statins, all the patients were kept on regular therapeutic diet and medications throughout the study (no changes in medications were made). The study protocol was approved by the ethics committee of Kurume University School of Medicine.
Cells
HUVECs were maintained in endothelial basal medium (EBM) supplemented with 2% fetal bovine serum, 0.4% bovine brain extract, 10 ng/mL human epidermal growth factor, and 1 μg/mL hydro-cortisone according to the supplier’s instructions (15). When the cells were treated with patient serum, the medium was changed to EBM supplemented with 10% patient serum.
Primers
Sequences of sense and antisense primers for detecting human RAGE, monocyte chemoattractant protein–1 (MCP–1), and vascular adhesion molecule–1 (VCAM–1) mRNAs were 5′–ATG-GAAACTGAACACAGGCC–3′ and 5′–CACACATGTCCCCACCTTAT–3′ (16), 5′–AACTGAAGCTCGCACTCTCG–3′ and 5′–TCAGCACAGATCTCCTTGGC–3′ (17), and 5′–TTCCTAGCGTGTACCCC-CTTGACC–3′ and 5′–CAGAAAGAG-GCTGTAGCTCCCCGT–3′ (18), respectively. Sequences of the upstream and downstream primers for detecting human β–actin mRNA were as described previously (15).
Semiquantitative RT–PCR
Poly(A)+ RNAs were isolated from the cells treated with patient serum for 12 h and analyzed by RT–PCR as described previously (19). The amounts of poly(A)+ RNA templates (30 ng) and cycle numbers (28 cycles for RAGE gene and VCAM–1 genes; 30 cycles for MCP–1 gene; 22 cycles for β–actin gene) for amplification were chosen in quantitative ranges, where reactions proceeded linearly, as determined by plotting signal intensity as a function of the template amounts and cycle numbers. We have previously shown that gene expression levels evaluated in this method correlate to protein expression levels (5,16,20).
CML Absorptive Property of AST–120 In Vitro
One milliliter CML solution (4 μg/mL) in phosphate–buffered saline was incubated with 10 mg AST–120 under stirring conditions at 37 °C. After 3 h, the reaction mixture was centrifuged, and the concentration of CML in the supernatant was determined. Adsorption rate (%) was calculated using the following formula: Adsorption rate (%) = [4 - CML concentration (μg/mL) in the supernatant/4] × 100.
Statistical Analysis
All values were expressed as mean ± SD. Statistical analysis was performed using paired Student t test. P < 0.05 was considered significant.
RESULTS
Laboratory Variables Before and After Treatments of AST–120
Laboratory variables of control subjects and AST–120-treated patients are shown in Table 1. Body weight, blood glucose, systolic and diastolic BP, BUN, and serum creatinine levels were not changed during the study period for both groups of subjects. Urinary protein excretion was modestly, but significantly, reduced by the treatment with AST–120.
Table 1.
Laboratory variables of control patients and AST–120-treated patients
| Control patients
|
AST–120-treated patients
|
|||
|---|---|---|---|---|
| Baseline | After 3 months | Before treatment | After 3 months of treatment | |
| Body weight (kg) | 57.9 ± 11.7 | 57.2 ± 12.7 | 58.3 ± 11.1 | 57.9 ± 11.4 |
| Fasting plasma glucose (mg/dL) | 85.2 ± 6.4 | 83.8 ± 4.6 | 90.8 ± 4.0 | 89.8 ± 4.8 |
| Systolic BP (mmHg) | 126 ± 21 | 136 ± 8.4 | 123 ± 16 | 125 ± 29 |
| Diastolic BP (mmHg) | 68 ± 8.8 | 72 ± 11 | 74 ± 9.4 | 72 ± 9.2 |
| BUN (mg/dL) | 44.4 ± 18.5 | 49.1 ± 20.9 | 52.3 ± 18.3 | 49.6 ± 21.7 |
| Serum creatinine (mg/dL) | 4.5 ± 2.0 | 4.9 ± 2.0 | 4.4 ± 1.7 | 4.8 ± 1.9 |
| Urinary protein excretion (g/day) | 2.3 ± 1.7 | 3.0 ± 3.3 | 1.8 ± 1.5 | 1.5 ± 1.3a |
P < 0.05 compared with the value before AST–120 treatment.
Effects of AST–120 Treatment on Serum Levels of AGEs
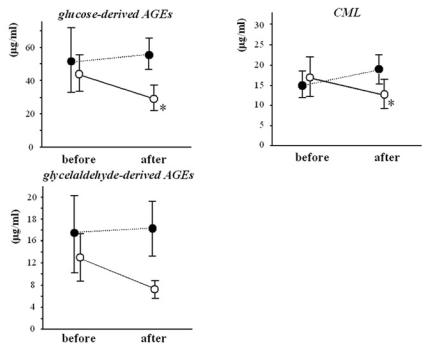
As shown in Figure 1, oral administration of AST–120 for 3 months significantly reduced serum levels of glucose–derived AGEs (44.1 ± 10.8 vs. 27.6 ± 6.0 μg/mL; P < 0.05) and CML (18.5 ± 5.0 vs. 11.0 ± 2.5 μg/mL; P < 0.05). Serum levels of glyceraldehyde–derived AGEs were marginally reduced by the treatment with AST–120 (13.2 ± 4.4 vs. 6.2 ± 0.9 μg/mL; P = 0.06). Decreases in serum AGE levels were not correlated with those in urinary protein excretion. Serum levels of AGEs remained unchanged in control subjects (not treated with AST–120) during the study period (Figure 1).
Figure 1.
Effects of oral administration of AST–120 on serum levels of AGEs in patients with nondiabetic CRF. ○, AST–treated patients (n = 10); •, control patients without AST treatment (n = 6). *P < 0.05 compared with the value before treatment.
Effects of Patient Serum on Atherosclerosis–Related Gene Expression in HUVECs
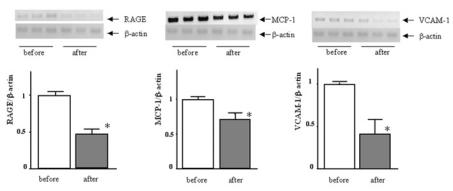
To show the clinical relevance of the reduction of serum AGEs in atherosclerosis, we next examined the effects of patient serum after AST–120 treatment on atherosclerosis–related gene expression in HUVECs using patient serum before treatment as a control. As shown in Figure 2, patient serum after AST–120 treatment significantly reduced mRNA levels of RAGE, MCP–1, and VCAM–1 in HUVECs, compared with serum before treatment. Because there was no change in clinical study parameters except AGEs in the serum (Table 1), our present observations suggest that reduction of serum AGEs by AST–120 treatment could be clinically relevant. Atherosclerosis–related gene expression in endothelial cells (ECs) is modulated by various metabolic factors other than AGEs (21). Therefore, the comparison between patient serum and normal blood serum could confound the effects of serum AGEs on atherosclerosis–related gene expression in HUVECs. This is a reason we did not use normal blood serum as a control.
Figure 2.
Effects of patient serum on atherosclerosis–related gene expression in HUVECs. HUVECs were treated with patient serum before or after AST–120 treatment for 12 h, and RAGE, VCAM, and MCP–1 mRNA levels were determined. Upper panel shows representative RT–PCR bands of these genes. Each lower panel shows the quantitative representation of each gene induction. Data were normalized by the intensity of β–actin mRNA–derived signals and related to the value of the control. *P < 0.05 compared with the value before AST–120 treatment.
CML Adsorptive Property of AST–120 In Vitro
Under our experiment conditions, AST–120 was found to completely adsorb CML, one of the well–characterized, digested food-derived AGEs; adsorption rate was 100%. When the concentration of CML was increased to 8 μg/mL, AST–120 completely adsorbed it.
DISCUSSION
In the present study, we demonstrated for the first time that oral administration of AST–120 for 3 months significantly reduced serum levels of AGEs in nondiabetic patients with CRF. Moreover, patient serum after AST–120 treatment significantly decreased mRNA levels of atherosclerosis–related genes such as RAGE, MCP–1, and VCAM–1 in cultured HUVECs. Because AST–120 was found to adsorb CML, one of the well–characterized, digested food-derived AGEs in vitro, our present findings suggest that AST–120 could exert atheroprotective properties by adsorbing diet–derived AGEs and subsequently decreasing serum levels of AGEs in patients with CRF.
AST–120 has been considered to exert beneficial effects on CRF progression by removing uremic toxins or their precursors in the digestive tract. However, the target uremic toxins of AST–120 had not been well characterized. Recently, it has been demonstrated that AST–120 decreases serum and urinary levels of in-doxyl sulphate by adsorbing its precursor, indole, in the intestine (7,22). Because indoxyl sulphate is involved in the pathogenesis of glomerular sclerosis and interstitial fibrosis and its serum levels are elevated in CRF patients (22–24), indole is considered to be one of the molecular targets for AST–120. However, whether AST–120 could exert favorable effects on renal injury and atherosclerosis by removing other uremic toxins remains obscure.
There is a growing body of evidence that AGEs represent an important class of uremic toxins (2,10). Indeed, serum levels of AGEs are markedly elevated in patients with renal failure, thus raising the speculation that they have a role as cardiovascular risk factors in this population. Furthermore, digested food-derived AGEs were recently found to play an important role in the development and progression of chronic kidney disease and atherosclerosis (10–12,25). As far as we know, the present study is the first report to show that digested food-derived AGEs such as CML may be a novel molecular target for oral adsorbent AST–120. Although we did not examine the CML adsorptive property of AST–120 under stimulated gastric and intestinal fluid conditions in vitro, our present observations suggest that adsorption of diet–derived AGEs or their precursors in the intestine by AST–120 would be a promising therapeutic strategy for preventing progressive renal disease and atherosclerotic complications in patients with CRF. Further study should be required to clarify how much amounts of dietary AGEs could be potentially adsorbed by AST–120.
We, along with others, have previously shown that engagement of RAGE by AGEs activates its downstream signaling via oxidative stress generation in ECs (5,6). The AGE–RAGE interaction enhances EC gene expression of chemokines and adhesion molecules such as MCP–1 and VCAM–1 (6,26). AGEs increase EC RAGE expression levels as well (5,6). These observations suggest that the AGE–RAGE system may form a positive feedback loop, thereby exacerbating vascular injury in patients with diabetes and/or CRF (6). Therefore, our present study suggests that the atheroprotective properties of AST–120 could be ascribed, at least in part, to its suppressive effects on EC RAGE expression.
At present, we do not know whether the AGE–lowering ability of AST–120 could actually contribute to the prevention of atherosclerosis in nondiabetic CRF patients. However, the findings that patient serum after AST–120 treatment significantly reduced EC gene expression of MCP–1 and VCAM–1, which are key molecules of the recruitment and firm adhesion of inflammatory cells to ECs, an initial step of atherosclerosis (27–29), suggest the clinical relevance of the AGE–reducing property of AST–120 in vivo. Further, because AST–120 has been reported to prevent carotid IMT and arterial stiffness, one of the surrogate markers for atherosclerosis, in CRF patients (9), the atheroprotective property of AST–120 could be ascribed, at least in part, to its AGE–lowering ability in vivo. In any case, more long–term observational study is needed to clarify whether reduction of serum AGE levels by AST–120 could exert beneficial effects on atherosclerosis in patients with CRF.
ACKNOWLEDGMENTS
Sources of support include a grant from Grant–in–Aid for Scientific Research from the Ministry of Education, Science and Culture, Tokyo; a grant from Japan Foundation of Cardiovascular Research, Tokyo; and a grant from the Ishibashi Foundation for the Promotion of Science, Kurume.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Vlassara H. Advanced glycation end–products and atherosclerosis. Ann Med. 1996;28:419–26. doi: 10.3109/07853899608999102. [DOI] [PubMed] [Google Scholar]
- 2.Glorieux G, et al. In vitro evidence for immune activating effect of specific AGE structures retained in uremia. Kidney Int. 2004;66:1873–80. doi: 10.1111/j.1523-1755.2004.00961.x. [DOI] [PubMed] [Google Scholar]
- 3.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–21. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 4.Bucala R, Crerami A. Chemistry, biology, and implications for diabetes and aging. Adv Pharmacol. 1992;23:1–34. doi: 10.1016/s1054-3589(08)60961-8. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor–alpha through nuclear factor–kappa B, and by 17beta–estradiol through Sp–1 in human vascular endothelial cells. J Biol Chem. 2000;275:25781–90. doi: 10.1074/jbc.M001235200. [DOI] [PubMed] [Google Scholar]
- 6.Wautier JL, Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ Res. 2004;95:233–8. doi: 10.1161/01.RES.0000137876.28454.64. [DOI] [PubMed] [Google Scholar]
- 7.Niwa T, Nomura T, Sygiyama S, Miyazaki T, Tsukushi S, Tsutsui S. The protein metabolite hypothesis, a model for the progression of renal failure: an oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int. 1997;62:S23–8. [PubMed] [Google Scholar]
- 8.Owada A, Nakao M, Koike J, Ujiie K, Tomita K, Shiigai T. Effects of oral adsorbent AST–120 on the progression of chronic renal failure: a randomized controlled study. Kidney Int. 1997;63:S188–90. [PubMed] [Google Scholar]
- 9.Nakamura T, et al. Oral adsorbent AST–120 decreases carotid intima–media thickness and arterial stiffness in patients with chronic renal failure. Kidney Blood Press Res. 2004;27:121–6. doi: 10.1159/000077536. [DOI] [PubMed] [Google Scholar]
- 10.Henle T. AGEs in foods: do they play a role in uremia? Kidney Int. 2003;84:S145–7. doi: 10.1046/j.1523-1755.63.s84.16.x. [DOI] [PubMed] [Google Scholar]
- 11.Lin RY, et al. Lowing of dietary advanced glycation endproducts (AGE) reduces neointimal formation after arterial injury in genetically hypercholesterolemic mice. Atherosclerosis. 2002;163:303–11. doi: 10.1016/s0021-9150(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 12.Cai W, et al. High levels of dietary advanced glycation end products transform low–density lipoprotein into a potent redox–sensitive mitogen–activated protein kinase stimulant in diabetic patients. Circulation. 2004;110:285–91. doi: 10.1161/01.CIR.0000135587.92455.0D. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed N, Babaei–Jadidi R, Howell SK, Beisswenger PJ, Thornalley PJ. Degradation products of proteins damaged by glycation, oxidation and nitration in clinical type 1 diabetes. Diabetologia. 2005;48:1590–603. doi: 10.1007/s00125-005-1810-7. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi M, Makita Z, Bucala R, Koike T, Kameda Y. Immunological evidence that non–carboxymethyllysine advance glycation endproducts are produced from short chain sugars and decarbonyl compounds in vivo. Mol Med. 2000;6:114–25. [PMC free article] [PubMed] [Google Scholar]
- 15.Yamagishi S, Nakamura K, Ueda S, Kato S, Imaizumi T. Pigment epithelium–derived factor (PEDF) blocks angiotensin II signaling in endothelial cells via suppression of NADPH oxidase: a novel anti–oxidative mechanism of PEDF. Cell Tissue Res. 2005;320:437–45. doi: 10.1007/s00441-005-1094-8. [DOI] [PubMed] [Google Scholar]
- 16.Yamagishi S, Takeuchi M. Nifedipine inhibits gene expression of receptor for advanced glycation end products (RAGE) in endothelial cells by suppressing reactive oxygen species generation. Drugs Exp Clin Res. 2004;30:169–75. [PubMed] [Google Scholar]
- 17.Yamagishi S, Inagaki Y, Kikuchi S. Nifedipine inhibits tumor necrosis factor–alpha–induced monocyte chemoattractant protein–1 overexpression by blocking NADPH oxidase–mediated reactive oxygen species generation. Drugs Exp Clin Res. 2003;29:147–52. [PubMed] [Google Scholar]
- 18.Yamagishi S, Takeuchi M. Nifedipine inhibits tumor necrosis factor–alpha–induced leukocyte adhesion to endothelial cells by suppressing vascular cell adhesion molecule–1 (VCAM–1) expression. Drugs Exp Clin Res. 2004;30:163–8. [PubMed] [Google Scholar]
- 19.Fukami K, et al. AGEs activate mesangial TGF–β–Smad signaling via an angiotensin II type I receptor interaction. Kidney Int. 2004;66:2137–47. doi: 10.1111/j.1523-1755.2004.66004.x. [DOI] [PubMed] [Google Scholar]
- 20.Inagaki Y, Yamagishi S, Okamoto T, Takeuchi M, Amano S. Pigment epithelium–derived factor prevents advanced glycation end products–induced monocyte chemoattractant protein–1 production in microvascular endothelial cells by suppressing intracellular reactive oxygen species generation. Diabetologia. 2003;46:2284–7. doi: 10.1007/s00125-002-1013-4. [DOI] [PubMed] [Google Scholar]
- 21.Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome: insights from therapeutic interventions. J Am Coll Cardiol. 2005;46:1978–895. doi: 10.1016/j.jacc.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 22.Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med. 1994;124:96–104. [PubMed] [Google Scholar]
- 23.Miyazaki T, Ise M, Seo H, Niwa T. Indoxyl sulfate increases the gene expressions of TGF–β1, TIMP–1 and pro–α1(I)collagen in uremic rat kidneys. Kidney Int. 1997;62:S15–22. [PubMed] [Google Scholar]
- 24.Miyazaki T, Aoyama I, Ise M, Seo H, Niwa T. An oral sorbent reduces overload of in-doxyl sulphate and gene expression of TGF–beta1 in uraemic rat kidneys. Nephrol Dial Transplant. 2000;15:1773–81. doi: 10.1093/ndt/15.11.1773. [DOI] [PubMed] [Google Scholar]
- 25.Koschinsky T, et al. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci U S A. 1997;94:6474–9. doi: 10.1073/pnas.94.12.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basta G, Lazzerini G, Del Turco S, Ratto GM, Schmidt AM, De Caterina R. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule–1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol. 2005;25:1401–7. doi: 10.1161/01.ATV.0000167522.48370.5e. [DOI] [PubMed] [Google Scholar]
- 27.Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992;6:2591–9. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- 28.Gu L, et al. Absence of monocyte chemoattractant protein–1 reduces atherosclerosis in low density lipoprotein receptor–deficient mice. Mol Cell. 1998;2:275–81. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 29.Dansky HM, et al. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule–1 gene dosage. Arterioscler Thromb Vasc Biol. 2001;21:1662–7. doi: 10.1161/hq1001.096625. [DOI] [PubMed] [Google Scholar]