Abstract
To test whether tocopherols (vitamin E) are essential in the protection against oxidative stress in plants, a series of Arabidopsis thaliana vitamin E (vte) biosynthetic mutants that accumulate different types and levels of tocopherols and pathway intermediates were analyzed under abiotic stress. Surprisingly subtle differences were observed between the tocopherol-deficient vte2 mutant and the wild type during high-light, salinity, and drought stresses. However, vte2, and to a lesser extent vte1, exhibited dramatic phenotypes under low temperature (i.e., increased anthocyanin levels and reduced growth and seed production). That these changes were independent of light level and occurred in the absence of photoinhibition or lipid peroxidation suggests that the mechanisms involved are independent of tocopherol functions in photoprotection. Compared with the wild type, vte1 and vte2 had reduced rates of photoassimilate export as early as 6 h into low-temperature treatment, increased soluble sugar levels by 60 h, and increased starch and reduced photosynthetic electron transport rate by 14 d. The rapid reduction in photoassimilate export in vte2 coincides with callose deposition exclusively in phloem parenchyma transfer cell walls adjacent to the companion cell/sieve element complex. Together, these results indicate that tocopherols have a more limited role in photoprotection than previously assumed but play crucial roles in low-temperature adaptation and phloem loading.
INTRODUCTION
Tocopherols are the best-studied class of lipid-soluble antioxidants and are produced only by photosynthetic organisms, including all plants and algae and some cyanobacteria. Structurally, all four tocopherols (α-, β-, γ-, and δ-tocopherols) consist of a chromanol head group attached to a phytyl tail and differ only in the number and positions of methyl groups on the chromanol ring (Figure 1). Tocopherols are amphipathic molecules, and in vitro studies using artificial membranes have shown that tocopherols form complexes with specific lipid constituents and physically stabilize membranes (Wassall et al., 1986; Stillwell et al., 1996; Wang and Quinn, 2000; Bradford et al., 2003). Tocopherols can efficiently quench singlet oxygen, scavenge various radicals, particularly lipid peroxy radicals, and thereby terminate lipid peroxidation chain reactions (Liebler and Burr, 1992; Bramley et al., 2000; Schneider, 2005). In animals, vitamin E deficiency results in muscular weakness and neurological dysfunction, which often coincide with increased lipid peroxidation (Machlin et al., 1977; Yokota et al., 2001). Recent studies have shown that tocopherols also have functions in animals unrelated to their antioxidant activity, such as modulation of cell signaling and transcriptional regulation (Ricciarelli et al., 1998; Jiang et al., 2000; Rimbach et al., 2002; Kempna et al., 2004). In contrast with the extensive studies of tocopherol functions in animals, we are only beginning to understand tocopherol functions in the photosynthetic organisms in which they are produced. In plants, tocopherols are synthesized and localized in plastid membranes that are also highly enriched in polyunsaturated fatty acids (PUFAs) (Bucke, 1968; Soll et al., 1980, 1985; Lichtenthaler et al., 1981; Soll, 1987; Vidi et al., 2006), and increased tocopherol content has been correlated in the response of photosynthetic tissues to a variety of abiotic stresses, including high-intensity light (HL), salinity, drought, and low temperatures (Munne-Bosch et al., 1999; Keles and Oncel, 2002; Bergmuller et al., 2003; Collakova and DellaPenna, 2003).
Figure 1.
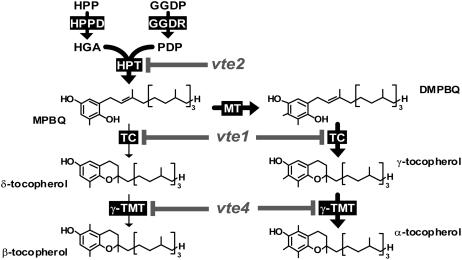
Tocopherol Biosynthetic Pathway and vte Mutations in Arabidopsis.
Enzymes are indicated by black boxes, and mutations are indicated by gray letters and lines. Thick arrows show the primary biosynthetic route in wild-type leaves. DMPBQ, 2,3-dimethyl-6-phytyl-1,4-benzoquinol; GGDP, geranylgeranyl diphosphate; GGDR, GGDP reductase; HGA, homogentisic acid; HPP, hydroxyphenylpyruvate; HPPD, HPP dioxygenase; HPT, HGA phytyltransferase; MPBQ, 2-methyl-6-phytyl-1,4-benzoquinol; MT, MPBQ methyltransferase; PDP, phytyl diphosphate; TC, tocopherol cyclase; γ-TMT, γ-tocopherol methyltransferase; vte1, vte2, and vte4, mutants of TC, HPT, and γ-TMT, respectively.
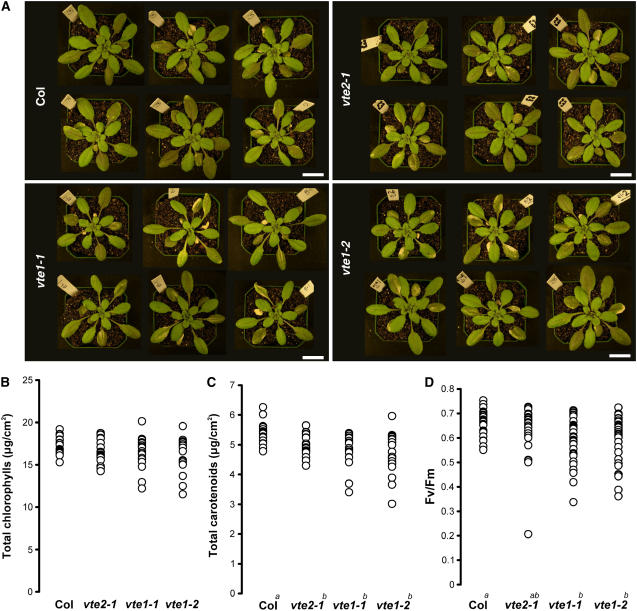
Such data, together with the evolutionary conservation of tocopherol synthesis among photosynthetic organisms, has led to the assumption that a primary function of tocopherols is to protect photosynthetic membranes from oxidative stresses by acting as lipid-soluble antioxidants (Fryer, 1992; Munne-Bosch and Alegre, 2002). Although plausible, such hypotheses are based primarily on correlations and circumstantial evidence and have yet to be rigorously tested in planta. The isolation of Arabidopsis thaliana mutants disrupting steps of the tocopherol biosynthetic pathway provides powerful tools with which to directly investigate tocopherol functions in plants (Figure 1) (Shintani and DellaPenna, 1998; Collakova and DellaPenna, 2001; Porfirova et al., 2002; Cheng et al., 2003; Sattler et al., 2003; DellaPenna and Pogson, 2006). The vitamin E2 (vte2) mutant is defective in homogentisate phytyl transferase (HPT) and lacks all tocopherols and pathway intermediates (Figure 1, Table 1). vte2 mutants are severely impaired in seed longevity and early seedling development as a result of the massive and uncontrolled peroxidation of storage lipids (Sattler et al., 2004; S.E. Sattler, L. Mene-Saffrane, E.E. Farmer, M. Krischke, M.J. Mueller, and D. DellaPenna, unpublished data), consistent with the loss of the lipid-soluble antioxidant functions of tocopherols (Ham and Liebler, 1995, 1997). Interestingly, the vte2 mutants that do survive early seedling development become virtually indistinguishable from wild-type plants under standard growth conditions (Sattler et al., 2004; this study), suggesting that unlike seed longevity and germination, tocopherols are dispensable in mature plants in the absence of stress. Consistent with this, constitutive overexpression of VTE2 in Arabidopsis increased total leaf tocopherols by 4.5-fold but had no discernible effect relative to the wild type on plant growth or chlorophyll and carotenoid content in the absence of stress or under combined nutrient and HL stresses (Collakova and DellaPenna, 2003).
Table 1.
Tocopherol and DMPBQ Contents in Leaves of Wild-Type and Tocopherol Biosynthetic Mutant Plants Grown under Permissive Conditions
| Plant | αa | β | γ | δ | Total | DMPBQ |
|---|---|---|---|---|---|---|
| Col | 15.6 ± 3.0 | 0b | 0.6 ± 0.1 | 0 | 16.2 ± 3.0 | 0 |
| vte2-1c | 0 | 0 | 0 | 0 | 0 | 0 |
| vte1-1c | 0 | 0 | 0 | 0 | 0 | 19.6 ± 1.3 |
| vte1-2c | 0 | 0 | 0 | 0 | 0 | 17.3 ± 1.8 |
| Ws | 12.3 ± 2.2 | 0 | 0 | 0 | 12.3 ± 2.2 | 0 |
| vte2-2d | 0 | 0 | 0 | 0 | 0 | 0 |
| vte4-3d | 0 | 0 | 17.7 ± 1.2 | 0 | 17.7 ± 1.2 | 0 |
Plants were grown for 4 weeks under permissive conditions, and mature leaves were harvested for analysis. Data are means ± sd (n = 3 or 4) and are expressed as picomoles per milligram fresh weight.
α, β, γ, and δ indicate α-, β-, γ-, and δ-tocopherol, respectively.
0 indicates that the compound was below detection (typically ≤0.1 pmol/mg fresh weight).
Col background.
Ws background.
The vte1 mutant is defective in tocopherol cyclase activity and deficient in all tocopherols, but unlike vte2, it accumulates the redox-active biosynthetic intermediate 2,3-dimethyl-6-phytyl-1,4-benzoquinol (DMPBQ) (Figure 1, Table 1) (Sattler et al., 2003). When grown at 100 to 120 μmol·m−2·s−1, vte1 plants are virtually identical to wild-type plants at all developmental stages (Porfirova et al., 2002; Sattler et al., 2003, 2004). The lipid-peroxidation phenotype observed in germinating vte2 seedlings was not observed in vte1, indicating that DMPBQ can fully compensate for tocopherols as a lipid-soluble antioxidant in seedlings (Sattler et al., 2004). Under HL stress (5 d at 850 μmol·m−2·s−1 [Porfirova et al., 2002]) or a combination of low-temperature and HL stresses (5 d at 6 to 8°C and 1100 μmol·m−2·s−1 [Havaux et al., 2005]), vte1 was nearly identical to the wild type for all parameters measured, including lipid peroxidation, with the exception of a slight decrease in maximum photosynthetic efficiency (Fv/Fm). Only under extreme conditions (24 h at 3°C and continuous 1500 to 1600 μmol·m−2·s−1) did vte1 show a more rapid induction of lipid peroxidation than the wild type, although this difference was transient, and after 48 h of treatment lipid peroxidation was increased similarly in vte1 and wild-type plants (Havaux et al., 2005). These studies with vte1 and vte2 mutants suggest that a primary function of tocopherols is to control nonenzymatic lipid oxidation, especially during seed storage and early germination, and also probably in photosynthetic tissues, but only under the most extreme of combined HL and low-temperature stresses.
Interestingly, a maize (Zea mays) tocopherol cyclase mutant (sucrose export defective1 [sxd1]) was identified several years before the identification of Arabidopsis vte1, not because of its impact on tocopherol synthesis but because of the accumulation of carbohydrates and anthocyanins in sxd1 source leaves, which coincided with aberrant plasmodesmata between the bundle sheath and vascular parenchyma cells (Russin et al., 1996). Cloning of the SXD1 locus did not provide insight into the biochemical activity of the nucleus-encoded, chloroplast-localized protein (Provencher et al., 2001), and it is only in retrospect that SXD1 has been demonstrated to have tocopherol cyclase activity (Sattler et al., 2003). The maize sxd1 carbohydrate-accumulation phenotype was intriguing in that it suggested an unexpected link between the tocopherol pathway and primary carbohydrate metabolism, although the mechanism involved was unclear. A similar carbohydrate phenotype did not occur in the orthologous Arabidopsis vte1 mutant (Sattler et al., 2003) but was observed in VTE1 RNA interference (RNAi) knockdown lines in potato (Solanum tuberosum) (Hofius et al., 2004).
In this study, we further define and clarify the physiological role(s) of tocopherols in photosynthetic plant tissues by subjecting and analyzing the response of a suite of Arabidopsis tocopherol mutants to a variety of abiotic stresses. We report that in contrast with long-held assumptions about tocopherol functions in plants, tocopherol-deficient mutants are remarkably similar to wild-type plants in their response to most abiotic stresses, with the notable exception of an increased sensitivity to nonfreezing low temperatures. Detailed physiological, biochemical, and ultrastructural data demonstrate that the earliest impact of tocopherol deficiency during low-temperature treatment is an inhibition of photoassimilate transport associated with dramatic structural changes in phloem parenchyma transfer cells, a bottleneck for photoassimilate transport. The resulting accumulation of carbohydrates in source leaves affects the physiology and response of the entire plant to low temperatures.
RESULTS
Tocopherol Biosynthetic (vte) Mutants Used in This Study
vte1-1, vte1-2, and vte2-1 are previously isolated and characterized ethyl methanesulfonate mutants in the Columbia (Col) ecotype that are deficient in the tocopherol cyclase (vte1-1 and vte1-2) and HPT (vte2-1) enzymes (Sattler et al., 2003, 2004) (Figure 1). vte2-2 and vte4-3 are T-DNA insertion mutants in the Wassilewskija (Ws) ecotype in genes encoding HPT and γ-tocopherol methyltransferase, respectively. Leaves of all mutants, vte2-1, vte2-2, vte1-1, vte1-2, and vte4-3, lack α-tocopherol, the major tocopherol in wild-type Arabidopsis leaves (Figure 1, Table 1) (Sattler et al., 2003). vte2-1 and vte2-2 lack all tocopherols and pathway intermediates. vte1-1 and vte1-2 lack all tocopherols but accumulate the biosynthetic pathway intermediate DMPBQ at a level comparable to α-tocopherol in Col. The vte4-3 mutant accumulates γ-tocopherol at an equivalent or slightly higher level than α-tocopherol in Ws. Three- to 5-week-old plants of all mutant genotypes grown under permissive conditions (12 h of 120 μmol·m−2·s−1 light at 22°C and 12 h of darkness at 18°C) were virtually identical to their respective wild-type backgrounds, consistent with previous reports that mutations disrupting tocopherol synthesis have little impact on the normal growth of mature plants (Porfirova et al., 2002; Bergmuller et al., 2003; Sattler et al., 2003, 2004).
Response of Tocopherol-Deficient Mutants to HL Stress
HL stress results in excessive excitation of chlorophyll and consequently generates reactive oxygen species, which in turn attack various biochemical targets in the cell, including PUFA-enriched photosynthetic membranes. Tocopherols are most abundant in these photosynthetic membranes (Bucke, 1968; Lichtenthaler et al., 1981; Soll et al., 1985), and leaf tocopherol levels increase up to 18-fold during HL stress in Arabidopsis (Collakova and DellaPenna, 2003). Therefore, it has been assumed that the elimination of tocopherols from photosynthetic membranes would have a dramatic effect on plant survival during HL stress. To test this hypothesis, Col, vte2-1, vte1-1, and vte1-2 plants were grown for 4 weeks under permissive conditions and then subjected to two levels of HL stress, 1000 and 1800 μmol·m−2·s−1 light for 16 h and 8 h darkness at 22°C (hereafter referred to as HL1000 and HL1800, respectively). HL1000 did not result in differential visible or biochemical phenotypes between vte2-1 and Col (see Supplemental Figure 1 online). When Col, vte2-1, vte1-1, and vte1-2 were subjected to HL1800, which approaches the intensity of full sunlight, this led to bleaching of some mature leaves in all genotypes. vte2-1 had a slight tendency toward more bleached leaves than Col, but this finding was not reproducible or significant, whereas vte1-1 and vte1-2 reproducibly had as many or more bleached mature leaves than Col or vte2-1 (Figure 2A; see Supplemental Figure 2 online). vte2-2 and vte4-3 subjected to HL1800 responded similarly to Ws, the corresponding wild type (data not shown).
Figure 2.
Phenotypic and Photosynthetic Responses of Col and the vte2 and vte1 Mutants to HL Stress.
Plants were grown under permissive conditions for 4 weeks and then transferred to HL stress in the middle of the day. When significance was observed between genotypes (analysis of variance [ANOVA], P < 0.05), pair-wise comparison of least-square means was evaluated; nonsignificant groups are indicated by a, b, or c, with a being the highest group.
(A) Six representative plants after 3 d of HL1800. Bars = 2 cm.
(B) and (C) Individual values of total chlorophyll (B) and carotenoid (C) contents from 19 leaves after 4 d of HL1800.
(D) Individual values of Fv/Fm from 30 leaves after 24 h of HL1800.
To assess changes in photosynthetic pigment and tocopherol levels in response to HL stress, the seventh to ninth oldest leaves were harvested before and after 4 d of HL1800 for HPLC analysis. Before HL1800, all levels were similar between genotypes except for the slightly lower β-carotene content in vte2-1 and vte1-1 relative to Col and the absence of tocopherols in all vte genotypes (see Supplemental Table 1 online). After 4 d of HL1800, the vte mutants generally had lower total and individual chlorophyll levels than Col, but these differences were not significant in all cases after 4 d of HL1800, even with n = 19 (Figure 2B, Table 2; see Supplemental Figure 2 online). Total carotenoids were consistently and significantly lower in Col than in vte1-1 and vte1-2, but not always in vte2-1, after 4 d of HL1800. Neoxanthin and violaxanthin were significantly lower in all vte mutants, whereas lutein was significantly lower only in vte1-1 and vte1-2. Interestingly, zeaxanthin was 70% higher in Col than in vte2-1 but unchanged relative to Col in both vte1 alleles (Table 2).
Table 2.
Contents of Photosynthetic Pigments and Tocopherols of Col and the vte2 and vte1 Mutants after HL1800 Treatment at 22°C
| After HL1800
|
||||
|---|---|---|---|---|
| Pigments and Tocopherols | Col | vte2-1 | vte1-1 | vte1-2 |
| Total tococpherols | 1.58 ± 0.25a | 0 ± 0b | 0 ± 0b | 0 ± 0b |
| Total chlorophylls | 17.28 ± 1.01 | 16.60 ± 1.37 | 16.51 ± 1.84 | 16.21 ± 2.05 |
| Chlorophyll a | 12.25 ± 0.87 | 11.69 ± 1.04 | 11.61 ± 1.38 | 11.52 ± 1.58 |
| Chlorophyll b | 5.02 ± 0.26 | 4.90 ± 0.35 | 4.90 ± 0.51 | 4.69 ± 0.50 |
| Chlorophyll a/chlorophyll b | 2.44 ± 0.16 | 2.38 ± 0.09 | 2.37 ± 0.13 | 2.45 ± 0.14 |
| Total carotenoids | 5.37 ± 0.35a | 4.98 ± 0.36b | 4.85 ± 0.54b | 4.75 ± 0.70b |
| β-Carotene | 0.66 ± 0.08 | 0.64 ± 0.07 | 0.63 ± 0.09 | 0.64 ± 0.11 |
| Lutein | 2.45 ± 0.14a | 2.39 ± 0.15a,b | 2.26 ± 0.22b,c | 2.20 ± 0.27c |
| Neoxanthin | 0.57 ± 0.03a | 0.51 ± 0.04b | 0.51 ± 0.06b | 0.50 ± 0.07b |
| Violaxanthin | 1.01 ± 0.12a | 0.57 ± 0.10c | 0.78 ± 0.17b | 0.77 ± 0.22b |
| Antheraxanthin | 0.39 ± 0.04a,b | 0.39 ± 0.04a | 0.36 ± 0.04b,c | 0.36 ± 0.04c |
| Zeaxanthin | 0.28 ± 0.05b | 0.48 ± 0.06a | 0.30 ± 0.06b | 0.29 ± 0.03b |
| A+Z+V | 1.69 ± 0.13a | 1.44 ± 0.12b | 1.44 ± 0.18b | 1.42 ± 0.26b |
| A+Z/A+Z+V | 0.40 ± 0.05c | 0.61 ± 0.05a | 0.46 ± 0.07b | 0.47 ± 0.07b |
Plants were grown for 4 weeks under permissive growth conditions (120 μmol·m−2·s−1), and pigment contents were analyzed after 4 d of HL (1800 μmol·m−2·s−1) treatment. Data are means ± sd and are expressed as micrograms per square centimeter (n = 19). When significance was observed between genotypes (ANOVA; P < 0.05), pair-wise comparisons of least-square means were evaluated, and nonsignificant groups are indicated by a, b, or c, with a being the highest group. A+Z+V, antheraxanthin + zeaxanthin + violaxanthin.
In vivo chlorophyll a fluorescence was also analyzed to assess photosystem II (PSII) function during HL stress. Typically, when plants are under oxidative stress, PSII is inactivated as a result of enhanced turnover of the D1 protein, a process termed photoinhibition, and Fv/Fm decreases (Bjorkman and Demmig, 1987; Maxwell and Johnson, 2000). Four-week-old Col, vte2-1, vte1-1, and vte1-2 plants grown under permissive conditions had identical Fv/Fm values of between 0.8 and 0.85, typical values for healthy leaves (Bjorkman and Demmig, 1987; Maxwell and Johnson, 2000) (data not shown). After 24 h of HL1800 (8 h of HL1800, 8 h of darkness, and 8 h of HL1800), a few vte2-1 leaves showed a dramatic reduction in Fv/Fm (<0.5), but the majority had values similar to Col, and the average Fv/Fm of vte2-1 was not significantly different from Col, even with n = 30 (Figure 2D; see Supplemental Figure 2 online). By contrast, vte1-1 and vte1-2 both had more leaves with Fv/Fm < 0.5 and average Fv/Fm values that were significantly lower than Col (Figure 2D; see Supplemental Figure 2 online).
These combined results indicate that the elimination of tocopherols in vte2 has surprisingly little impact on the response of the photosynthetic apparatus to HL stress in comparison with Col, with the exception of altered xanthophyll cycle carotenoids. Equally surprising is the fact that although the vte2 and vte1 genotypes are identical with regard to their tocopherol deficiencies, vte1 alleles are slightly more susceptible to HL1800 than vte2. As the primary biochemical difference between these two genotypes is that vte1 mutants accumulate the redox-active intermediate DMPBQ and vte2 mutants do not, the presence of DMPBQ in vte1 may have negative effects on HL stress tolerance in Arabidopsis.
Tocopherol-Deficient Mutants Exhibit a Low-Temperature-Sensitive Phenotype
In searching for a condition that more obviously affects the wild type and tocopherol-deficient mutants in a differential manner, vte2-1 and Col plants were subjected to abiotic stress treatments other than HL, including salinity (100, 150, and 200 mM NaCl), drought, and various low-temperature treatments. Like HL stress, the salinity and drought stress conditions used also did not result in obvious phenotypic differences between vte2-1 and Col (see Supplemental Figure 1 online), and further analyses will be required to determine any consequences of tocopherol deficiency during these stresses. However, when plants were transferred from permissive conditions to nonfreezing low-temperature conditions, both vte2-1 and vte2-2 grew more slowly than their respective wild types, Col and Ws, and their mature leaves changed color to purple (Figure 3). These phenotypic differences were consistently observed in conditions ranging from 3 to 12°C and light intensities from 15 to 200 μmol·m−2·s−1 (data not shown). Differences were most obvious and consistent under 12 h of 75 μmol·m−2·s−1 light and 12 h of darkness at 7.5°C (Figure 3), and this low-temperature regime (hereafter referred to as 7.5°C-treated) was used for all subsequent experiments.
Figure 3.
Visible Phenotypes of vte Mutants during Extended Low-Temperature Treatment.
Plants were grown under permissive conditions for 3 weeks and then subjected to 7.5°C treatment for the indicated time periods.
(A) to (D) Representative plants of 3-week-old wild type (Col and Ws), vte1-1 and vte2-1 (Col background), and vte2-2 and vte4-3 (Ws background) after 0 d (A), 1 month (B), 2 months (C), and 4 months (D) of 7.5°C treatment. Bars = 2 cm.
(E) Representative siliques from Col, vte1-1, and vte2-1 plants after 5 months of low-temperature treatment. Arrows denote aborted seeds in vte1-1 and vte2-1 siliques. Bar = 0.2 cm.
After transfer to 7.5°C, vte2-1 and Col did not differ in time to bolting (53 ± 4 and 51 ± 3 d, respectively) or in the number of leaves produced at the start of bolting (32 ± 3 and 31 ± 2 leaves, respectively), indicating that the process of vernalization was not affected by the lack of tocopherols. However, after prolonged growth at 7.5°C, vte2-1 siliques were shorter, produced significantly fewer seeds per silique and per plant compared with Col, and 35% of the seeds in vte2-1 siliques were aborted compared with <1% in Col siliques (Figure 3E, Table 3). These results indicate that tocopherols play a crucial role in low-temperature adaptation in Arabidopsis.
Table 3.
Yields and Abortion Rates of Seeds Produced during Low-Temperature (7.5°C) Treatment
| Variable | Col | vte2-1 | vte1-1 |
|---|---|---|---|
| Seeds per silique | 31.1 ± 1.6 | 19.5 ± 3.4a | 27.3 ± 2.5b |
| Aborted seeds per silique | 0.1 ± 0.4 | 6.8 ± 2.4a | 2.0 ± 1.0b |
| Percentage of abortion | 0.4 | 34.6a | 7.3b |
| Yield (mg seeds/plant) | 373 ± 77 | 87 ± 27a | 223c |
Data are means ± sd (n = 3 for yields; n = 7 for aborted seeds).
P < 0.01 by Student's t test relative to Col.
P < 0.05 by Student's t test relative to Col.
An average of duplicate plants (225.7 and 219.4 mg seeds/plant).
Subjecting vte1-1 to 7.5°C treatment resulted in a phenotype intermediate between vte2-1 and Col in terms of overall growth, mature leaf color, silique size, number of seeds per silique, percentage of aborted seeds, and seed yield per plant (Figure 3, Table 3). These phenotypes in 7.5°C-treated vte4-3 were virtually indistinguishable from those in wild type Ws (Figures 3A to 3C). These results indicate that during low-temperature adaptation in Arabidopsis, the quinol biosynthetic intermediate DMPBQ partially compensates for the lack of tocopherols in vte1-1, whereas the γ-tocopherol accumulated in vte4-3 leaves can functionally replace α-tocopherol in this regard.
Photooxidative Damage Is Not Associated with the vte2 Low-Temperature Phenotype
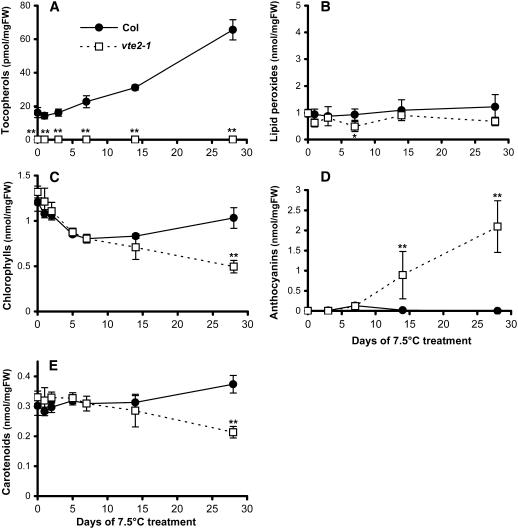
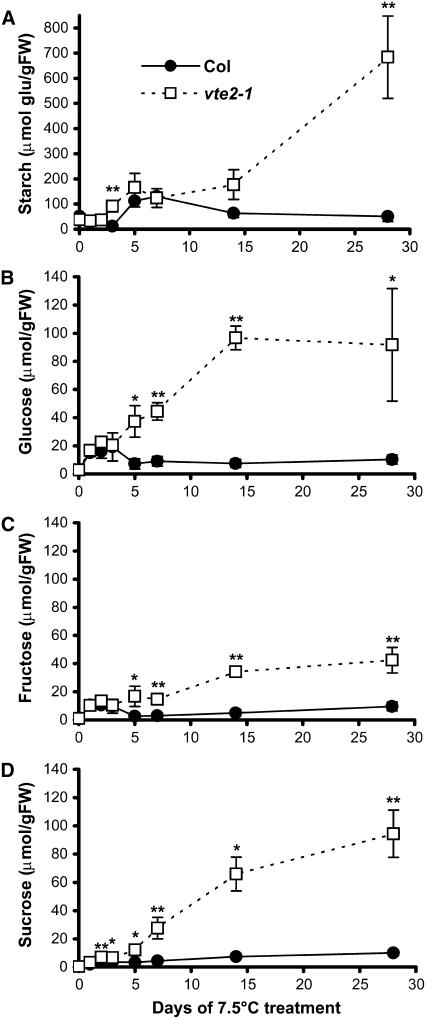
To further examine changes during the time course of 7.5°C treatment, vte2-1 and Col were subjected to detailed comparative biochemical analyses. Plants grown for 4 weeks in permissive conditions were transferred to 7.5°C, and the seventh to ninth oldest fully expanded rosette leaves were harvested at various time points for analysis. The tocopherol content in Col started to increase after 3 d of 7.5°C treatment, reaching levels fivefold higher than the initial levels by 28 d, whereas vte2-1 lacked tocopherols at all time points (Figure 4A).
Figure 4.
Tocopherol, Lipid Peroxide, Anthocyanin, and Photosynthetic Pigment Contents of Col and the vte2 Mutant during 4 Weeks of Low-Temperature Treatment.
Col (closed circles) and vte2-1 (open squares) were grown under permissive conditions for 4 weeks and then transferred to 7.5°C conditions at the beginning of the light cycle for the indicated times. Data are means ± sd (n = 3 or 4). * P < 0.05, ** P < 0.01 by Student's t test of vte2-1 relative to Col at each time point. FW, fresh weight.
(A) Total tocopherols.
(B) Lipid peroxides.
(C) Total chlorophylls.
(D) Anthocyanins.
(E) Total carotenoids.
Consistent with the purple color of mature leaves of 7.5°C-treated vte2 mutants (Figure 3B), vte2-1 accumulated significantly higher levels of anthocyanins than Col after 14 d of 7.5°C (Figure 4D). In Col, anthocyanins were detected only at 7 d. Because anthocyanin accumulation is often associated with plant responses to stress (Leyva et al., 1995; Chalker-Scott, 1999) and tocopherols are well-characterized lipid-soluble antioxidants in animals (Ham and Liebler, 1995, 1997), it seemed plausible that increased lipid peroxidation might be occurring in vte2-1 during low-temperature treatment. However, the lipid peroxide levels of vte2-1 and Col analyzed by the ferrous oxidation xylenol orange assay were found to be similar and near background levels at all time points (Figure 4B), indicating that the observed phenotypic differences between 7.5°C-treated vte2-1 and Col are not associated with a detectable increase in lipid peroxidation.
Given the reported localization of tocopherols and tocopherol biosynthetic enzymes to plastids (Bucke, 1968; Soll et al., 1980, 1985; Lichtenthaler et al., 1981; Soll, 1987), it seems reasonable to hypothesize that tocopherol deficiency might affect the components and function of the photosynthetic apparatus during 7.5°C treatment. Under permissive growth conditions, the levels of individual and total photosynthetic pigments (chlorophylls and carotenoids) were nearly identical in vte2-1 and Col (Figures 4C and 4E; see Supplemental Table 2 online, day 0). The chlorophyll and carotenoid contents of both vte2-1 and Col changed in parallel during the first 2 weeks of 7.5°C treatment and became significantly different only at 28 d (Figures 4C and 4E; see Supplemental Table 2 online). It is especially noteworthy that zeaxanthin, a xanthophyll cycle carotenoid that accumulates under HL stress (Muller et al., 2001) (Table 2), was not detectable at any time point in 7.5°C-treated Col and vte2-1 (see Supplemental Table 2 online), suggesting that the plants were not experiencing photooxidative stress under the low-temperature conditions used.
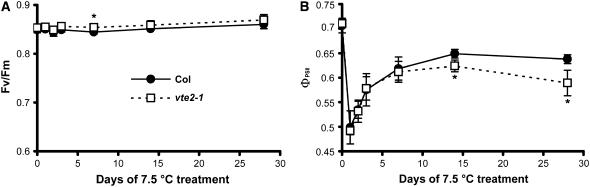
To assess the response of the photosynthetic apparatus to 7.5°C, changes in photosynthetic parameters were analyzed. Fv/Fm was unchanged in both Col and vte2-1 at any time point (Figure 5A), indicating that photoinhibition is not occurring in either genotype during permissive or 7.5°C conditions. The quantum yield of PSII (ΦPSII) was also identical between Col and vte2-1 under permissive growth conditions (Figure 5B, day 0), suggesting that tocopherol deficiency also does not affect the efficiency of electron transport via PSII in the absence of stress (Genty et al., 1989). During the first 7 d of 7.5°C treatment, ΦPSII responded identically in Col and vte2-1: ΦPSII decreased sharply during the first day followed by a gradual recovery by 7 d. However, at 14 d, the vte2-1 ΦPSII was significantly lower than that in Col and declined further by 28 d, whereas the ΦPSII of Col remained stable from day 14 onward (Figure 5B).
Figure 5.
Photosynthetic Status of Col and the vte2 Mutant during 4 Weeks of Low-Temperature Treatment.
Col (closed circles) and vte2-1 (open squares) were grown under permissive conditions for 4 weeks and then transferred to 7.5°C conditions at the beginning of the light cycle for the indicated times. Analysis was conducted in the middle of the light cycle. Data are means ± sd (n = 4). * P < 0.05 by Student's t test of vte2-1 relative to Col at each time point.
(A) Maximum photosynthetic efficiency (Fv/Fm).
(B) Quantum yield of PSII (ΦPSII).
Tocopherol-Deficient Mutants Accumulate Carbohydrates during Low-Temperature Treatment
The reduced ΦPSII in vte2-1 after 14 d could result from feedback inhibition of photosynthesis attributable to the accumulation of downstream carbon metabolites (Goldschmidt and Huber, 1992; Koch, 1996; Paul and Foyer, 2001; Paul and Peliny, 2003). To assess this possibility, starch, glucose, fructose, and sucrose contents were analyzed during the time course of 7.5°C treatment. Starch represents the main plastidic carbohydrate storage pool, sucrose and fructose are cytosolic pools, and glucose is present in both subcellular compartments. Col and vte2-1 had identical carbohydrate contents at the end of the light period under permissive growth conditions (Figure 6, day 0). During the first 7 d of 7.5°C treatment, starch content increased similarly in both Col and vte2-1 to ∼120 μmol glucose equivalents/g fresh weight. After 7 d, vte2-1 starch content increased steadily to 680 μmol glucose equivalents/g fresh weight, whereas Col starch levels decreased to near initial levels (Figure 6A). Likewise, the glucose, fructose, and sucrose contents of Col and vte2-1 increased similarly during the first 3 d of low-temperature treatment (Figures 6B to 6D), likely as a component of the well-documented cold acclimation response(s) in Arabidopsis (Wanner and Junttila, 1999; Taji et al., 2002). After 3 d, Col soluble sugar levels decreased, whereas those in vte2-1 continued to increase, reaching 35, 43, and 255 times the initial levels of glucose, fructose, and sucrose, respectively, after 28 d of low-temperature treatment. The timing of the increase and accumulation of carbohydrates in vte2-1 is consistent with this being the root cause of the reduction in ΦPSII observed after 14 d at 7.5°C (Figure 5B).
Figure 6.
Changes in Starch and Soluble Sugar Levels in Col and the vte2 Mutant during 4 Weeks of Low-Temperature Treatment.
Col (closed circles) and vte2-1 (open squares) were grown under permissive conditions for 4 weeks and then transferred to 7.5°C conditions at the beginning of the light cycle for the indicated times. Samples were harvested at the end of the light cycle. Day 0 of cold treatment indicates the end of the light cycle and the day before initiating 7.5°C treatment. Starch is expressed as micromoles of glucose equivalents per gram fresh weight (FW). Data are means ± sd (n = 3 or 4). * P < 0.05, ** P < 0.01 by Student's t test of vte2-1 relative to Col at each time point.
(A) Starch.
(B) Glucose.
(C) Fructose.
(D) Sucrose.
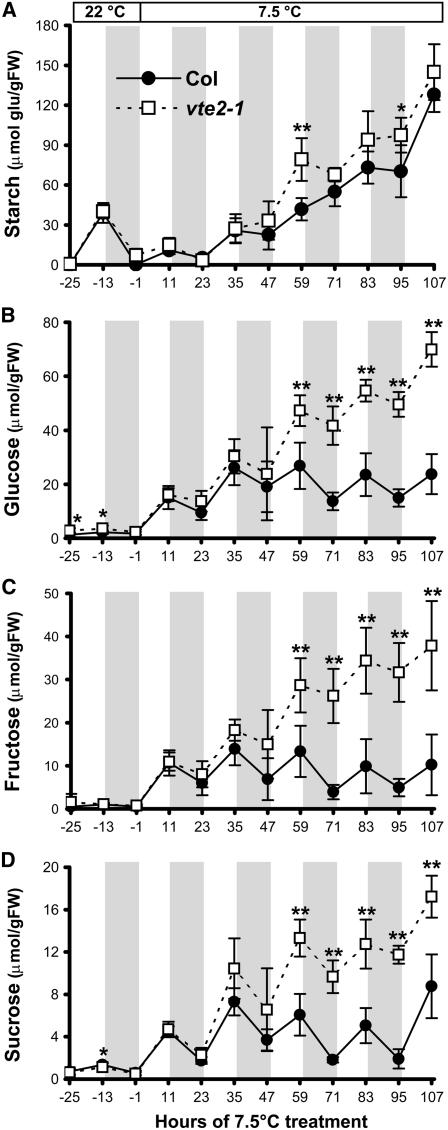
To further investigate any differences in carbohydrate accumulation between vte2-1 and Col during the initial 5 d of low-temperature treatment, diurnal changes in carbohydrate contents were analyzed 1 h before the end of the light and dark cycles. During the 25 h before low-temperature treatment (Figure 7, −25, −13, and −1 h, with 0 h being the transfer time of plants to low temperature at the start of the light cycle), starch, glucose, fructose, and sucrose contents were almost identical in vte2-1and Col. These data indicate that the lack of tocopherols does not have a significant impact on carbohydrate metabolism under permissive growth conditions. After transfer to low temperature, the soluble sugar content increased similarly in vte2-1 and Col for the first two diurnal cycles, with significant differences first being observed between genotypes at the end of the third low-temperature light period (Figures 7B to 7D, 59 h). By contrast, starch levels did not become significantly different between genotypes until 14 d of low-temperature treatment (Figures 6A and 7A). The differential increase of soluble sugars before starch accumulation in vte2-1 indicates that the increase in cytosolic soluble sugars precedes starch accumulation in the chloroplast and that soluble sugars are not being efficiently metabolized or mobilized in 7.5°C-treated vte2-1.
Figure 7.
Diurnal Changes in Starch and Soluble Sugar Levels in Col and the vte2 Mutant during the First 4 d of Low-Temperature Treatment.
Col (closed circles) and vte2-1 (open squares) were grown under permissive conditions for 4 weeks and then transferred to 7.5°C conditions at the beginning of the light cycle for the indicated times. Samples were harvested at the end of the dark and light cycles. Gray areas indicate the 12-h dark cycles. Hour 0 of cold treatment indicates the beginning of the first light cycle of the low-temperature treatment. Starch is expressed as micromoles of glucose equivalents per gram fresh weight (FW). Data are means ± sd (n = 5). * P < 0.05, ** P < 0.01 by Student's t test of vte2-1 relative to Col at each time point.
(A) Starch.
(B) Glucose.
(C) Fructose.
(D) Sucrose.
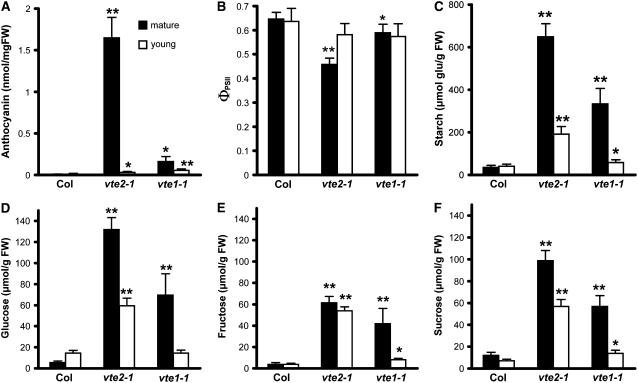
The vte2 and vte1 Cold-Sensitive Phenotypes Are Attenuated in Young Leaves
Mature (7th to 9th oldest) and young (13th to 16th oldest) leaves of vte2-1 and vte1-1 mutants showed obvious visible differences in their responses to low temperature; young leaves of vte2-1 and vte1-1 did not change their color to purple even after 2 months of 7.5°C treatment (Figure 3C). Consistent with these visual observations, mature leaves of vte1-1 had an anthocyanin content 10% that of vte2-1 but still higher than that of Col, whereas young vte2-1 and vte1-1 leaves accumulated many fewer anthocyanins compared with their respective mature leaves after 28 d of 7.5°C treatment (Figure 8A). Fv/Fm was >0.8 in all cases, indicating that photoinhibition was not occurring in either young or mature leaves of any genotype (data not shown). The ΦPSII of mature vte2-1 leaves was reduced to 70% of that of mature Col leaves, consistent with Figure 5, whereas the ΦPSII of mature vte1-1 leaves was only slightly decreased relative to mature Col leaves. However, the ΦPSII of mature and young Col leaves and young vte2-1 and vte1-1 leaves was not significantly different (Figure 8B). Levels of all carbohydrates in mature vte2-1 leaves were greatly increased compared with Col, consistent with Figure 6. Mature vte1-1 leaves contained intermediate levels of starch, glucose, fructose, and sucrose (51, 53, 68, and 58% of vte2-1 levels, respectively) (Figures 8C to 8F). Young vte2-1 and vte1-1 leaves contained substantially reduced starch, glucose, and sucrose levels compared with their respective mature leaves. These results indicate that the initiation and development of young vte2-1 and vte1-1 leaves under 7.5°C conditions attenuates the biochemical phenotypes observed in mature leaves of both genotypes and that the DMPBQ accumulated in vte1-1 further suppresses these biochemical phenotypes in both mature and young leaves.
Figure 8.
Biochemical Phenotypes in Mature and Young Leaves of Col and the vte2 and vte1 Mutants after 4 Weeks of Low-Temperature Treatment.
Col, vte2-1, and vte1-1 were grown under permissive conditions for 4 weeks and then transferred to 7.5°C conditions at the beginning of the light cycle for an additional 4 weeks. Mature leaves (7th to 9th oldest; black bars) and young leaves (13th to 16th oldest; white bars) were harvested at the end of the light cycle for analyses in (A) and (C) to (F). The photosynthetic parameter in (B) was measured in the middle of the light cycle. Data are means ± sd (n = 4 or 5). * P < 0.05, ** P < 0.01 by Student's t test of mutant leaves relative to corresponding Col young or mature leaves. FW, fresh weight.
(A) Anthocyanin content.
(B) Quantum yield of PSII (ΦPSII).
(C) Starch content expressed as micromoles of glucose equivalents per gram fresh weight.
(D) to (F) Glucose (D), fructose (E), and sucrose (F) contents.
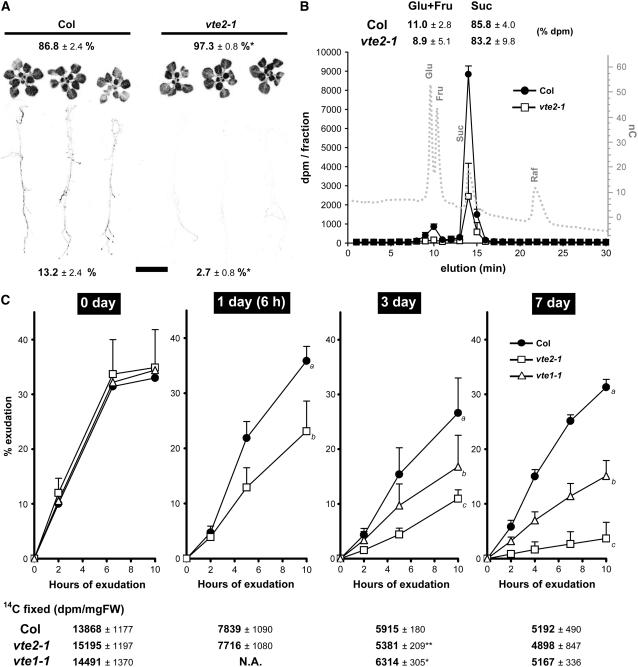
Tocopherol-Deficient Mutants Have Reduced Source Leaf Photoassimilate Export Capacity at Low Temperatures
The reduced seed yield (Table 3) and attenuated carbohydrate accumulation in young leaves relative to mature leaves (Figure 8) in 7.5°C-treated vte2-1 suggested an impaired translocation of photoassimilates from mature source tissues to young sink tissues. To test this possibility, 14CO2 labeling experiments were conducted. Col and vte2-1 were grown on plates under permissive conditions for 3 weeks and then transferred to 7.5°C for an additional 7 d. Whole plants were labeled with 14CO2 at 7.5°C, transferred to high humidity in darkness at 7.5°C for 2 h to allow for photoassimilate transport, and subsequently exposed to a phosphor screen to visualize the movement of 14C-labeled photoassimilate. Immediately after labeling, >99% of the 14CO2 incorporated was present in leaf tissue (data not shown). Col and vte2-1 incorporated similar amounts of 14CO2 into photosynthate, suggesting that their carbon fixation rates do not differ, consistent with the similar ΦPSII within the first 7 d at 7.5°C (Figure 5B; data not shown). After the 2-h dark period, Col had translocated 13.2% of the 14C-labeled photoassimilate fixed in leaves to roots, whereas only 2.7% was translocated in vte2-1 (Figure 9A). These results demonstrate that vte2-1 translocates significantly less photoassimilate from source to sink than Col after 7 d of 7.5°C treatment.
Figure 9.
Translocation and Export of 14C-Labeled Photoassimilates in Low-Temperature-Treated Col and the vte2 and vte1 Mutants.
(A) 14C-labeled photoassimilate translocation of Col and vte2-1 treated for 7 d at 7.5°C. Percentage label detected in leaves (top) and roots (bottom) is indicated as means ± sd (n = 3). * P < 0.05 by Student's t test relative to Col.
(B) HPLC analysis of phloem exudates collected from mature leaves of Col and vte2-1 treated for 10 d at 7.5°C. The HPLC trace of sugar standards is shown as a dotted gray line. The percentage of label detected in the glucose/fructose and sucrose fractions is indicated as means ± sd (n = 3). Fru, fructose; Glu, glucose; Raf, raffinose; Suc, sucrose.
(C) Phloem exudation of 14C-labeled photoassimilates from Col and vte2-1 and vte1-1 mature leaves during 7 d of 7.5°C treatment. Total 14C fixed per milligram fresh weight (FW) of each sample at the indicated time after transfer to 7.5°C is shown below each graph. Data are means ± sd (n = 6 to 8). Two-factor ANOVA using end points (values at 10 h of exudation) indicates that interactions are significant (P < 0.05, with days of 7.5°C treatment and genotype as factors). The pair-wise comparisons of least-square means between genotypes at 1, 3, and 7 d of 7.5°C treatment are indicated as a, b, or c; day-0 values were not significant. N.A., data not available.
Impaired photoassimilate translocation in 7.5°C-treated vte2-1 could be attributable to reduced sink strength or impaired photoassimilate export from source leaves (Stitt, 1996; Herbers and Sonnewald, 1998; Gottwald et al., 2000). To address these possibilities, phloem exudation experiments were conducted (King and Zeevaart, 1974). Col and vte2-1 were grown for 4 weeks under permissive conditions and transferred to 7.5°C for an additional 0, 1, 3, or 7 d. Mature (seventh to ninth oldest) leaves were excised from plants and labeled with 14CO2. The petioles of labeled leaves were then transferred to an EDTA solution to induce phloem exudation, and radioactivity in the EDTA solution was determined at various time points (King and Zeevaart, 1974). Again, total 14CO2 fixed in mature leaves was similar in all genotypes at each time point (Figure 9C). Before 7.5°C treatment (day 0), Col, vte1-1, and vte2-1 leaves exuded similar amounts of labeled photoassimilates, accounting for ∼34% of the total 14CO2 fixed in each genotype. During 7.5°C treatment, the percentage exudation by Col decreased slightly after 3 and 7 d (to 27 and 31% of the total 14CO2 fixed, respectively), whereas that of vte2-1 was greatly reduced (to 11 and 4% at 3 and 7 d, respectively). Even more intriguingly, exudation in vte2-1 was significantly lower than that in Col during the first day of 7.5°C treatment, which corresponds to only 6 h of 7.5°C treatment. The vte1-1 mutant exuded 17 and 15% of the total 14CO2 fixed after 3 and 7 d at 7.5°C, respectively, levels intermediate between Col and vte2-1 (Figure 9C).
In apoplastic loaders such as Arabidopsis, sucrose is almost the exclusive translocated photoassimilate (Vanbel, 1993). To assess the chemical nature of the labeled compounds exuded from Col and vte2-1, phloem exudates were collected and separated by anion-exchange chromatography together with sugar standards. As shown in Figure 9B, ∼85% of the label in Col and vte2-1 exudates comigrated with the sucrose standard and 10% with glucose/fructose standards. The high proportion of sucrose indicates that the label collected is almost entirely from phloem exudate rather than sugars from the cytosol of damaged cells.
Overall, the results obtained from 14CO2 labeling experiments indicate that tocopherol deficiency in both vte1-1 and vte2-1 results in a dramatically reduced capacity of photoassimilate export from source leaves in response to 7.5°C treatment. The rapidity of the reduction in photoassimilate export in 7.5°C-treated vte2-1 strongly suggests that impairment of photoassimilate export is the root cause of the sugar accumulation phenotype observed in mature leaves of 7.5°C-treated tocopherol-deficient mutants.
Structural Changes in Low-Temperature-Treated Tocopherol-Deficient Mutants
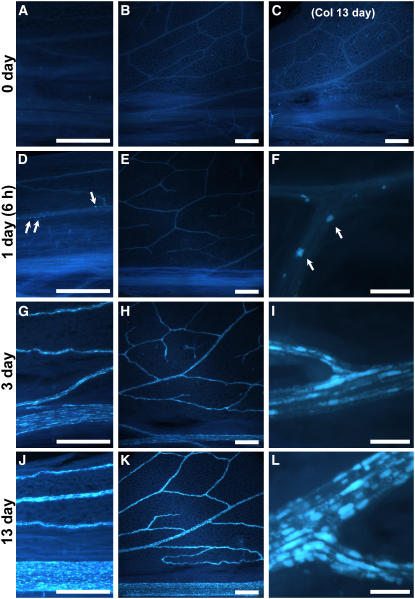
Previously, callose was reported to accumulate at the bundle sheath/vascular parenchyma interface of the maize sxd1 mutant and in vascular tissue of potato VTE1-RNAi lines, both of which are defective in tocopherol cyclase (Botha et al., 2000; Hofius et al., 2004). To determine whether callose deposition also occurs in Col, vte2-1, and vte1-1, leaves were harvested at 0, 1, 3, and 13 d of 7.5°C treatment and aniline blue–positive fluorescence was assessed. Under permissive conditions, aniline blue–positive fluorescence was absent or sporadic and no significant differences were observed in any genotypes. Aniline blue–positive fluorescence also was not altered in Col during the entire 7.5°C treatment period (e.g., 13 d at 7.5°C) (Figure 10C). By contrast, aniline blue–positive fluorescence strongly increased in the vascular tissue of 7.5°C-treated vte2-1 (Figure 10) and to a slightly lesser extent in vte1-1 (see Supplemental Figure 3 online). In both vte2-1 and vte1-1, fluorescence initially appeared in a limited number of vascular cells in the petiole as early as 6 h after transfer to 7.5°C conditions (Figures 10D and 10F; see Supplemental Figures 3A and 3B online). The number of aniline blue–fluorescing cells in the vasculature and their fluorescence intensity subsequently increased in an acropetal manner in both vte2-1 and vte1-1 during the course of 7.5°C treatment (Figure 10; see Supplemental Figure 3 online). Intriguingly, the induction, intensity, and acropetal spread of vasculature-specific aniline blue–positive fluorescence in vte2-1 at 7.5°C was unaffected by light levels ranging from 1 to 800 μmol·m−2·s−1 (Figures 11A to 11F). Aniline blue–positive fluorescence was not observed in the vasculature of Col at any light level at 7.5°C (data not shown) and was also absent from the vasculature of both Col and vte2-1 subjected to HL1800 at 22°C for up to 4 d (Figures 11G and 11H; data not shown).
Figure 10.
Aniline Blue–Positive Fluorescence in Leaves of Col and the vte2 Mutant during Low-Temperature Treatment.
Col (C) and vte2-1 (all panels except [C]) were grown under permissive conditions for 4 weeks and then transferred to 7.5°C at the beginning of the light cycle. Leaves were harvested in the middle of the day before 7.5°C treatment (0 d; [A] and [B]) and after 1 d (6 h; [D] to [F]), 3 d ([G] to [I]), and 13 d ([C] and [J] to [L]) of 7.5°C treatment, and aniline blue–positive fluorescence were observed at leaf petioles ([A], [D], [G], and [J]), the lower half of leaves ([B], [C], [E], [H], and [K]), and vein junctions ([F], [I], and [L]). Arrows in (D) and (F) denote highly fluorescent spots that initially appear in side veins of vte2-1 petioles after 6 h of 7.5°C treatment. Bars = 50 μm for (F), (I), and (L) and 500 μm for all other panels.
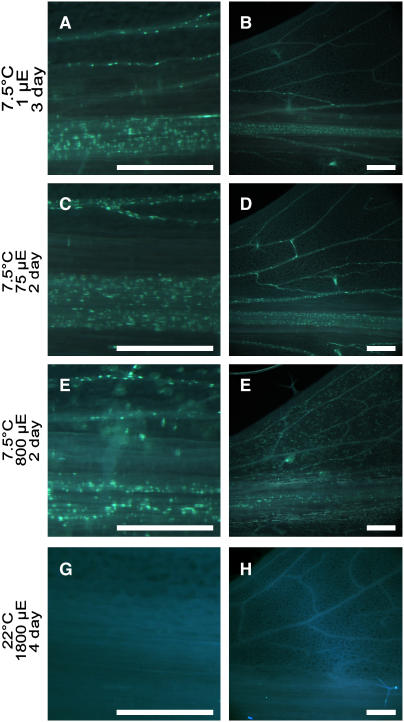
Figure 11.
Aniline Blue–Positive Fluorescence in Leaves of the vte2 Mutant at Various Light Intensities under Permissive and Low-Temperature Conditions.
vte2-1 was grown under permissive conditions for 4 weeks and then transferred at the beginning of the light cycle to 12 h of light and 12 h of darkness at the indicated light levels at 7.5°C ([A] to [F]) and in the middle of the day to HL1800 at 22°C ([G] and [H]). Leaves were harvested in the middle of the day. Aniline blue–positive fluorescence was observed at leaf petioles ([A], [C], [E], and [G]) and the lower half of leaves ([B], [D], [F], and [H]). Bars = 500 μm.
(A) and (B) 7.5°C at 1 μmol·m−2·s−1 for 3 d (54 h).
(C) and (D) 7.5°C at 75 μmol·m−2·s−1 for 2 d (30 h).
(E) and (F) 7.5°C at 800 μmol·m−2·s−1 for 2 d (30 h).
(G) and (H) 22°C at 1800 μmol·m−2·s−1 for 4 d (72 h).
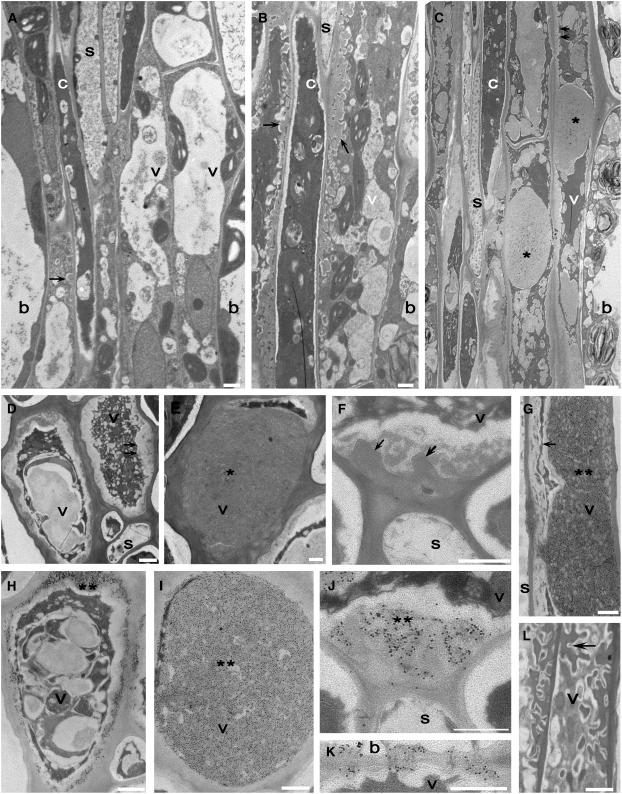
To confirm whether or not aniline blue–positive fluorescence was cell-specific and could be attributed to callose deposition, serial sections of 0- and 14-d 7.5°C-treated Col and vte2-1 vascular tissue were examined at the level of the transmission electron microscope. The spatial organization of cells and types of cells constituting the phloem and xylem of both Col and vte2-1 were identical to what has been described previously for Arabidopsis (Haritatos et al., 2000) (data not shown). Notably, at day 0, phloem vascular parenchyma cells of both Col and vte2-1 contained transfer cell wall ingrowths adjacent to sieve elements and companion cells (Figure 12A). Treatment of Col at 7.5°C for 14 d did not result in obvious ultrastructural changes in any vascular cell type except for a noticeable increase in phloem parenchyma transfer cell differentiation and transfer cell wall deposition exclusively adjacent to sieve elements and companion cells in all vascular tissues (Figure 12B), although to a lesser degree in the midvein.
Figure 12.
Cellular Structure and Immunodetection of Callose in Col and vte2-1 before and after 14 d of Low-Temperature Treatment.
(A) vte2-1 before 7.5°C treatment.
(B) to (L) vte2-1 ([C] to [K]) and Col ([B] and [L]) after 14 d of 7.5°C treatment.
(G) to (L) are immunolabeled with anti-β-1,3-glucan antibody. (D) to (F) are controls with only secondary antibody. Single arrows denote phloem parenchyma transfer cell wall ingrowths. Double arrows denote abnormal thickening of phloem parenchyma transfer cell wall ingrowths. Single asterisks mark massive wall ingrowths of phloem parenchyma transfer cells. Double asterisks mark wall ingrowths immunolabeled with anti-β-1,3-glucan. Paradermal ([C] and [G]) and transverse ([E] and [I]) sections show the entire phloem parenchyma transfer cell occluded with callose. Paradermal ([C] and [G]) and transverse ([D] and [H]) sections show the peripheral callose sheath of phloem parenchyma transfer cells. Note the callose at the boundary between the phloem parenchyma transfer cell and the sieve element ([F] and [J]). Plasmodesmata between the bundle sheath (top cell) and the phloem parenchyma transfer cell immunolabeled with anti-β-1,3-glucan are shown in (K). b, bundle sheath; c, companion cell; s, sieve element; v, vascular parenchyma transfer cell. Bars = 1 μm (all panels except [C]) and 5 μm (C).
During the same time course of 7.5°C treatment in vte2-1, changes in cell fine structure occurred exclusively within the phloem parenchyma transfer cells of all vascular traces. Phloem parenchyma transfer cells in 14-d-treated vte2-1 exhibited irregularly thickened cell wall depositions with ultrastructural features characteristic of callose (Nishimura et al., 2003) (Figures 12C to 12F). Large callose-like masses that dissected the cell lumen corresponded in shape to aniline blue–positive fluorescent regions (cf. Figures 12C and 12E with Figures 10F, 10I, and 10L). The callose-like wall material also formed a sheath around the cells (Figure 12D) and was deposited over transfer cell wall ingrowths (Figure 12F) and between the end walls of adjoining transfer cells, including plasmodesmata (data not shown). Immunolocalization using monoclonal antibodies against callose confirmed the presence of callose at each location (Figures 12G to 12J) and at plasmodesmata between the phloem parenchyma transfer cells and the bundle sheath (Figure 12K). No immunolabeling was present in controls using secondary antibody only (Figures 12D to 12F), and immunolabeling was rare to absent in all cell types of untreated Col and vte2-1 and in 14-d, 7.5°C-treated Col, including phloem parenchyma transfer cells (Figure 12L).
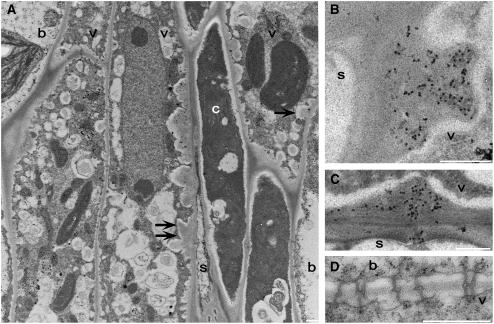
Serial sections of vascular tissue from Col and vte2-1 treated at 7.5°C for 3 and 7 d were subsequently examined at the level of the transmission electron microscope to determine the spatial and temporal development of callose deposition within phloem parenchyma cells. At 3 d, phloem parenchyma transfer cell wall deposition in Col was confined to the sieve element or companion cell boundary (data not shown), but in vte2-1, wall deposition was present around the entire transfer cell periphery (Figure 13A). Cell wall deposition in 3-d-treated vte2-1 resulted in abnormally thickened and irregular shaped ingrowths with callose-like depositions adjacent to sieve elements and companion cells (Figure 13A) that grew increasingly prominent by day 7 (data not shown). In 3-d, 7.5°C-treated vte2-1, positive immunolocalization with monoclonal antibodies to callose was present exclusively at the phloem parenchyma transfer cell wall/sieve element boundary (Figure 13B) and included phloem parenchyma transfer cell–sieve element plasmodesmata connections (Figure 13C). In contrast with 14 d, plasmodesmata between bundle sheath and phloem vascular parenchyma cells in 3-d, 7.5°C-treated vte2-1 were continuous and immunonegative for callose (cf. Figures 12K and 13D).
Figure 13.
Cellular Structure and Immunodetection of Callose in vte2-1 after 3 d of Low-Temperature Treatment.
(A) Single arrows denote phloem parenchyma transfer cell wall ingrowths adjacent to the bundle sheath. Double arrows denote abnormal thickening of phloem parenchyma transfer cell wall ingrowths adjacent to the companion cell. Note that transfer cell wall ingrowths are present around the entire phloem parenchyma transfer cell.
(B) to (D) are immunolabeled with anti-β-1,3-glucan antibody.
(B) Transverse section of wall ingrowths immunolabeled with anti-β-1,3-glucan at the phloem parenchyma transfer cell and sieve element boundary.
(C) Plasmodesmata between the phloem parenchyma transfer cell (top cell) and the sieve element are immunopositive for anti-β-1,3-glucan.
(D) Plasmodesmata between the bundle sheath (top cell) and the phloem parenchyma cell are continuous, lack callose-like wall depositions, and are immunonegative for anti-β-1,3-glucan.
b, bundle sheath; c, companion cell; s, sieve element; v, vascular parenchyma transfer cell. Bars = 0.5 μm.
DISCUSSION
The chemistry of tocopherols as lipid-soluble antioxidants and terminators of PUFA free radical chain reactions has been well established from analyses in artificial membranes and animal-derived membrane systems (Liebler and Burr, 1992; Ham and Liebler, 1995). It has long been assumed that similar chemistry occurs in the tocopherol-containing PUFA-enriched membranes of photosynthetic organisms, and this assumption has recently been supported by studies of tocopherol-deficient photosynthetic organisms (Sattler et al., 2004; Havaux et al., 2005; Maeda et al., 2005). During the first several days of germination, a period of high oxidative metabolism, Arabidopsis vte2 seedlings contain levels of oxidized lipids >100-fold higher than wild-type or vte1 seedlings, which were indistinguishable (Sattler et al., 2004; S.E. Sattler, L. Mene-Saffrane, E.E. Farmer, M. Krischke, M.J. Mueller, and D. DellaPenna, unpublished data). Thus, a chemical role for tocopherols (or DMPBQ in vte1) in limiting lipid peroxidation appears to be conserved between photosynthetic organisms and animals. By 18 d of growth, the lipid peroxide levels of vte2 seedlings had decreased to near wild-type and vte1 levels (Sattler et al., 2004), and they became indistinguishable from those of the wild type and vte1 after 4 weeks of growth (Figure 4B), consistent with tocopherols being dispensable in mature photosynthetic tissues in the absence of stress (Porfirova et al., 2002; Sattler et al., 2003, 2004).
Although a chemical role for tocopherols in controlling lipid peroxidation at specific points of the plant life cycle now seems clear, the physiological roles of tocopherols during plant stresses do not. In this study, we used a suite of Arabidopsis vte mutants that accumulate different types and levels of tocopherols and pathway intermediates (Table 1) to directly assess tocopherol-specific functions in photosynthetic tissues in planta in response to abiotic stress treatments.
A Limited Role for Tocopherols in Protecting Arabidopsis Plants from HL Stress
Tocopherols have long been assumed to play crucial roles in HL protection, presumably by acting as singlet oxygen quenchers and lipid peroxy radical scavengers (Fryer, 1992; Munne-Bosch and Alegre, 2002; Trebst et al., 2002). In this study, the biochemical and photosynthetic responses of the tocopherol-deficient vte2 mutant exposed to HL1000 and HL1800 at 22°C for up to 11 d were surprisingly similar to those of the wild type (Figure 2; see Supplemental Figures 1 and 2 online). Likewise, the mutation corresponding to Arabidopsis vte2 in the cyanobacterium Synechocystis sp PCC6803 (slr1736) had a similarly limited impact on growth and photosynthesis under permissive growth conditions and during HL stress (Collakova and DellaPenna, 2001; Maeda et al., 2005). In both organisms, it was only when HL stress was combined with other stresses, with lipid peroxidation–inducing chemicals in the Synechocystis slr1736 mutant (Maeda et al., 2005) or with low temperatures (2 to 3°C) in Arabidopsis vte2 and vte1 mutants, that differential effects on photosynthetic parameters or lipid oxidation were observed (Havaux et al., 2005). These combined data indicate that tocopherols are not essential for the adaptation and tolerance of photosynthetic tissues subjected to HL stress alone. Such a conclusion runs counter to long-held assumptions that a primary function of tocopherols is to protect photosynthetic tissues against HL stress (Fryer, 1992; Munne-Bosch and Alegre, 2002).
One possible explanation for this surprisingly limited role of tocopherols during HL stress is that other mechanisms compensate for their absence. The zeaxanthin level of HL1800 vte2 was nearly twice that of Col (Table 2). vte2 (and vte1) also had a higher xanthophyll deepoxidation state (A+Z/A+Z+V) after HL1800 treatment (see Table 2 for definitions), as did vte1 during HL stress combined with low or high temperatures (Havaux et al., 2005). Growth of a tocopherol-deficient Synechocystis mutant was also much more susceptible than that of the wild type to treatment with a biosynthetic inhibitor of carotenoid synthesis during HL stress (Maeda et al., 2005). Similarly, a double mutant of vte1 and npq1 (for nonphotochemical quenching1), which cannot accumulate zeaxanthin in response to HL and hence cannot induce nonphotochemical quenching (Niyogi et al., 1998), was reportedly more susceptible than either single mutant to the combination of HL and low-temperature stresses (Havaux et al., 2005). Conversely, the young npq1 leaves were also tolerant of short- and long-term HL stress (up to HL1800) and accumulated higher levels of tocopherols than did wild-type leaves (Havaux et al., 2000; Golan et al., 2006). These data suggest that tocopherols and carotenoids, particularly zeaxanthin, have overlapping functions in protecting photosynthetic organisms against HL stress.
In previous studies, it was demonstrated that, although vte1 and vte2 are both tocopherol-deficient, the two genotypes behave quite differently during early seedling development: vte2 exhibited a >100 fold increase in nonenzymatic lipid peroxidation during germination, whereas lipid peroxidation in vte1 was identical to that in the wild type (Sattler et al., 2004; S.E. Sattler, L. Mene-Saffrane, E.E. Farmer, M. Krischke, M.J. Mueller, and D. DellaPenna, unpublished data). In this study, vte1 was again found to behave differently from vte2. In response to HL1800 stress, vte1 had a slightly, but reproducibly, higher degree of photoinhibition and a higher level of photobleaching than either vte2 or the wild type (Figure 2, Table 2; see Supplemental Figure 2 online), suggesting that vte1 negatively affects HL stress tolerance beyond its tocopherol deficiency. Why would vte1 respond so differently from vte2 during germination and HL1800, given that both mutant genotypes are tocopherol-deficient? The most likely explanation lies in the singularly unique biochemical feature of vte1: it accumulates the redox-active quinol biosynthetic intermediate DMPBQ in place of tocopherols. DMPBQ is absent from vte2 and Col (Table 1) (Sattler et al., 2003), and its presence in vte1 can clearly have significant, unintended experimental consequences that are independent of the tocopherol deficiency in vte1. Thus, when attempting to define tocopherol functions based on vte mutant phenotypes, one must be careful to delineate genuine tocopherol functions, which would occur in both vte1 and vte2, from potentially confounding artifacts attributable to the presence of DMPBQ, which would occur only in vte1. Such DMPBQ-dependent artifacts can have negative (HL1800), positive (seedling germination), or partially positive (low-temperature adaptation) consequences depending on the treatment condition and phenotype assessed. These concerns are not relevant for vte2.
Arabidopsis Tocopherol-Deficient Mutants Exhibit a Cold-Sensitive Phenotype Independent of Photooxidative Damage
In contrast with the equivocal results of HL, salinity, and drought stress treatments with tocopherol-deficient photosynthetic organisms (Figure 2; see Supplemental Figures 1 and 2 online) (Porfirova et al., 2002; Havaux et al., 2005; Maeda et al., 2005), both tocopherol-deficient vte1 and vte2 genotypes were found susceptible to nonfreezing low-temperature treatments compared with their respective wild types (Figure 3). These results clearly indicate that tocopherols play a critical role in the responses of mature Arabidopsis plants to nonfreezing low temperatures. Given the well-defined role of tocopherols as lipid-soluble antioxidants, we initially hypothesized that tocopherol deficiency at low temperature would result in increased photooxidative damage relative to the wild type and that this might lead to the low-temperature-sensitive phenotype observed in vte2. However, the hallmarks of photooxidative stress and photoinhibition, decreased Fv/Fm and chlorophyll levels and increased zeaxanthin accumulation and lipid peroxidation (Havaux and Niyogi, 1999; Maxwell and Johnson, 2000; Broin and Rey, 2003), were not observed during the first 2 weeks of low-temperature treatment (Figures 4B, 4C, and 5A; see Supplemental Table 2 online), the time frame during which the vte2 carbohydrate-accumulation phenotype fully develops (Figures 6 and 7). Likewise, ΦPSII, although altered by low temperature, was identical in the wild type and vte2 during the first week of low-temperature treatment (Figure 5B). These data indicate that the tocopherol deficiency has no discernible impact on photosynthesis under the low-temperature conditions used and that the low-temperature-sensitive phenotype of vte2 is not associated with increased photooxidative damage or photoinhibition resulting from the absence of tocopherols.
Tocopherols Are Required for Photoassimilate Export from Source Leaves during Low-Temperature Adaptation
A well-documented response of plants to low temperatures is the accumulation of soluble sugars and other osmoprotectants, which are critical components for the process of cold acclimation leading to freezing tolerance (Wanner and Junttila, 1999; Gilmour et al., 2000). The subsequent recovery of photosynthesis and sucrose metabolism is an important component of low-temperature adaptation in that it provides carbon to sustain growth under low temperatures (Strand et al., 1997, 1999). During the first 2 d of low-temperature treatment, soluble sugar levels increased similarly in both vte2 and the wild type (Figure 7), suggesting that tocopherols have little impact on the initial accumulation of soluble sugars in response to low temperature. However, the accumulation of sucrose and other soluble sugars was much greater in vte2 than in the wild type after 60 h of low-temperature treatment (Figures 7B to 7D), although the rates of photosynthesis and carbon fixation were indistinguishable between the two genotypes until 14 d at low temperature (Figures 4B and 9C). vte2 also reduced soluble sugar levels more slowly at night than did the wild type after 3 d of low-temperature treatment (Figures 7B to 7D). These results suggest that tocopherol deficiency affects carbohydrate use/mobilization rather than the supply of fixed carbon from photosynthesis during low-temperature adaptation.
14CO2-labeling experiments demonstrated that compared with the wild type, low-temperature-treated vte2 translocated significantly less 14C-labeled photoassimilate from leaves (source tissue) to roots (sink tissue) (Figure 9A). The long-distance transport of photoassimilates occurs through phloem, and the transport rate is determined either by the rate of export from source leaves to phloem (loading) or by removal into sink tissues (unloading) (Vanbel, 1993; Stitt, 1996; Herbers and Sonnewald, 1998). Phloem exudation experiments with excised leaves showed that vte2 source leaves exported significantly less 14C-labeled photoassimilate than did the wild type as early as 6 h after transfer to low temperature (Figure 9C). The rapidity of this reduction in photoassimilate export compared with the increased sugar accumulation in vte2 starting at 60 h (cf. Figures 7 and 9) indicates that impaired photoassimilate export is an early, upstream event in the vte2 low-temperature phenotype and the likely root cause of the increased sugar accumulation in low-temperature-treated vte2. Together, these analyses demonstrate that tocopherols are required for the proper regulation of photoassimilate export from source leaves and thereby play a critical role in low-temperature adaptation in Arabidopsis.
Previous studies of the maize sxd1 mutant and potato VTE1-RNAi lines (both affecting tocopherol cyclase activity) had suggested a linkage between carbohydrate metabolism and tocopherol biosynthesis, as in both cases carbohydrates accumulated to high levels in mature leaves at normal growth temperatures (19 to 30°C) (Russin et al., 1996; Provencher et al., 2001; Hofius et al., 2004). The absence of this phenotype in Arabidopsis vte1 and vte2 at 22°C raised questions regarding the universality of any interaction between tocopherol synthesis and carbohydrate metabolism (Sattler et al., 2003) (Figures 6 and 7, day 0). We now know that tocopherol-deficient Arabidopsis mutants do indeed exhibit a phenotype that is analogous to sxd1 but that it is inducible only at low temperatures. Thus, the linkage between tocopherol biosynthesis and carbohydrate metabolism is conserved among all tocopherol-deficient mutants identified in higher plants to date (maize sxd1, potato VTE1-RNAi, and low-temperature-treated Arabidopsis vte1 and vte2).
Although the maize sxd1 mutant and the potato VTE1-RNAi line suggested that tocopherol chromanol ring cyclization was somehow related to the regulation of carbohydrate metabolism, it was unclear whether the phenotype was attributable to the lack of tocopherols or to the accumulation of the redox-active quinol intermediate DMPBQ (Sattler et al., 2003; Hofius et al., 2004). Analysis of the full suite of Arabidopsis vte mutants now allows a conclusive answer to this question. Given that vte2 lacks DMPBQ (Table 1) and exhibits a more severe carbohydrate-accumulation phenotype than vte1 (Figure 8), we can conclude that it is the absence of tocopherols, rather than the accumulation of DMPBQ, that causes the carbohydrate-accumulation phenotype. The reduced severity of the carbohydrate-accumulation phenotype in vte1 suggests that DMPBQ partially suppresses the low temperature-inducible vte2 carbohydrate-accumulation phenotype.
Tocopherol Deficiency Results in a Cell-Specific Response by Phloem Parenchyma Transfer Cells at Low Temperature
The carbohydrate-accumulation phenotype of maize sxd1 was reported to be associated with altered structural features within vascular tissue. Plasmodesmata at the sxd1 bundle sheath/vascular parenchyma boundary were reportedly occluded by wall materials (Russin et al., 1996; Provencher et al., 2001) and subsequently suggested to correspond to aniline blue–positive fluorescence (Botha et al., 2000). This structural aberration in sxd1 plasmodesmata was posited to be the basis of the sxd1 carbohydrate-accumulation phenotype, because it would lead to a block in the symplastic movement of photoassimilate. Callose was also observed in vascular tissue of potato VTE1-RNAi plants by light microscopy with monoclonal antibodies against β-1,3-glucan (Hofius et al., 2004). In the absence of high-resolution microscopy, Hofius et al. (2004) also suggested that this vascular-associated callose somehow interrupts photoassimilate transport. However, in both the sxd1 and potato VTE1-RNAi studies, it was impossible to determine whether callose deposition was a cause or an effect of carbohydrate accumulation. A critical observation from this study is that the low-temperature-inducible photoassimilate export defect in Arabidopsis tocopherol-deficient mutants was temporally associated with callose deposition in a specific vascular tissue cell type (cf. Figures 9C and 10). These results are significant in that they provide a direct link between defective photoassimilate export and callose deposition (or events tightly associated with callose deposition) in tocopherol-deficient mutants and exclude the possibility that callose deposition is a secondary effect caused by carbohydrate accumulation.
The low-temperature-inducible callose deposition in Arabidopsis vte2 occurred selectively in phloem parenchyma transfer cells (Figures 12 and 13). Importantly, initial callose deposition was site-specific within these cells and resulted in a callose boundary between the phloem parenchyma transfer cell and the sieve element/companion cell complex where transfer cell wall ingrowths occur (Figure 13). We saw no evidence of callose deposition or occlusion of plasmodesmata at the bundle sheath/vascular parenchyma boundary during induction of the export-defective phenotype in vte2 (e.g., 3 d of low-temperature treatment) (Figure 13D). However, by 14 d of low-temperature treatment, when vte2 contains high levels of starch and anthocyanins (Figures 4D and 6A) and more closely resembles the phenotype of maize sxd1, the entire parenchyma transfer cell became encased in a callose sheath associated with abnormally shaped transfer cell wall ingrowths, and it was at this point that callose deposition was also observed in vte2 plasmodesmata at the bundle sheath/vascular parenchyma boundary (Figure 12K). When one compares the development, polarity, and morphology of transfer cell walls in 7.5°C-treated vte2 and Col (cf. Figures 12G and 12L), it becomes clear that tocopherols play an important role in transfer cell wall synthesis at low temperatures.
Results from previous structural studies on the minor vein structure of Arabidopsis have suggested that phloem parenchyma transfer cells are the site of apoplastic unloading of photoassimilates arriving symplastically from bundle sheath cells (Haritatos et al., 2000). The coincidence of the reduction of photoassimilate export with callose deposition in the spatially distinct subcellular site in the vte2 mutant during low-temperature treatment (Figures 9, 10, and 13) provides direct support for the role of transfer cells in photoassimilate export from source leaves via delivery to the phloem apoplast. This callose deposition (or events associated with the callose deposition) in phloem parenchyma transfer cells of 7.5°C-treated vte2 would form a barrier to symplast-to-apoplast but not symplast-to-symplast transport. The limited export that still occurs in 7.5°C-treated vte2 source leaves (Figure 9) may be attributable to apoplastic unloading from bundle sheath cells and subsequent loading to the sieve element/companion cell complex (Haritatos et al., 2000). The special characteristics of phloem parenchyma transfer cells that lead them, compared with other cell types in the leaf, to be so specifically and differentially affected by tocopherol deficiency during low-temperature treatment remain to be determined.
Tocopherol Functions in Plant Stress Physiology
In this study, the tocopherol-deficient vte2 mutant was found to be remarkably similar to the wild type in its response to most abiotic stresses, with the notable exception of nonfreezing low-temperature treatments. Tocopherol deficiency specifically results in abnormal phloem parenchyma transfer cell wall development at low temperature. This leads to the rapid impairment of photoassimilate export that profoundly affects cellular metabolism and whole plant physiology during both short- and long-term low-temperature treatments. That this occurs in both vte2 and vte1 strongly suggests that tocopherols play a crucial, previously unrecognized role in low-temperature adaptation, specifically in phloem loading. Several studies have suggested that vascular tissues, including vascular parenchyma, are metabolically distinct, sensitive to changing environmental conditions, and hence critical sites for stress responses (Orozco-Cardenas et al., 2001; Hibberd and Quick, 2002; Fryer et al., 2003; Koiwai et al., 2004; Narvaez-Vasquez and Ryan, 2004). Our data are consistent with this thesis and suggest that tocopherols have important function(s) in regulating the responses of these specific cell types to environmental stress, such as low temperatures.
Our findings that photooxidative damage and photoinhibition are not associated with the vte2 low-temperature phenotype and that HL1800 (which approaches full sunlight) at 22°C has little impact on vte2 compared with the wild type suggest a more limited role for tocopherols in protecting plants from photooxidative stress than has been assumed. This seems in direct contradiction with a recent report using Arabidopsis vte mutants that concluded that tocopherols protect Arabidopsis against photoinhibition and photooxidative stress (Havaux et al., 2005). However, the conclusions of that work were based entirely on the differential responses of wild-type and vte plants exposed to low temperatures (2 to 8°C) in combination with HL (1000 to 1600 μmol·m−2·s−1) for durations of up to 7 d. We now know that such low-temperature treatments would rapidly block photoassimilate export in tocopherol-deficient genotypes but not in the wild type, independent of any light regime imposed (Figures 9 to 11), and likely would confound any interpretations with respect to previously proposed HL-specific tocopherol functions. Thus, the photoprotective functions of tocopherols in plants remain an open question, and a critical reassessment is needed to clarify this issue.
METHODS
Growth Conditions and HL and Low-Temperature Treatments
Arabidopsis thaliana seeds were stratified for 4 to 7 d (4°C), planted in a vermiculite and soil mixture fertilized with 1× Hoagland solution, and grown in a chamber under permissive conditions: 12 h of 120 μmol·m−2·s−1 light at 22°C and 12 h of darkness at 18°C with 70% RH. Plants were watered every other day and fertilized with 0.5× Hoagland solution once per week. For HL treatments, 4-week-old plants were transferred in the middle of the light cycle to 16 h of 1800 μmol·m−2·s−1 light and 8 h of darkness at 22°C. For low-temperature treatments, 3- to 4-week-old plants were transferred at the beginning of the light cycle to 12 h of 75 μmol·m−2·s−1 light and 12 h of darkness at 7.5 ± <3°C.
Tocopherol, Anthocyanin, Chlorophyll, and Carotenoid Analyses
Leaf samples (12 to 15 mg) were harvested directly into liquid nitrogen at the end of the light cycle, and lipids were extracted in the presence of 0.01% (w/v) butylated hydroxytoluene using tocol as an internal standard, as described (Collakova and DellaPenna, 2001). After phase separation, the aqueous phase was transferred to a new tube and acidified by adding an equal volume of 1 N HCl, and anthocyanin content was measured spectrophotometrically at 520 nm as described (Merzlyak and Chivkunova, 2000). The lipid phase was used for reverse-phase HPLC analyses to identify and quantify each tocopherol, chlorophyll, and carotenoid species as described previously (Collakova and DellaPenna, 2001; Tian and DellaPenna, 2001).
Lipid Peroxide Analysis
Lipid peroxide content was measured using the ferrous oxidation xylenol orange assay as described previously (DeLong et al., 2002; Sattler et al., 2004) with the following modifications. Leaf samples (25 to 30 mg) harvested at the end of the light cycle were immediately extracted with 200 μL of methanol containing 0.01% (w/v) butylated hydroxytoluene, 200 μL of dichloromethane, and 50 μL of 150 mM acetic acid using three 3-mm glass beads and a commercial paint shaker. After shaking for 4 min, 100 μL of water and 100 μL of dichloromethane were added for phase separation. Half of the organic phase was incubated with an equal volume of 50 mM triphenyl phosphine in methanol for 30 min to reduce lipid peroxides, and half was incubated with an equal volume of methanol for 30 min. The triphenyl phosphine–treated and untreated samples (100 μL) were incubated with 900 μL of ferrous oxidation xylenol orange reagent (90% [v/v] methanol, 4 mM butylated hydroxytoluene, 25 mM sulfuric acid, 250 μM ferrous ammonium sulfate, and 100 μM xylenol orange) at room temperature for exactly 20 min, and A560 was measured. Lipid peroxide content was calculated based on a standard curve of hydrogen peroxide, as described previously (DeLong et al., 2002).
Chlorophyll Fluorescence Measurements
In vivo chlorophyll a fluorescence was measured in the middle of the light cycle using a pulse amplitude modulation fluorometer (FMS2; PP Systems). Attached leaves were dark-adapted for at least 15 min before measurement, and fluorescence parameters were determined according to Maxwell and Johnson (2000). ΦPSII was calculated as (F′m − Ft)/F′m, where F′m and Ft are maximum fluorescence and steady state fluorescence in the light, respectively.
Carbohydrate Analyses
Soluble sugar (glucose, fructose, and sucrose) and starch levels of leaves were quantified as described (Jones et al., 1977; Lin et al., 1988) with minor modifications. Unshaded leaf tissue (<50 mg) was harvested, immediately frozen in liquid nitrogen, and extracted twice with 700 μL of 80% ethanol at 80°C. The ethanol extract was evaporated and redissolved in 200 μL of distilled water (Jones et al., 1977). For starch analysis, the extracted leaf residue was ground in 200 μL of 0.2 n KOH and boiled at 95°C for 45 min. After cooling, the sample was neutralized to pH 5.0 with 50 μL of 1 n acetic acid and centrifuged, and 50 μL of the supernatant was mixed with 492.5 μL of 0.2 M sodium acetate, pH 4.8, 150 μL of water, 4 μL of α-amylase (4 units), and 3.5 μL of amyloglucosidase (2 units) and incubated at 37°C overnight. Glucose, fructose, and sucrose levels in the soluble sugar extract and the glucose level of the digested starch extract were determined enzymatically (Jones et al., 1977).
14CO2-Labeling Experiments
For phloem exudation experiments, ∼20 mature leaves (seventh to ninth oldest) were detached in the middle of the day, 0.5 cm of petiole was recut under water, and the petiole of each leaf was submerged in water and placed in a tightly sealed 10-liter glass chamber. 14CO2 was generated in the chamber by adding 3 mL of 0.25 N H2SO4 to 0.1 mCi (7 μmol) of NaH14CO3 and unlabeled 93 μmol of NaHCO3 to give a carbon dioxide concentration of 522 ppm. After labeling for 30 min at 120 μmol·m−2·s−1, the petiole of each leaf was submerged in 0.45 mL of 20 mM EDTA, pH 7.0, and kept in the dark with high humidity to induce phloem exudation (King and Zeevaart, 1974). All of these procedures were performed at 7.5°C for low-temperature-treated leaf samples. Exudation of radiolabel into the EDTA solution was then periodically measured over the course of 10 h by liquid scintillation counting (Tri-Carb 2800TR; Perkin-Elmer). After 10 h of exudation, radiolabel remaining in the leaves was determined by liquid scintillation counting. Total radiolabel fixed per leaf was calculated by adding total radiolabel exuded and remaining in each leaf.
To analyze the translocation of radiolabeled photoassimilates in whole plants, plants were grown on half-strength Murashige and Skoog plates under permissive conditions for 3 weeks and transferred to low-temperature conditions for 7 d with lids partially ajar to supply atmospheric CO2. Whole plants were labeled at 7.5°C for 40 min as described above and placed in darkness at 7.5°C and high humidity for 2 h to allow for translocation. Roots were excised from leaves, and both were exposed to a phosphor screen to visualize the location of radioactivity (Storm; GE Healthcare).
Carbohydrate analysis of phloem exudate was performed by high-pH anion-exchange chromatography. Excised leaves were treated as described for phloem exudation experiments, except that at the end of the initial 2-h exudation period, the petiole of each leaf was transferred to 0.45 mL of water for 4 h to collect exudates for analysis. The water exudates were dried under vacuum and dissolved in 50 μL of water, and 20 μL of the sample was mixed with 5 μL of standards (25 nmol of glucose, 50 nmol of fructose, 125 nmol of sucrose, and 100 nmol of raffinose) and injected onto the high-pH anion-exchange chromatograph. The mixtures were separated on a CarboPac PA-10 column (Dionex) using a 30-min linear gradient of 20 to 140 mM NaOH with a flow rate of 1 mL/min. One-milliliter fractions were collected, and radioactivity was determined by liquid scintillation counting; sugar standards were detected by pulsed amperometric detection.
Fluorescence and Transmission Electron Microscopy
Leaves were prepared for aniline blue fluorescence microscopy (n = 2 leaves/plant, 4 to 6 plants/sample time) (Martin, 1959) and transmission electron microscopy (n = 1 leaf/plant, 2 to 3 plants/sample time) (Sage and Williams, 1995) at the same sampling times described above for export studies. The presence or absence of callose was determined using immunolocalization at the level of the transmission electron microscope as described by Lam et al. (2001) with monoclonal antibodies to β-1,3-glucan (Biosupplies). Primary and secondary antibody (18-nm anti-mouse IgG gold conjugate; Jackson Immunoresearch) dilutions were 1:100 and 1:20, respectively. Incubation times in the primary and secondary antibodies were 2 and 1 h, respectively. Controls were run by omitting primary antibody. Images were captured on the Leica MZ 16F fluorescence microscope (Wetzlar) and the Phillips 201 transmission electron microscope equipped with an Advantage HR camera system (Advanced Microscopy Techniques).
Statistical Analysis
One-way ANOVA was used for the data in Table 2 and Figure 2 using genotype as a factor. Two-way ANOVA was used for the data in Figure 9C using days of cold treatment and genotype as factors. When significance was observed (P < 0.05), pair-wise comparison of least-square means was evaluated. SAS software was used for these analyses (SAS Institute). Student's t test was used for the rest of the data to compare the statistical significance of mutant data relative to Col data (P < 0.05) using Microsoft Excel.
Accession Numbers
Sequence data for the Arabidopsis tocopherol biosynthetic enzymes described in this article can be found in the GenBank nucleotide sequence database under the following accession numbers: VTE1 (At4g32770), NM119430; VTE2 (At2g18950), NM179653; VTE4 (At1g64970), NM105171.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table 1. Contents of Photosynthetic Pigments and Tocopherols in Col and the vte2 and vte1 Mutants Grown under Permissive Conditions.
Supplemental Table 2. Contents of Individual Photosynthetic Pigments in Col and the vte2 Mutant during Low-Temperature (7.5°C) Treatment.
Supplemental Figure 1. Phenotypes of the vte2 Mutant and Col during HL, Drought, and Salinity Stresses.
Supplemental Figure 2. Phenotypic and Photosynthetic Responses of Col and the vte2 and vte1 Mutants to HL Stress.
Supplemental Figure 3. Aniline Blue–Positive Fluorescence in Leaves of the vte2 and vte1 Mutants during Low-Temperature Treatment.
Supplementary Material
Acknowledgments
We thank Maria Magallanes-Lundback for isolation of the vte4-3 allele, Kathy Sault for technical assistance with microscopy, Ashok Ragavendran at the Michigan State University College of Agriculture and Natural Resources Biometry Group for assistance with statistical analyses, and Andreas Weber, Lars Voll, and members of the DellaPenna laboratory for their critical advice, discussions, and manuscript review. This work was supported by National Science Foundation Grant MCB-023529 to D.P. and by a Connaught Award and a Natural Sciences and Engineering Research Council of Canada Discovery Grant to T.L.S.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Dean DellaPenna (dellapen@msu.edu).
Online version contains Web-only data.
References
- Bergmuller, E., Porfirova, S., and Dormann, P. (2003). Characterization of an Arabidopsis mutant deficient in gamma-tocopherol methyltransferase. Plant Mol. Biol. 52 1181–1190. [DOI] [PubMed] [Google Scholar]
- Bjorkman, O., and Demmig, B. (1987). Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 170 489–504. [DOI] [PubMed] [Google Scholar]
- Botha, C.E.J., Cross, R.H.M., van Bel, A.J.E., and Peter, C.I. (2000). Phloem loading in the sucrose-export-defective (SXD-1) mutant maize is limited by callose deposition at plasmodesmata in bundle sheath-vascular parenchyma interface. Protoplasma 214 65–72. [Google Scholar]
- Bradford, A., Atkinson, J., Fuller, N., and Rand, R.P. (2003). The effect of vitamin E on the structure of membrane lipid assemblies. J. Lipid Res. 44 1940–1945. [DOI] [PubMed] [Google Scholar]
- Bramley, P.M., Elmadfa, I., Kafatos, A., Kelly, F.J., Manios, Y., Roxborough, H.E., Schuch, W., Sheehy, P.J.A., and Wagner, K.H. (2000). Vitamin E. J. Sci. Food Agric. 80 913–938. [Google Scholar]
- Broin, M., and Rey, P. (2003). Potato plants lacking the CDSP32 plastidic thioredoxin exhibit overoxidation of the BAS1 2-cysteine peroxiredoxin and increased lipid peroxidation in thylakoids under photooxidative stress. Plant Physiol. 132 1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucke, C. (1968). Distribution and stability of alpha-tocopherol in subcellular fractions of broad bean leaves. Phytochemistry 7 693–700. [Google Scholar]
- Chalker-Scott, L. (1999). Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 70 1–9. [Google Scholar]
- Cheng, Z.G., Sattler, S., Maeda, H., Sakuragi, Y., Bryant, D.A., and DellaPennaa, D. (2003). Highly divergent methyltransferases catalyze a conserved reaction in tocopherol and plastoquinone synthesis in cyanobacteria and photosynthetic eukaryotes. Plant Cell 15 2343–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collakova, E., and DellaPenna, D. (2001). Isolation and functional analysis of homogentisate phytyltransferase from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol. 127 1113–1124. [PMC free article] [PubMed] [Google Scholar]
- Collakova, E., and DellaPenna, D. (2003). The role of homogentisate phytyltransferase and other tocopherol pathway enzymes in the regulation of tocopherol synthesis during abiotic stress. Plant Physiol. 133 930–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaPenna, D., and Pogson, B. (2006). Vitamin synthesis in plants: Tocopherols and carotenoids. Annu. Rev. Plant Biol. 57 711–738. [DOI] [PubMed] [Google Scholar]
- DeLong, J.M., Prange, R.K., Hodges, D.M., Forney, C.F., Bishop, M.C., and Quilliam, M. (2002). Using a modified ferrous oxidation-xylenol orange (FOX) assay for detection of lipid hydroperoxides in plant tissue. J. Agric. Food Chem. 50 248–254. [DOI] [PubMed] [Google Scholar]
- Fryer, M.J. (1992). The antioxidant effects of thylakoid vitamin E (alpha-tocopherol). Plant Cell Environ. 15 381–392. [Google Scholar]
- Fryer, M.J., Ball, L., Oxborough, K., Karpinski, S., Mullineaux, P.M., and Baker, N.R. (2003). Control of Ascorbate Peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J. 33 691–705. [DOI] [PubMed] [Google Scholar]
- Genty, B., Briantais, J.M., and Baker, N.R. (1989). The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990 87–92. [Google Scholar]
- Gilmour, S.J., Sebolt, A.M., Salazar, M.P., Everard, J.D., and Thomashow, M.F. (2000). Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol. 124 1854–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan, T., Muller-Moule, P., and Niyogi, K.K. (2006). Photoprotection mutants of Arabidopsis thaliana acclimate to high light by increasing photosynthesis and specific antioxidants. Plant Cell Environ. 29 879–887. [DOI] [PubMed] [Google Scholar]
- Goldschmidt, E.E., and Huber, S.C. (1992). Regulation of photosynthesis by end-product accumulation in leaves of plants storing starch, sucrose, and hexose sugars. Plant Physiol. 99 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald, J.R., Krysan, P.J., Young, J.C., Evert, R.F., and Sussman, M.R. (2000). Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc. Natl. Acad. Sci. USA 97 13979–13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham, A.J.L., and Liebler, D.C. (1995). Vitamin E oxidation in rat-liver mitochondria. Biochemistry 34 5754–5761. [DOI] [PubMed] [Google Scholar]
- Ham, A.J.L., and Liebler, D.C. (1997). Antioxidant reactions of vitamin E in the perfused rat liver: Product distribution and effect of dietary vitamin E supplementation. Arch. Biochem. Biophys. 339 157–164. [DOI] [PubMed] [Google Scholar]
- Haritatos, E., Medville, R., and Turgeon, R. (2000). Minor vein structure and sugar transport in Arabidopsis thaliana. Planta 211 105–111. [DOI] [PubMed] [Google Scholar]
- Havaux, M., Bonfils, J.P., Lutz, C., and Niyogi, K.K. (2000). Photodamage of the photosynthetic apparatus and its dependence on the leaf developmental stage in the npq1 Arabidopsis mutant deficient in the xanthophyll cycle enzyme violaxanthin de-epoxidase. Plant Physiol. 124 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux, M., Eymery, F., Porfirova, S., Rey, P., and Dormann, P. (2005). Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 17 3451–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux, M., and Niyogi, K.K. (1999). The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 96 8762–8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers, K., and Sonnewald, U. (1998). Molecular determinants of sink strength. Curr. Opin. Plant Biol. 1 207–216. [DOI] [PubMed] [Google Scholar]
- Hibberd, J.M., and Quick, W.P. (2002). Characteristics of C-4 photosynthesis in stems and petioles of C-3 flowering plants. Nature 415 451–454. [DOI] [PubMed] [Google Scholar]
- Hofius, D., Hajirezaei, M.R., Geiger, M., Tschiersch, H., Melzer, M., and Sonnewald, U. (2004). RNAi-mediated tocopherol deficiency impairs photoassimilate export in transgenic potato plants. Plant Physiol. 135 1256–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Q., Elson-Schwab, I., Courtemanche, C., and Ames, B.N. (2000). gamma-Tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl. Acad. Sci. USA 97 11494–11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M.G.K., Outlaw, W.H., and Lowry, O.H. (1977). Enzymic assay of 10−7 to 10−14 moles of sucrose in plant tissues. Plant Physiol. 60 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keles, Y., and Oncel, I. (2002). Response of antioxidative defence system to temperature and water stress combinations in wheat seedlings. Plant Sci. 163 783–790. [Google Scholar]
- Kempna, P., Reiter, E., Arock, M., Azzi, A., and Zingg, J.M. (2004). Inhibition of HMC-1 mast cell proliferation by vitamin E—Involvement of the protein kinase B pathway. J. Biol. Chem. 279 50700–50709. [DOI] [PubMed] [Google Scholar]
- King, R.W., and Zeevaart, J.A. (1974). Enhancement of phloem exudation from cut petioles by chelating agents. Plant Physiol. 53 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, K.E. (1996). Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47 509–540. [DOI] [PubMed] [Google Scholar]
- Koiwai, H., Nakaminami, K., Seo, M., Mitsuhashi, W., Toyomasu, T., and Koshiba, T. (2004). Tissue-specific localization of an abscisic acid biosynthetic enzyme, AAO3, in Arabidopsis. Plant Physiol. 134 1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, B.C.H., Sage, T.L., Bianchi, F., and Blumwald, E. (2001). Role of SH3 domain-containing proteins in clathrin-mediated vesicle trafficking in Arabidopsis. Plant Cell 13 2499–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva, A., Jarillo, J.A., Salinas, J., and Martinezzapater, J.M. (1995). Low temperature induces the accumulation of phenylalanine ammonia-lyase and chalcone synthase messenger-RNAs of Arabidopsis thaliana in a light-dependent manner. Plant Physiol. 108 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler, H.K., Prenzel, U., Douce, R., and Joyard, J. (1981). Localization of prenylquinones in the envelope of spinach chloroplasts. Biochim. Biophys. Acta 641 99–105. [DOI] [PubMed] [Google Scholar]
- Liebler, D.C., and Burr, J.A. (1992). Oxidation of vitamin E during iron-catalyzed lipid peroxidation—Evidence for electron-transfer reactions of the tocopheroxyl radical. Biochemistry 31 8278–8284. [DOI] [PubMed] [Google Scholar]
- Lin, T.P., Caspar, T., Somerville, C., and Preiss, J. (1988). Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) Heynh lacking ADPglucose pyrophosphorylase activity. Plant Physiol. 86 1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlin, L.J., Filipski, R., Nelson, J., Horn, L.R., and Brin, M. (1977). Effects of a prolonged vitamin E deficiency in rat. J. Nutr. 107 1200–1208. [DOI] [PubMed] [Google Scholar]
- Maeda, H., Sakuragi, Y., Bryant, D.A., and DellaPenna, D. (2005). Tocopherols protect Synechocystis sp strain PCC 6803 from lipid peroxidation. Plant Physiol. 138 1422–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, F.W. (1959). Staining and observing pollen tubes in the style by means of fluorescence. Stain Technol. 34 125–128. [DOI] [PubMed] [Google Scholar]
- Maxwell, K., and Johnson, G.N. (2000). Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 51 659–668. [DOI] [PubMed] [Google Scholar]
- Merzlyak, M.N., and Chivkunova, O.B. (2000). Light-stress-induced pigment changes and evidence for anthocyanin photoprotection in apples. J. Photochem. Photobiol. B 55 155–163. [DOI] [PubMed] [Google Scholar]
- Muller, P., Li, X.P., and Niyogi, K.K. (2001). Non-photochemical quenching. A response to excess light energy. Plant Physiol. 125 1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munne-Bosch, S., and Alegre, L. (2002). The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 21 31–57. [Google Scholar]
- Munne-Bosch, S., Schwarz, K., and Alegre, L. (1999). Enhanced formation of alpha-tocopherol and highly oxidized abietane diterpenes in water-stressed rosemary plants. Plant Physiol. 121 1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaez-Vasquez, J., and Ryan, C.A. (2004). The cellular localization of prosystemin: A functional role for phloem parenchyma in systemic wound signaling. Planta 218 360–369. [DOI] [PubMed] [Google Scholar]
- Nishimura, M.T., Stein, M., Hou, B.H., Vogel, J.P., Edwards, H., and Somerville, S.C. (2003). Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301 969–972. [DOI] [PubMed] [Google Scholar]
- Niyogi, K.K., Grossman, A.R., and Bjorkman, O. (1998). Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cardenas, M.L., Narvaez-Vasquez, J., and Ryan, C.A. (2001). Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13 179–191. [PMC free article] [PubMed] [Google Scholar]
- Paul, M.J., and Foyer, C.H. (2001). Sink regulation of photosynthesis. J. Exp. Bot. 52 1383–1400. [DOI] [PubMed] [Google Scholar]
- Paul, M.J., and Peliny, T.K. (2003). Carbon metabolite feedback regulation of leaf photosynthesis and development. J. Exp. Bot. 54 539–547. [DOI] [PubMed] [Google Scholar]
- Porfirova, S., Bergmuller, E., Tropf, S., Lemke, R., and Dormann, P. (2002). Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc. Natl. Acad. Sci. USA 99 12495–12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher, L.M., Miao, L., Sinha, N., and Lucas, W.J. (2001). Sucrose Export Defective1 encodes a novel protein implicated in chloroplast-to-nucleus signaling. Plant Cell 13 1127–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciarelli, R., Tasinato, A., Clement, S., Ozer, N.K., Boscoboinik, D., and Azzi, A. (1998). alpha-Tocopherol specifically inactivates cellular protein kinase C alpha by changing its phosphorylation state. Biochem. J. 334 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbach, G., Minihane, A.M., Majewicz, J., Fischer, A., Pallauf, J., Virgli, F., and Weinberg, P.D. (2002). Regulation of cell signalling by vitamin E. Proc. Nutr. Soc. 61 415–425. [DOI] [PubMed] [Google Scholar]
- Russin, W.A., Evert, R.F., Vanderveer, P.J., Sharkey, T.D., and Briggs, S.P. (1996). Modification of a specific class of plasmodesmata and loss of sucrose export ability in the sucrose export defective1 maize mutant. Plant Cell 8 645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage, T.L., and Williams, E.G. (1995). Structure, ultrastructure, and histochemistry of the pollen-tube pathway in the milkweed Asclepias exaltata L. Sex. Plant Reprod. 8 257–265. [Google Scholar]
- Sattler, S.E., Cahoon, E.B., Coughlan, S.J., and DellaPenna, D. (2003). Characterization of tocopherol cyclases from higher plants and cyanobacteria. Evolutionary implications for tocopherol synthesis and function. Plant Physiol. 132 2184–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler, S.E., Gilliland, L.U., Magallanes-Lundback, M., Pollard, M., and DellaPenna, D. (2004). Vitamin E is essential for seed longevity, and for preventing lipid peroxidation during germination. Plant Cell 16 1419–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler, S.E., Mene-Saffrane, L., Farmer, E.E., Krischke, M., Mueller, M.J., and DellaPenna, D. (2006). Non-enzymatic lipid peroxidation reprograms gene expression and activates defense markers in tocopherol deficient mutants. Submitted to Plant Cell. [DOI] [PMC free article] [PubMed]
- Schneider, C. (2005). Chemistry and biology of vitamin E. Mol. Nutr. Food Res. 49 7–30. [DOI] [PubMed] [Google Scholar]
- Shintani, D., and DellaPenna, D. (1998). Elevating the vitamin E content of plants through metabolic engineering. Science 282 2098–2100. [DOI] [PubMed] [Google Scholar]
- Soll, J. (1987). Alpha-tocopherol and plastoquinone synthesis in chloroplast membranes. Methods Enzymol. 148 383–392. [Google Scholar]
- Soll, J., Douce, R., and Schultz, G. (1980). Site of biosynthesis of alpha-tocopherol in spinach chloroplasts. FEBS Lett. 112 243–246. [Google Scholar]
- Soll, J., Schultz, G., Joyard, J., Douce, R., and Block, M.A. (1985). Localization and synthesis of prenylquinones in isolated outer and inner envelope membranes from spinach chloroplasts. Arch. Biochem. Biophys. 238 290–299. [DOI] [PubMed] [Google Scholar]
- Stillwell, W., Dallman, T., Dumaual, A.C., Crump, F.T., and Jenski, L.J. (1996). Cholesterol versus alpha-tocopherol: Effects on properties of bilayers made from heteroacid phosphatidylcholines. Biochemistry 35 13353–13362. [DOI] [PubMed] [Google Scholar]
- Stitt, M. (1996). Plasmodesmata play an essential role in sucrose export from leaves: A step toward an integration of metabolic biochemistry and cell biology. Plant Cell 8 565–571. [Google Scholar]
- Strand, A., Hurry, V., Gustafsson, P., and Gardestrom, P. (1997). Development of Arabidopsis thaliana leaves at low temperatures releases the suppression of photosynthesis and photosynthetic gene expression despite the accumulation of soluble carbohydrates. Plant J. 12 605–614. [DOI] [PubMed] [Google Scholar]
- Strand, A., Hurry, V., Henkes, S., Huner, N., Gustafsson, P., Gardestrom, P., and Stitt, M. (1999). Acclimation of Arabidopsis leaves developing at low temperatures. Increasing cytoplasmic volume accompanies increased activities of enzymes in the Calvin cycle and in the sucrose-biosynthesis pathway. Plant Physiol. 119 1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taji, T., Ohsumi, C., Iuchi, S., Seki, M., Kasuga, M., Kobayashi, M., Yamaguchi-Shinozaki, K., and Shinozaki, K. (2002). Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J. 29 417–426. [DOI] [PubMed] [Google Scholar]
- Tian, L., and DellaPenna, D. (2001). Characterization of a second carotenoid beta-hydroxylase gene from Arabidopsis and its relationship to the LUT1 locus. Plant Mol. Biol. 47 379–388. [DOI] [PubMed] [Google Scholar]
- Trebst, A., Depka, B., and Hollander-Czytko, H. (2002). A specific role for tocopherol and of chemical singlet oxygen quenchers in the maintenance of photosystem II structure and function in Chlamydomonas reinhardtii. FEBS Lett. 516 156–160. [DOI] [PubMed] [Google Scholar]
- Vanbel, A.J.E. (1993). Strategies of phloem loading. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44 253–281. [Google Scholar]
- Vidi, P.A., Kanwischer, M., Baginsky, S., Austin, J.R., Csucs, G., Dormann, P., Kessler, F., and Brehelin, C. (2006). Tocopherol cyclase (VTE1) localization and vitamin E accumulation in chloroplast plastoglobule lipoprotein particles. J. Biol. Chem. 281 11225–11234. [DOI] [PubMed] [Google Scholar]
- Wang, X.Y., and Quinn, P.J. (2000). The location and function of vitamin E in membranes (review). Mol. Membr. Biol. 17 143–156. [DOI] [PubMed] [Google Scholar]
- Wanner, L.A., and Junttila, O. (1999). Cold-induced freezing tolerance in Arabidopsis. Plant Physiol. 120 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassall, S.R., Thewalt, J.L., Wong, L., Gorrissen, H., and Cushley, R.J. (1986). Deuterium NMR study of the interaction of alpha-tocopherol with a phospholipid model membrane. Biochemistry 25 319–326. [DOI] [PubMed] [Google Scholar]
- Yokota, T., Igarashi, K., Uchihara, T., Jishage, K., Tomita, H., Inaba, A., Li, Y., Arita, M., Suzuki, H., Mizusawa, H., and Arai, H. (2001). Delayed-onset ataxia in mice lacking alpha-tocopherol transfer protein: Model for neuronal degeneration caused by chronic oxidative stress. Proc. Natl. Acad. Sci. USA 98 15185–15190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.