Abstract
Most seed storage proteins of the prolamin class accumulate in the endoplasmic reticulum (ER) as large insoluble polymers termed protein bodies (PBs), through mechanisms that are still poorly understood. We previously showed that a fusion between the Phaseolus vulgaris vacuolar storage protein phaseolin and the N-terminal half of the Zea mays prolamin γ-zein forms ER-located PBs. Zeolin has 6 Cys residues and, like γ-zein with 15 residues, is insoluble unless reduced. The contribution of disulfide bonds to zeolin destiny was determined by studying in vivo the effects of 2-mercaptoethanol (2-ME) and by zeolin mutagenesis. We show that in tobacco (Nicotiana tabacum) protoplasts, 2-ME enhances interactions of newly synthesized proteins with the ER chaperone BiP and inhibits the secretory traffic of soluble proteins with or without disulfide bonds. In spite of this general inhibition, 2-ME enhances the solubility of zeolin and relieves its retention in the ER, resulting in increased zeolin traffic. Consistently, mutated zeolin unable to form disulfide bonds is soluble and efficiently enters the secretory traffic without 2-ME treatment. We conclude that disulfide bonds that lead to insolubilization are a determinant for PB-mediated protein accumulation in the ER.
INTRODUCTION
In a number of cereals such as maize (Zea mays), sorghum (Sorghum bicolor), millet (Panicum miliaceum), and rice (Oryza sativa), storage proteins of the prolamin class accumulate within the endoplasmic reticulum (ER) as large polymers termed protein bodies (PBs) (Shewry and Halford, 2002). The mechanisms of prolamin retention within the ER and of PB formation are still largely unknown. The ER constitutively exchanges membrane and proteins with the Golgi complex, which is the key sorting compartment of the secretory pathway (Lee et al., 2004; Hanton et al., 2005). This allows proper traffic of newly synthesized proteins destined for the cell surface or the different types of vacuoles and retrieval of those that will perform their functions within the ER itself. Thus, many ER residents indeed leave this compartment but have sorting signals that promote their efficient retrieval from the Golgi complex, such as the KDEL/HDEL tetrapeptide present in soluble proteins and the dilysine or diarginine motifs on the cytosolic tails of transmembrane proteins (Lee et al., 2004). Individual subunits of polymeric ER residents can be devoid of these signals, but in such cases they form heterooligomers with partners that have retrieval signals (Vuori et al., 1992; Trombetta et al., 1996). True protein retention, as opposed to retrieval, also occurs in the ER (Sonnichsen et al., 1994; Isidoro et al., 1996; Andersson et al., 1999; Szczesna-Skorupa and Kemper, 2000). ER-located prolamins do not have known retrieval signals. Rice prolamins show unusually prolonged interactions with the ER molecular chaperone BiP, which has the HDEL signal (Li et al., 1993), but the contribution of these interactions to PB formation and prolamin residence within the ER is not clear (Vitale and Ceriotti, 2004). PBs are nearly spherical polymers that can reach diameters of 2 μm, a size that is much larger than that of the COP II vesicles that leave the ER for the Golgi complex; it is thus possible, but not proven, that size exclusion determines, or contributes to, the ER retention of PBs (Vitale and Ceriotti, 2004). The inclusion of newly synthesized prolamin polypeptides into PBs and their ER retention or retrieval before such inclusion must be very efficient to avoid the escape of unpolymerized molecules.
PBs are heteropolymers composed of the products of gene families. Prolamins have variable structures, but they share the common property of being soluble in water mixed with a high percentage of alcohol. In many cases, they contain a repetitive domain (made of building blocks that are not conserved between the different prolamins) inserted within other regions that are rich in Cys residues (Shewry and Halford, 2002). The latter contain domains homologous with those of the 2S storage albumins abundant in oil seeds, which are water-soluble, monomeric vacuolar storage proteins (Shewry et al., 1995). The Cys residues of prolamins form intrachain and often interchain disulfide bonds, most likely contributing to PB assembly.
The expression of individual prolamin genes in vegetative tissues can lead to the formation of PBs, indicating that PB assembly and retention does not require cereal seed–specific ER proteins other than the prolamins themselves (Geli et al., 1994; Bagga et al., 1995). Accumulation of different prolamins in transgenic plants is polypeptide-specific and indicates that certain polypeptides are more important than others for the formation of a stable PB (Coleman et al., 1996; Bagga et al., 1997). The prolamins of maize are divided into four groups: α-, β-, δ-, and γ-zein, the most abundant being the α-zeins, followed by γ-zein. At the end of seed maturation, the central part of the PB is mainly occupied by α-zeins, whereas γ-zein is located peripherally (Lending and Larkins, 1989). However, the synthesis of γ- and β-zein starts earlier than that of the other zein classes during maize seed development, and coexpression experiments in transgenic tobacco (Nicotiana tabacum) indicate that γ- and β-zein have a stabilizing effect on α- and δ-zein, respectively (Lending and Larkins, 1989; Coleman et al., 1996; Bagga et al., 1997). Therefore, γ-zein seems to be a fundamental protein for maize PB biogenesis.
We have expressed in tobacco a fusion between the 7S vacuolar storage protein of Phaseolus vulgaris, phaseolin, and approximately the first half of γ-zein (Mainieri et al., 2004). The zein portion includes the repeated region and 6 of the total 15 Cys residues, excludes the domains homologous with the 2S albumins, and has been shown in deletion experiments to be able to accumulate in the ER (Geli et al., 1994; Shewry et al., 1995). Phaseolin does not contain Cys residues and is a soluble trimeric globulin. The fusion protein, termed zeolin, has the solubility and assembly properties of γ-zein: it is insoluble unless its disulfide bonds are reduced, and it forms PBs in the ER (Mainieri et al., 2004). Therefore, zeolin is a good model to analyze PB formation within the plant ER, focusing on the repeated domain and a limited number of Cys residues. In this study, we have investigated the role played by disulfide bonds in the ER retention of zeolin by studying the in vivo effect of the reducing agent 2-mercaptoethanol (2-ME) and by zeolin mutagenesis.
RESULTS
2-ME Inhibits Traffic of a Protein That Does Not Contain Cys Residues
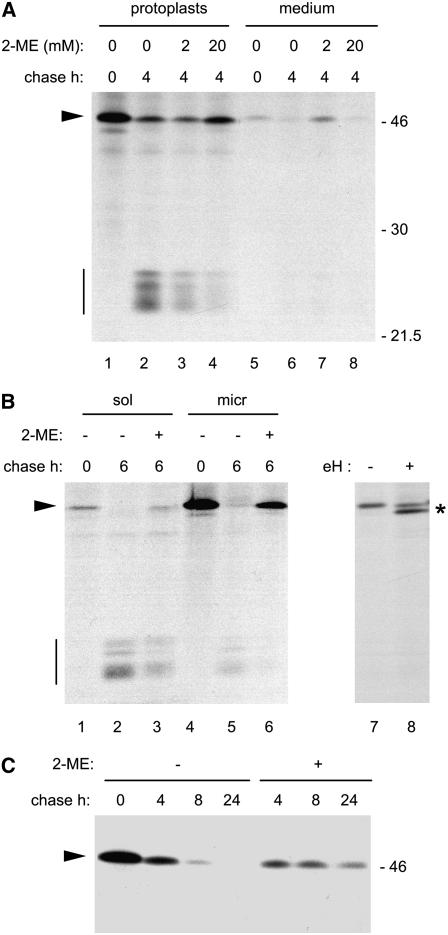
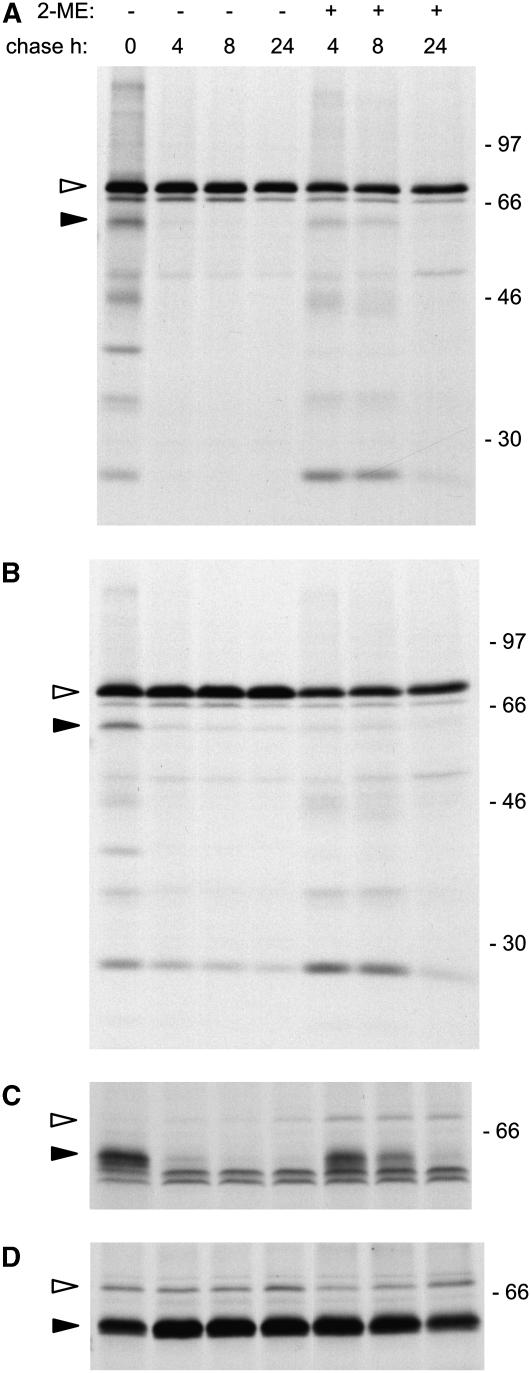
We first tested whether reducing agents have a general effect on the traffic machinery of the plant secretory pathway. We used as a marker protein phaseolin, which does not contain Cys residues; therefore, any effect of the reducing agent on phaseolin traffic would be indirect and attributable to altered functions of one or more constituents of the traffic machinery. Leaf protoplasts from transgenic tobacco expressing the phaseolin T343F construct (Pedrazzini et al., 1997) were subjected to 1 h of pulse-labeling with a mixture of 35S-labeled Met and Cys followed by a 0- or 4-h chase. Phaseolin was immunoprecipitated with anti-phaseolin antiserum and analyzed by SDS-PAGE and fluorography. Consistent with similar previous experiments (Pedrazzini et al., 1997), T343F phaseolin was recovered as intact polypeptides (molecular mass of ∼45 kD) at the end of the pulse, and typical vacuolar fragmentation products were formed during the chase (Figure 1A, lanes 1 and 2). Inclusion of 2 or 20 mM 2-ME in the protoplast incubation medium at the beginning of the chase partially inhibited the formation of vacuolar fragments, and this effect was more marked at higher concentrations (Figure 1, lanes 3 and 4). Treatment with the much stronger dithiol reducing agent DTT leads to an even greater inhibition of vacuolar fragmentation (data not shown). When protoplasts were pretreated with 20 mM 2-ME and the reducing agent was maintained during labeling, there was a dramatic inhibition of protein synthesis (Klein et al., 2006), which made subsequent analysis very difficult. Pretreatment with 10 mM DTT had an even worse effect, inhibiting protein synthesis by >99% (Klein et al., 2006). It has been reported that 10 mM DTT reduces tobacco leaf protoplast viability by 50% and reduces the expression of transgenes to undetectable levels (Piñeiro et al., 1994). Strong reduction in the synthesis of secretory proteins by the inclusion of reducing agents during pulse-labeling has also been observed in mammalian cells (Chanat et al., 1993; Lodish and Kong, 1993). Reducing agents induce the unfolded protein response, a complex series of effects that include attenuation of protein synthesis (Rutkowski and Kaufman, 2004). Because our purpose was to investigate traffic rather than synthesis, and we wanted to minimize stress, subsequent experiments were performed including 20 mM 2-ME during the chase.
Figure 1.
Effect of 2-ME on the Synthesis of Phaseolin.
Leaf protoplasts prepared from transgenic tobacco expressing T343F phaseolin ([A] and [B]) or Δ418 phaseolin (C) were pulse-labeled with [35S]Met and [35S]Cys for 1 h and chased for the indicated times. Immunoprecipitations were with anti-phaseolin antiserum. Analysis was by SDS-PAGE and fluorography. The positions of intact phaseolin (arrowheads), phaseolin vacuolar fragments (vertical bars), deglycosylated phaseolin (asterisk), and molecular mass markers (numbers at right; in kD) are indicated.
(A) Chase was performed in the absence or presence of 2-ME at the indicated concentrations. Phaseolin was immunoprecipitated from protoplast or incubation medium homogenates.
(B) Lanes 1 to 6, chase was performed in the absence (−) or presence (+) of 20 mM 2-ME. Phaseolin was immunoprecipitated from soluble (sol) or microsomal (micr) fractions prepared from protoplast homogenates. Lanes 7 and 8, phaseolin was immunoprecipitated from an aliquot of the subcellular fraction shown in lane 6 and incubated with (+) or without (−) endoglycosidase H (eH).
(C) Chase was performed in the absence (−) or presence (+) of 20 mM 2-ME. Phaseolin was immunoprecipitated from protoplast homogenates.
In normal conditions, there is virtually no secretion of T343F phaseolin in transgenic tobacco leaves, indicating very efficient vacuolar sorting (Pedrazzini et al., 1997) (Figure 1A, lanes 5 and 6). Intact phaseolin recovered at 0 h of chase in the medium is probably derived from contamination by protoplasts. However, treatment with 2 mM, but not 20 mM, 2-ME leads to partial secretion of intact phaseolin during the chase. These results indicate that at low concentration, 2-ME diverts part of the phaseolin from vacuolar sorting and leads to its secretion, suggesting that at higher concentration the reducing agent inhibits traffic of the vacuolar protein. It is possible, however, that the strong inhibition of phaseolin fragmentation observed at 20 mM 2-ME resulted partially or totally from inhibition of the vacuolar proteolytic activity rather than from a block of vacuolar delivery. To discriminate between these two possibilities, protoplasts were subjected to subcellular fractionation into a microsomal and a soluble fraction. The latter contains cytosolic proteins as well as soluble vacuolar proteins, because vacuoles break during homogenation (Pedrazzini et al., 1997). Consistently, after 6 h of chase, phaseolin fragments are mostly recovered in the soluble fraction (Figure 1B, lanes 2 and 5). After chase in the presence of 20 mM 2-ME, intact T343F phaseolin is almost completely recovered in the microsomal fraction, indicating that traffic is inhibited (Figure 1B, lanes 3 and 6).
The microsomal fraction contains ER and the Golgi complex as major components of the endomembrane system. To acquire more information on the compartment where the block in traffic occurs, we took advantage of the fact that the single oligosaccharide chain of T343F phaseolin is modified into a complex structure in the Golgi complex (Frigerio et al., 1998). N-linked oligosaccharide chains added to glycoproteins within the ER can be removed in vitro from the polypeptide chain by the enzyme endoglycosidase H, but extensive modifications by Golgi enzymes confer resistance to the glycosidase. T343F phaseolin accumulated in the microsomal fraction of protoplasts treated with 2-ME is for the most part susceptible to digestion by endoglycosidase H (Figure 1B, lanes 7 and 8), indicating that most polypeptides are still located in the ER or the cis-cisternae of the Golgi complex after 6 h of chase.
To investigate whether 2-ME also inhibits the traffic of secreted proteins, we analyzed its effect on Δ418 phaseolin, a mutated form that does not contain the vacuolar sorting signal and is efficiently secreted from protoplasts of transgenic tobacco (Frigerio et al., 1998). A concentration of 20 mM 2-ME partially inhibited the secretion of Δ418 phaseolin, which remained detectable in protoplast extracts even after 24 h of chase (Figure 1C). Similarly, intact T343F polypeptides were also detectable after 24 h of chase in the presence of 20 mM 2-ME (data not shown). However, it should be noted that secretion of Δ418 was less severely inhibited than vacuolar delivery of T343F phaseolin, particularly during the first hour of chase.
We conclude that 20 mM 2-ME strongly inhibits the traffic of phaseolin, causing mainly accumulation in the ER or cis-Golgi cisternae, and that the inhibitory effect is more marked on the vacuolar sorting machinery.
2-ME Inhibits the Release of Newly Synthesized Polypeptides from BiP
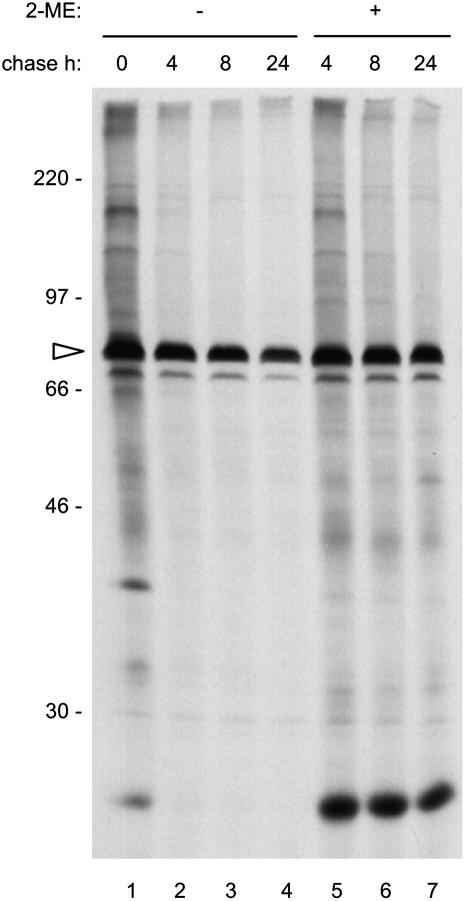
Reducing agents perturb the proper folding of a number of newly synthesized polypeptides that emerge into the ER (Braakman et al., 1992; Tatu et al., 1993). This could result in prolonged interactions with ER chaperones such as BiP (Jämsä et al., 1994; Ben-Zeev et al., 2002). Association with BiP is part of the quality-control mechanism that prevents the intracellular traffic of protein folding and assembly intermediates and of defective, misfolded proteins (Sitia and Braakman, 2003). Therefore, we investigated whether 2-ME alters the association of newly synthesized polypeptides with BiP. Protoplasts prepared from wild-type tobacco leaves were subjected to pulse–chase, and proteins were immunoprecipitated with anti-BiP antiserum. As reported previously (Crofts et al., 1998), in normal conditions many newly synthesized tobacco polypeptides were coimmunoprecipitated with BiP after 1 h of pulse-labeling but not after the subsequent chase points, reflecting the expected physiological transient association with the chaperone (Figure 2, lanes 1 to 4). In the presence of 2-ME, this association was dramatically extended in time (Figure 2, lanes 5 to 7). The qualitative SDS-PAGE banding pattern of BiP-coimmunoprecipitated polypeptides was not markedly altered by 2-ME, except for the intense band of <30 kD. However, unlike the other radioactive polypeptides, this component was resistant to in vitro release from BiP by ATP treatment, suggesting peculiar interactions (see Supplemental Figure 1 online). We conclude that 2-ME markedly slows the release from BiP of many natural transient ligands of this chaperone.
Figure 2.
2-ME Inhibits the Release of Newly Synthesized Polypeptides from BiP.
Leaf protoplasts from wild-type tobacco were pulse-labeled with [35S]Met and [35S]Cys for 1 h. Chase was performed for the indicated times in the presence (+) or absence (−) of 20 mM 2-ME. BiP was immunoprecipitated with anti-BiP antiserum from protoplast homogenates and analyzed by SDS-PAGE and fluorography. The positions of BiP (arrowhead) and molecular mass markers (in kD) are indicated at left.
2-ME Increases the Solubility of Zeolin and Relieves Its ER Retention
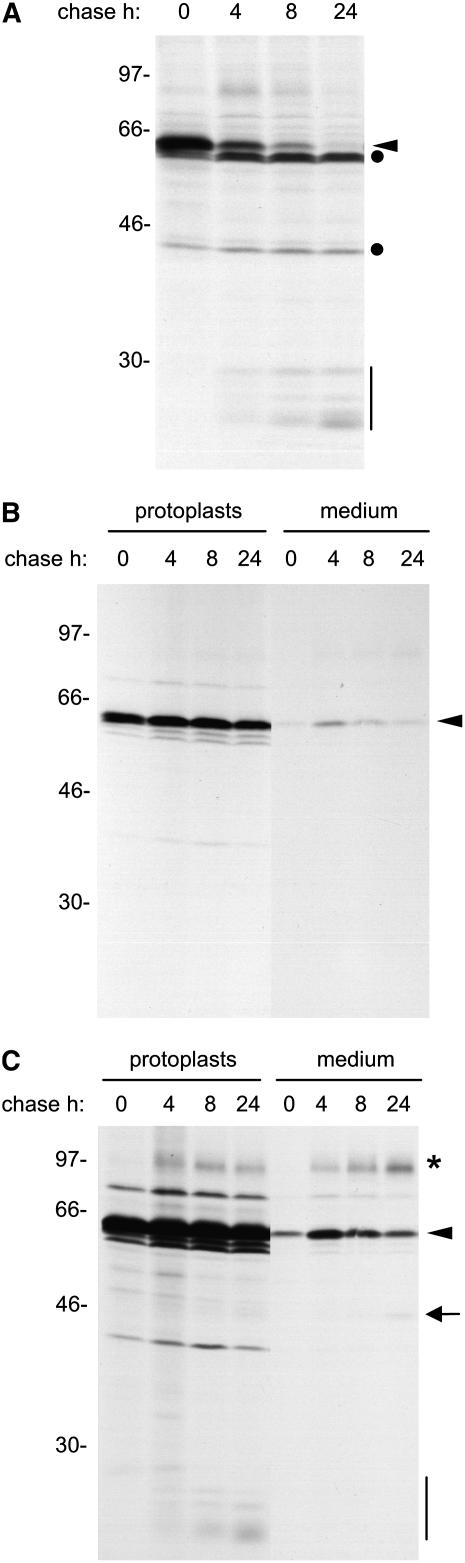
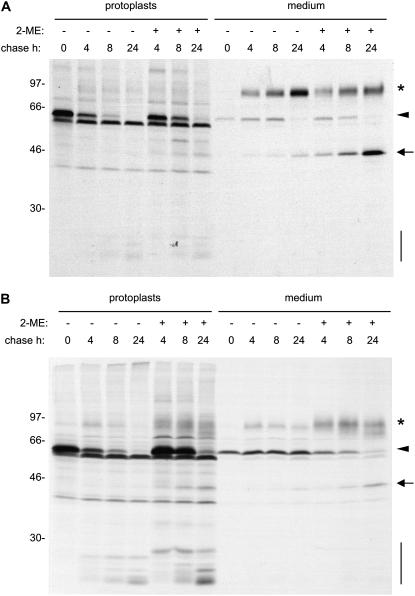
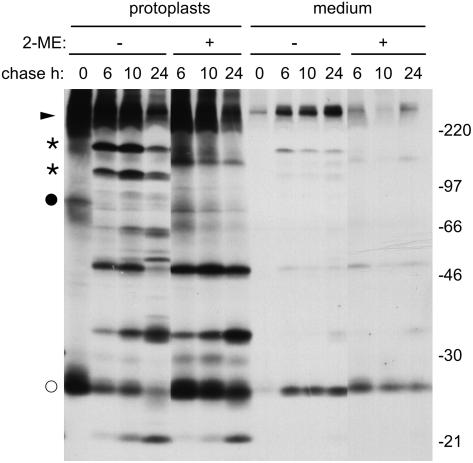
To study the effect of 2-ME on zeolin synthesis, we first analyzed in detail the behavior of this protein in pulse–chase experiments. We previously established that intact zeolin becomes rapidly insoluble after synthesis and is very stable (Mainieri et al., 2004). Interchain disulfide bonds determine zeolin insolubility and polymerization (Mainieri et al., 2004). The progressive loss of solubility can be followed by pulse–chase of protoplasts isolated from transgenic tobacco expressing zeolin. When homogenation was performed in the absence of added reducing agents, there was with time a progressive decrease in the amount of zeolin that could be immunoprecipitated with anti-phaseolin antiserum (Figure 3A, arrowhead). The polypeptides indicated by the dots are contaminant tobacco proteins immunoselected by the anti-phaseolin antiserum (see Figure 5 in Pedrazzini et al., 1997). When 2-ME was included in the homogenation buffer, the recovery of zeolin was almost constant over the 8-h chase time and decreased only marginally during the next 16 h (Figure 3B, protoplasts). This finding indicates that the marked time-dependent decrease shown in Figure 3A is attributable to posttranslational disulfide bond formation that makes the protein insoluble and therefore not immunoselectable. Zeolin accumulates mainly as intact polypeptides polymerized into ER-located PBs, but a small proportion undergoes posttranslational, traffic-dependent proteolysis that releases phaseolin trimers (Mainieri et al., 2004). Pulse–chase indicates that these trimers undergo further proteolysis into the typical fragments that are formed when phaseolin is delivered to the vacuoles of tobacco leaf cells (Figure 3A, vertical bar; cf. Figure 1). The fragments are not detected by protein blot (Mainieri et al., 2004), indicating that they are eventually fully degraded. Comparison of Figures 3A and 3B shows that the relative proportion between phaseolin vacuolar fragments produced during the chase and intact zeolin detected at the end of the pulse is much lower when immunoprecipitation is performed using extracts prepared in reducing conditions (in this case, the fragments are only detectable upon long exposure of the fluorographs that largely saturate the signal given by intact zeolin, as shown in Figure 3C). This is because a relevant proportion of intact zeolin is already insoluble at the end of 1 h of pulse; therefore, in nonreducing conditions, its recovery is nonquantitative (Mainieri et al., 2004).
Figure 3.
Solubility and Traffic of Zeolin.
Leaf protoplasts from transgenic tobacco expressing zeolin were pulse-labeled with [35S]Met and [35S]Cys for 1 h and subjected to chase for the indicated times. Analysis was by SDS-PAGE and fluorography. The positions of zeolin (arrowheads), the 45-kD form (arrow), phaseolin fragments (vertical bars), the 95-kD putative O-glycosylated zeolin (asterisk), and contaminant polypeptides (dots) are indicated. Numbers at left indicate the positions of molecular mass markers in kD.
(A) Total homogenates were prepared from protoplasts, using homogenation buffer without 2-ME, and immunoselected using anti-phaseolin antiserum.
(B) Total homogenates were prepared from protoplasts or incubation medium and immunoselected with anti-phaseolin antiserum, using homogenation and immunoprecipitation buffers supplemented with 2-ME.
(C) Longer exposure of the fluorograph shown in (B).
Figure 5.
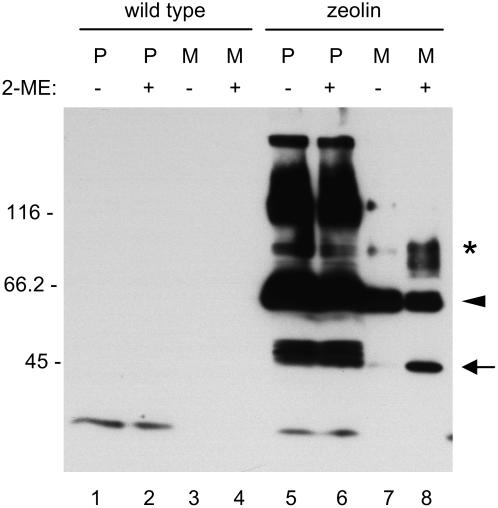
Accumulation of the Secreted 45- and 95-kD Forms.
Protoplasts were isolated from wild-type (lanes 1 to 4) or zeolin-expressing (lanes 5 to 8) tobacco leaves and incubated for 24 h in the presence (+) or absence (−) of 20 mM 2-ME. Aliquots of protoplast homogenates (P) and incubation medium (M) of an equivalent amount of protoplasts were analyzed by SDS-PAGE and protein blot using anti-phaseolin antiserum. The positions of zeolin (arrowhead), the 45-kD form (arrow), and the 95-kD putative O-glycosylated zeolin (asterisk) are indicated. Numbers at left indicate the positions of molecular mass markers in kD.
Immunoprecipitation of proteins secreted in the protoplast incubation medium (Figure 3B, medium) showed that there is little secretion of zeolin. There is no accumulation at longer chase times, suggesting extracellular degradation attributable to secreted proteases. The decrease of zeolin in the incubation medium between 4 and 24 h of chase is not the result of disulfide bond formation and insolubilization: in all of the experiments performed in this study, the incubation medium was homogenized in the presence of 2-ME to detect all secreted polypeptides. Besides intact zeolin, the anti-phaseolin antiserum immunoprecipitated from the incubation medium two other classes of polypeptides, of ∼95 and 45 kD, detected after long gel exposure (asterisk and arrow in Figure 3C). These are very minor radioactive components with respect to intracellular zeolin. Polypeptides with very similar molecular masses are detected by protein blot of total leaf proteins; also in that case, they represent a very minor proportion of total zeolin (Mainieri et al., 2004). The 45-kD polypeptide is intact, or nearly intact, phaseolin released from zeolin by posttranslational proteolysis and, like wild-type phaseolin, is assembled into trimers (Mainieri et al., 2004). This suggests that phaseolin released from zeolin upon traffic-dependent proteolysis is in part delivered to the vacuole and in part secreted, possibly because of a partial loss or inefficient exposure of the C-terminal phaseolin vacuolar sorting signal. The nature of the 95-kD polypeptide was not investigated further, but it could represent a fraction of zeolin that escapes insolubilization and undergoes Pro hydroxylation and subsequent O-glycosylation during intracellular traffic. The banding pattern of γ-zein expressed in leaves of transgenic Arabidopsis also suggested the hydroxylation of Pro residues (Geli et al., 1994), a process that does not occur in maize endosperm. The secreted polypeptides are immunoselected irrespective of the redox conditions of the homogenation buffer (see Supplemental Figure 2 online), indicating that they are soluble, but they represent a very minor proportion of radioactive zeolin.
The effect of 2-ME on the solubility, traffic, and processing of zeolin was next determined. The reducing agent was included in the protoplast incubation medium during the chase but not in the homogenation buffer. 2-ME increased the solubility of intact zeolin during the chase (Figures 4A and 4B, cf. zeolin in protoplasts at the different chase points in the presence and absence of 2-ME). In the experiment shown in Figure 4A, the major effect on the pattern of zeolin processing and traffic was a marked increase in the secretion of the 45-kD processed form. A polypeptide with the same molecular mass became detectable also intracellularly during the chase, probably representing the intermediate of secretion. In several repetitions of this experiment, we consistently observed a marked increase of zeolin solubility during the chase in the presence of 2-ME. In some experiments, the increase in secretion of the 45-kD form was less marked, whereas the secreted 95-kD form and/or phaseolin vacuolar fragments became more abundant (Figure 4B). Protein blot analysis of protoplasts and incubation medium confirmed the pulse–chase results: after 24 h of treatment with 2-ME, the 45- and 95-kD forms clearly accumulated in the incubation medium (Figure 5, cf. lanes 7 and 8).
Figure 4.
2-ME Enhances the Intracellular Traffic of Zeolin.
Protoplasts from transgenic tobacco expressing zeolin were pulse-labeled with [35S]Met and [35S]Cys for 1 h and subjected to chase for the indicated times in the presence (+) or absence (−) of 20 mM 2-ME. Total homogenates were prepared from protoplasts or incubation medium, using homogenation buffer supplemented with 2-ME (medium) or without the reducing agent (protoplasts), and immunoselected using anti-phaseolin antiserum. Analysis was by SDS-PAGE and fluorography. (A) and (B) show results from fully independent experiments. The positions of zeolin (arrowheads), the 45-kD form (arrows), phaseolin fragments (vertical bars), and the 95-kD putative O-glycosylated zeolin (asterisks) are indicated. Numbers at left indicate the positions of molecular mass markers in kD.
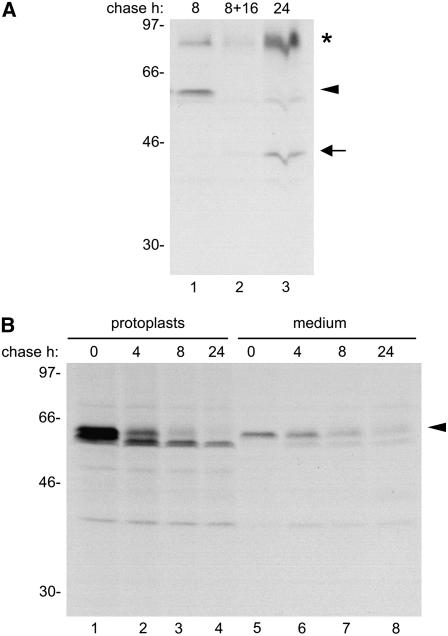
We next investigated the possibility that, at least in part, the 45-kD form could originate from extracellular processing of zeolin, because the latter is almost undetectable in the incubation medium after 24 h of chase in the presence of 2-ME (Figure 4). Protoplasts from plants expressing zeolin were subjected to a 1-h pulse followed by an 8-h chase; the medium was collected and divided into two aliquots: one was immunoselected with anti-phaseolin antiserum (Figure 6A, lane 1), and the other was adjusted to 20 mM 2-ME and used to substitute for the incubation medium of unlabeled protoplasts that had been pretreated for 8 h with 20 mM 2-ME; these protoplasts were incubated for an additional 16 h before immunoprecipitation of the medium with anti-phaseolin antiserum (Figure 6A, lane 2). As a control, the medium from protoplasts labeled for 1 h and subjected to 24 h of chase in the presence of 20 mM 2-ME was also immunoprecipitated (Figure 6A, lane 3). The results indicate that during the 16-h incubation, zeolin was degraded but the 45-kD form was not produced. Therefore, phaseolin is released from zeolin intracellularly and then largely secreted, in a process that is markedly stimulated by 2-ME.
Figure 6.
Secretion of the 45-kD Processed Form of Zeolin.
(A) All protoplasts were prepared from transgenic tobacco expressing zeolin. Protoplasts were pulse-labeled with [35S]Met and [35S]Cys for 1 h followed by an 8-h chase; the incubation medium was collected and divided into two equal aliquots: one was homogenated and immunoselected with anti-phaseolin antiserum (lane 1); the other was adjusted to 20 mM 2-ME and added to unlabeled protoplasts that had been pretreated for 8 h with 20 mM 2-ME, and after an additional 16 h of incubation, the medium was homogenated and immunoselected with anti-phaseolin antiserum (lane 2). As a control, the medium from protoplasts labeled for 1 h and subjected to a 24-h chase in the presence of 20 mM 2-ME was also immunoselected (lane 3).
(B) Protoplasts from transgenic tobacco expressing zeolin were preincubated for 45 min in the presence of BFA and maintained in the presence of the drug for the whole pulse–chase. Pulse with [35S]Met and [35S]Cys was for 1 h, and chase was for the indicated times, in the presence of 20 mM 2-ME. Total homogenates were prepared from protoplasts or incubation medium, using homogenation buffer supplemented with 2-ME (medium) or without the reducing agent (protoplasts), and immunoselected using anti-phaseolin antiserum.
Analysis was by SDS-PAGE and fluorography. The positions of zeolin (arrowheads), the 45-kD form (arrow), and the 95-kD putative O-glycosylated zeolin (asterisk) are indicated. Numbers at left indicate the positions of molecular mass markers in kilodaltons.
To determine whether secretion of the 45- and 95-kD forms used the major secretory pathway mediated by the Golgi complex, we performed pulse–chase in the presence of the fungal metabolite brefeldin A (BFA). BFA inhibits vesicle traffic between the ER and the Golgi complex, leading to merging of the two compartments and a block of post-Golgi traffic of newly synthesized polypeptides (Nebenführ et al., 2002). When treatment with 2-ME was performed in the presence of BFA, the 45- and 95-kD forms were not detectable in the incubation medium or within protoplasts, indicating that they were not produced (Figure 6B). Therefore, Golgi-mediated traffic is necessary for their formation and secretion. The recovery of intact zeolin in the medium was instead not affected by BFA, indicating that either these polypeptides are secreted by a Golgi-independent route or, perhaps more likely, are released from a small fraction of dead protoplasts.
We interpret the data shown in Figures 3 to 6 to indicate that 2-ME inhibits the insolubilization of zeolin and relieves its ER retention, leading to increased release of 45-kD phaseolin polypeptides that are either secreted or sorted to the vacuole, with a variable balance between the two destinies, and secretion of the 95-kD form. We conclude that, in the case of zeolin, the general partial inhibitory effect of 2-ME on the secretory pathway is overcome by the increase in solubility that makes the polypeptides competent for traffic. This finding suggests a role of disulfide bonds in the retention of zeolin within the ER, which was further investigated by mutagenesis of the Cys codons.
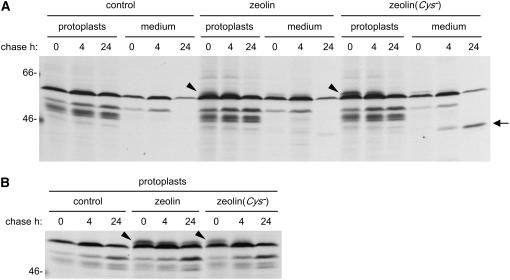
Mutated Zeolin Devoid of Cys Residues Is Secreted
The six Cys codons of zeolin were mutated to Ser codons to study the destiny of a zeolin mutant unable to form disulfide bonds. The mutated zeolin sequence, termed zeolin(Cys−) was transiently expressed in tobacco protoplasts. Transfections with the empty plasmid or with plasmid encoding unmodified zeolin were used as controls. After pulse–chase labeling, protoplasts and incubation medium were homogenized in the presence (Figures 7A, incubation medium, and 7B) or absence (Figure 7A, protoplasts) of 2-ME and the homogenates were immunoprecipitated with anti-phaseolin antiserum. Transiently transfected protoplasts produce on average a lower amount of foreign protein than transgenic protoplasts, because of the inefficiency of the transfection procedure. As a result, contamination of immunoprecipitates by endogenous tobacco polypeptides is quite marked. This is illustrated by transfection with the empty plasmid (Figure 7, control). Most zeolin is already insoluble at the end of the pulse because of disulfide bond formation; it then becomes almost fully insoluble during the chase and is secreted in very small amounts (Figures 7A and 7B, arrowheads; note that immunoprecipitations were performed on equal aliquots of protoplast homogenates but that the fluorograph in B was exposed for half of the time with respect to that in A). This finding indicates a very similar zeolin destiny in transiently transfected and transgenic protoplasts. Zeolin(Cys−) does not become insoluble (Figures 7A and 7B, arrowheads) and is mostly processed into secreted phaseolin (arrow) during the chase. Therefore, Cys residues are responsible for the insolubility of zeolin and its inability to enter traffic along the secretory pathway. The secreted, high molecular mass form of zeolin was not detectable in the pulse–chase results shown in Figure 7, but it was detectable in other repetitions of the same experiment (data not shown), again indicating that there is a subtle balance between proteolytic cleavage and other forms of posttranslational processing once zeolin enters traffic.
Figure 7.
Zeolin(Cys−) Is Secreted.
Protoplasts prepared from leaves of wild-type tobacco were transfected with either pDHA plasmid (control) or pDHA containing the coding sequence of zeolin or zeolin(Cys−). Transfected protoplasts were pulse-labeled with [35S]Met and [35S]Cys for 1 h and subjected to chase for the indicated times. Total homogenates were prepared from protoplasts or incubation medium, using homogenation buffer supplemented either with 2-ME ([B] and medium in [A]) or without the reducing agent (protoplasts in [A]), and immunoselected using anti-phaseolin antiserum. Analysis was by SDS-PAGE and fluorography. The gel in (A) was exposed for 10 d, and that in (B) was exposed for 5 d. The positions of zeolin and zeolin(Cys−) (arrowheads) and of the 45-kD form (arrow) are indicated. Numbers at left indicate the positions of molecular mass markers in kD.
2-ME Does Not Increase the Proportion of Zeolin Associated with BiP
BiP is found in association with both newly synthesized, soluble zeolin polypeptides and insoluble zeolin polymers (Mainieri et al., 2004). Having established that 2-ME inhibits the release of newly synthesized proteins from BiP but simulates traffic of zeolin, we investigated the effect of the reducing agent on interactions between the two proteins. Protoplast homogenation performed in nonreducing conditions and immunoprecipitation with anti-BiP antiserum indicated a substantial increase in coimmunoselected zeolin if the chase was performed in the presence of 2-ME (Figure 8A). This, however, did not correspond to an increase in the total amount of zeolin associated with the chaperone, as indicated by immunoprecipitation of homogenates prepared in the presence of 2-ME (Figure 8B). Immunoprecipitation of homogenates using anti-phaseolin antiserum confirmed that 2-ME supplemented during the chase did not increase the association between BiP and total zeolin (Figure 8D) but solubilized a proportion of zeolin that remained associated with the chaperone (Figure 8C). Therefore 2-ME inhibits in vivo zeolin polymerization but not its association with BiP. The lack of correlation between the increase in zeolin traffic stimulated by 2-ME and the extent of zeolin association with BiP suggests that release from BiP is not the key event leading to zeolin secretion.
Figure 8.
Association between Zeolin and BiP.
Protoplasts from transgenic tobacco expressing zeolin were pulse-labeled with [35S]Met and [35S]Cys for 1 h and subjected to chase for the indicated times in the presence (+) or absence (−) of 20 mM 2-ME. For all panels, chase points and in vivo 2-ME treatments are as in (A). Total homogenates were prepared from protoplasts, using homogenation buffer supplemented with 2-ME ([B] and [D]) or without the reducing agent ([A] and [C]), and immunoselected using anti-BiP ([A] and [B]) or anti-phaseolin ([C] and [D]) antiserum. Analysis was by SDS-PAGE and fluorography. The positions of zeolin (closed arrowheads) and BiP (open arrowheads) are indicated. Numbers at right indicate the positions of molecular mass markers in kD.
2-ME Inhibits the Assembly and Traffic of a Soluble Oligomeric Protein That Contains Intrachain and Interchain Disulfide Bonds
If the disulfide bond–dependent zeolin retention within the ER is attributable to insolubilization rather than directly to the formation of disulfide bonds, the traffic of an oligomeric protein that contains disulfide bonds but is soluble should be inhibited rather than stimulated by 2-ME. This was tested by studying the effect of the reducing agent on the traffic of a tetrameric immunoglobulin expressed in tobacco. We choose a well-characterized IgG/A because this protein is in part secreted, like wild-type IgGs, and in part delivered to the vacuole (Frigerio et al., 2000). Therefore, we could follow both routes. IgA/G has intrachain and interchain disulfide bonds, like IgGs, and a free Cys residue in the C-terminal domain of the heavy chains. Analysis of the immunoprecipitated immunoglobulins was performed by nonreducing SDS-PAGE to follow protein assembly (Frigerio et al., 2000). The protein rapidly assembles into disulfide-bound tetramers that are already prominent at the end of pulse-labeling in protoplasts of transgenic tobacco (Figure 9, arrowhead); assembled tetramers and free light chains (open circle) are secreted during the chase, but part of the assembled molecules is also delivered to the vacuole, where fragmentation products are formed (asterisks) (Frigerio et al., 2000). Secretion of free light chains is probably attributable to the unbalanced synthesis of heavy and light chains in these plants. Treatment with 2-ME inhibited the secretion of tetramers and, to a less extent, of free light chains (Figure 9, medium). Analysis of intracellular immunoglobulins showed an increased accumulation of free light chains and a reduction in the formation of vacuolar fragments (Figure 9, protoplasts). Upon longer exposure of the fluorograph, an increase in intracellular unassembled heavy chains became visible in the 2-ME–treated samples (data not shown). We conclude that 2-ME inhibits the assembly and traffic of IgA/G, supporting our hypothesis that the effect on zeolin is not related to the inhibition of disulfide bond formation per se but to the fact that these are necessary for zeolin assembly into insoluble PBs.
Figure 9.
2-ME Inhibits the Assembly and Traffic of IgA/G.
Protoplasts from transgenic tobacco expressing IgA/G (heavy and light chains) were pulse-labeled with [35S]Met and [35S]Cys for 1 h and subjected to chase for the indicated times in the presence (+) or absence (−) of 2-ME. Protoplast or incubation medium homogenates were then immunoselected using with anti-IgG antiserum. Polypeptides were analyzed by nonreducing SDS-PAGE and fluorography The positions of assembled IgA/G tetramers (arrowhead), unassembled heavy (closed circle) and light (open circle) chains, and IgA/G vacuolar fragmentation products (asterisks) are indicated. Numbers at right indicate molecular mass markers in kD.
DISCUSSION
We have shown here that the reducing agent 2-ME disrupts the process of insolubilization of zeolin and relieves its retention within the ER, resulting in enhanced zeolin traffic along the secretory pathway. This process occurs in spite of a general inhibitory effect of 2-ME on the activity of the plant secretory pathway and on the release of newly synthesized ligands from the chaperone BiP. We have also shown that mutated zeolin that cannot form disulfide bonds is soluble and enters traffic with high efficiency. These results indicate that the formation of insoluble PBs mediated by interchain disulfide bonds is the major, if not the exclusive, determinant of zeolin retention within the ER. It is possible that the same holds true for wild-type γ-zein, which has the same solubility properties and ability to form PBs of zeolin.
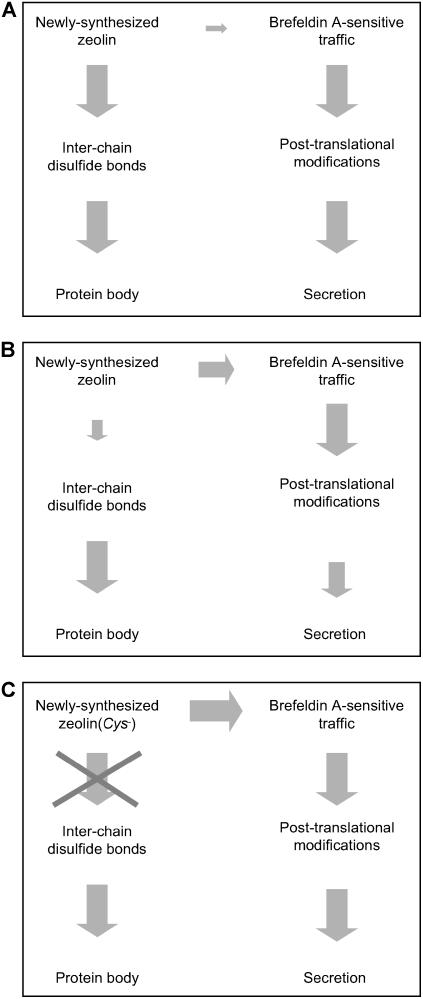
Zeolin solubilization and traffic are only partially stimulated by 20 mM 2-ME, probably because of the partial resistance of disulfide bonds to the reducing agent. Experiments with further increased concentrations of 2-ME or with the much more potent agent DTT, in an effort to increase the solubilization of zeolin, would be difficult to interpret, because of the severe effects on protoplast viability (Piñeiro et al., 1994; our unpublished results). We cannot exclude a possible additional role of other mechanisms (such as interactions of the zein repetitive domain with membrane lipids; see below) in zeolin ER retention, but the destiny of zeolin(Cys−) in transiently transfected protoplasts assigns a major role to disulfide bonds. A model that takes into account our results is shown in Figure 10.
Figure 10.
Model Illustrating the Influence of Disulfide Bonds on Zeolin Destiny.
The size of the gray arrows is roughly proportional to the efficiency of each step, independent of that of the preceding or competing step. Of course, an inefficient or impossible step (small arrows or crossed arrow) becomes limiting for a pathway even if the subsequent steps are efficient or are unaffected by a given treatment (large arrows), but such a cascade of events is not illustrated here because we wish to emphasize key effects.
(A) Destiny of zeolin in normal conditions. The formation of disulfide bonds is very efficient and leads to PB formation, almost completely avoiding traffic that would lead to processing and secretion.
(B) Destiny of zeolin in cells treated with 2-ME. General traffic and secretion are negatively affected, probably at multiple steps, but not fully blocked. Disulfide bond formation of zeolin is also inhibited, allowing the protein to enter traffic.
(C) Destiny of zeolin(Cys−). The formation of disulfide bonds is impossible; therefore, the protein enters traffic with high efficiency.
The Plant Secretory Pathway Is Sensitive to Reducing Agents
Many secretory proteins contain disulfide bonds, which often contribute to proper polypeptide folding and to the assembly of oligomeric proteins. Therefore, it is reasonable to hypothesize that treatment with reducing agents can negatively affect the functions of a number of proteins destined to secretion or vacuolar delivery and, more importantly, of proteins such as folding helpers and receptors that assist and regulate structural maturation and traffic within the plant secretory pathway. In yeast cells, the secretion of yeast pro-α-factor and invertase was not affected by treatment with 20 mM DTT (Jämsä et al., 1994). These proteins are, respectively, devoid of Cys residues and disulfide bonds. Similarly, the secretion of α1-antitrypsin (which is also devoid of disulfide bonds) by human hepatoma cells is only marginally inhibited by 2 mM DTT (Lodish and Kong, 1993). The same treatment of yeast and hepatoma cells, as well as similar treatment in other mammalian systems (Verde et al., 1995; Huuskonen et al., 1998), instead blocks the traffic of a number of proteins that contain disulfide bonds, leading to the conclusion that reducing agents cause ER retention of proteins that normally contain disulfide bonds, probably by the ER quality-control mechanism, but, perhaps surprisingly, do not affect the general activity of the secretory pathway (Lodish and Kong, 1993; Jämsä et al., 1994). Conversely, we have shown here that 2-ME inhibits the intracellular traffic of phaseolin and the secretion of a phaseolin mutant devoid of the vacuolar sorting signal. Because phaseolin does not contain Cys residues, the effect must be on one or more proteins of the secretory pathway machinery. The inhibitory effect is strong in the presence of 20 mM 2-ME but is already clearly detectable at 2 mM 2-ME. In 2-ME–treated protoplasts, phaseolin accumulates in a microsomal subcellular fraction and remains largely sensitive to endoglycosidase H treatment, indicating that the block occurs either in the ER or in cis-cisternae of the Golgi complex. Our results indicate that, at least in the widely used tobacco protoplast system, the plant secretory pathway has a generally higher sensitivity to changes in the redox conditions compared with those of yeast and of the animal cells that have been tested.
When cells are treated with reducing agents, the newly synthesized polypeptides of a number of mammalian or yeast secretory proteins that normally contain disulfide bonds increase their association with BiP and other ER chaperones (Jämsä et al., 1994; Ben-Zeev et al., 2002) and can form heterotypic aggregates excluding proteins that normally are devoid of disulfide bonds (Sawyer et al., 1994). Interactions with chaperones and the formation of large aggregates could in turn determine the inhibitory effect on intracellular traffic. Consistently, we have shown that 2-ME enhances the interactions of many tobacco newly synthesized polypeptides with BiP. However, this relatively simple explanation cannot hold for phaseolin. Therefore, it is likely that a reduction of one or more factors of the machinery necessary for protein folding and traffic causes its loss of function, with a cascade of effects that inhibit traffic and sorting along the plant secretory pathway, independent of the disulfide requirements of individual passenger proteins. The effect is more marked on vacuolar sorting; in this respect, it should be emphasized that the basic mechanisms of protein traffic are conserved in eukaryotes, but the different kingdoms have a number of distinct features, particularly in the traffic to the inner hydrolytic compartments (Jürgens, 2004; Vitale and Hinz, 2005).
Disulfide Bonds Play a Role in Zeolin Accumulation in the ER
The expression of a number of deletion mutants of γ-zein in Arabidopsis thaliana indicates that the N-terminal region, containing the repetitive domain, is fundamental for ER retention, whereas the C-terminal region is necessary for assembly into PBs (Geli et al., 1994). Our studies on zeolin indicate that the C-terminal region, which contains the domains homologous with the 2S albumins, is indeed not strictly necessary for PB formation (Mainieri et al., 2004). A synthetic version of the eight VHLPPP repeats of the γ-zein N-terminal region forms an amphipathic polyproline II structure and interacts with liposomes prepared with soybean (Glycine max) phosphatidylcholine, suggesting that this repeat may directly associate with the ER membrane in vivo (Kogan et al., 2004). This is consistent with the accumulation of γ-zein at the periphery of the PBs, in apparent contact with the ER membrane (Lending and Larkins, 1989). The interactions with membrane lipids could contribute to ER retention. However, the N-terminal region also contains six Cys residues, and we showed that disulfide bonds are necessary for zeolin polymerization and insolubilization (Mainieri et al., 2004). We show here that 2-ME or mutagenesis of the Cys codons enhances the in vivo solubility of zeolin and increases the secretion or vacuolar sorting of the phaseolin portion of zeolin. The release of phaseolin trimers from zeolin also occurs, to a much more limited extent, in normal conditions and is inhibited by the presence of BFA, indicating that it requires traffic (Mainieri et al., 2004). Consistently, the 2-ME–mediated secretion of the released phaseolin is also inhibited by BFA. The repartition between secretion and vacuolar delivery of the released phaseolin varies between different experiments, possibly because of variability in the maintenance of the vacuolar sorting signal after zeolin cleavage. 2-ME and mutagenesis also induce the increased formation and secretion of a 95-kD form of zeolin, which is probably O-glycosylated during traffic through the Golgi complex. Our results indicate that disulfide bonds between the Cys residues present in zeolin play an important role in the retention of the fusion protein within the ER, possibly because of their fundamental role in zeolin polymerization.
Zeolin solubilized in vivo by 2-ME interacts with BiP but becomes available for traffic. Because the amount of BiP-associated zeolin that we detect during the chase points is a very small proportion of total zeolin, we cannot rule out the possibility that it represents a subset of misfolded molecules that will remain unavailable for traffic. However, the results indicate that release from BiP is not the major limiting step in the 2-ME–induced traffic of zeolin molecules.
Comparison with Other Systems
Our results complement and extend a number of studies on the disulfide bond–mediated retention of specific proteins in the ER. Rice α-globulin is a vacuolar storage protein with the typical A, B, and C domains of seed storage proteins of the 2S albumin class. 2S albumins are soluble monomeric proteins that contain four conserved intrachain disulfide bonds and are deposited into storage vacuoles (Shewry et al., 1995). Mutations of individual Cys codons of α-globulin make orphan Cys residues available for interchain interactions in transgenic rice and cause its mistargeting to ER PBs, indicating that interchain disulfide bonds can inhibit intracellular traffic and induce incorporation into at least preexisting PBs (Kawagoe et al., 2005). The ABC domains are also present in many prolamins, which thus seem to have evolved by the insertion of a repetitive domain within the albumin sequence (Shewry et al., 1995). In γ-zein, the ABC domains constitute the C-terminal region, which is not included in zeolin and has nine Cys residues: eight of them possibly form the typical intrachain bonds of 2S albumins; theoretically, the additional residue can be involved in zein polymerization, but it is clearly disposable for PB formation, at least in the zeolin construct.
The role of disulfide bonds in the sorting of regulated secretory proteins of mammalian cells has been investigated extensively. In endocrine, neuroendocrine, and exocrine cells, these proteins are stored in post-Golgi secretory granules that secrete their content only upon stimulation. The cell biology of these proteins has attracted the interest of researchers who study seed storage proteins, because the formation of highly condensed polymers has a role in sorting into secretory granules (Arvan et al., 2002; Vitale and Hinz, 2005). Unstimulated secretion of newly synthesized chromogranin B was observed in PC-12 nerve cells treated with DTT, implying a role of disulfide bonds in the sorting into secretory granules and therefore in preventing default secretion (Chanat et al., 1993; Gorr et al., 1999). However, this is not a general feature of regulated secretion, because treatment with reducing agents differentially affects the traffic and sorting of regulated secretory proteins, depending on both the individual protein and the cell type (Gorr et al., 1999).
Finally, immunoglobulins are a well-characterized example of how disulfide interactions can prevent the traffic of secretory proteins. Exposed free Cys residues mediate the ER retention of the assembly intermediates of tetrameric IgG and polymeric IgA and IgM (Guenzi et al., 1994; Valetti and Sitia, 1994; Reddy et al., 1996). This is a form of ER quality control: the key element in the retention mechanism is the thiol-containing ER resident protein ER p44, but other residents forming the so-called ER matrix are probably also involved (Anelli et al., 2003). Treatment with 2-ME relieves this retention and leads to the enhanced secretion of unassembled light chains and partially assembled IgA and IgM, whereas the much more potent reducing agent DTT causes the reduction of both interchain and intrachain disulfide bonds, leading to misfolding that enhances instead of relieves ER retention, probably because of stronger interactions of exposed domains with chaperones such as BiP (Guenzi et al., 1994; Valetti and Sitia, 1994; Reddy et al., 1996). We have shown here that 2-ME inhibits the traffic and assembly of IgA/G and inhibits instead of enhances the secretion of light chains, again indicating that the plant secretory pathway is more sensitive than that of mammalian cells to reducing agents.
The examples described above indicate that at different steps of the secretory pathway, interchain disulfide bonds either between specific passenger proteins or between these and the folding machinery can lead to the retention of protein in the ER or in specialized secretory granules. We have shown that disulfide bonds also play a role in the formation and ER retention of plant PBs, structures that are formed by proteins that do not have counterparts in other eukaryotes.
METHODS
Plant Growth, Protoplast Preparation, and Pulse–Chase Labeling
Wild-type tobacco plants (Nicotiana tabacum cv Petit Havana SR1) or transgenic plants expressing zeolin (Mainieri et al., 2004), T343F phaseolin (Pedrazzini et al., 1997), Δ418 phaseolin (Frigerio et al., 1998), or IgA/G (Ma et al., 1994) were cultured in axenic conditions. Protoplasts were prepared from young (4 to 7 cm long) leaves as described (Pedrazzini et al., 1994). Pulse–chase labeling of protoplasts was performed using Pro-Mix (a mixture of [35S]Met and [35S]Cys; Amersham Biosciences) as described (Pedrazzini et al., 1997). For treatments with 2-ME (Sigma-Aldrich), the reducing agent was added at the end of pulse-labeling at the appropriate concentrations. For treatment with BFA (Roche), the inhibitor was supplemented to the protoplast incubation medium at 10 μg/mL at 45 min before radioactive labeling and was maintained at the same concentration throughout the pulse–chase. Protoplasts were collected immediately after labeling by the addition of 3 volumes of ice-cold W5 medium (154 mM NaCl, 5 mM KCl, 125 mM CaCl2·2H2O, and 5 mM glucose) and centrifugation at 60g and 4°C for 10 min. The supernatant (incubation medium) containing secreted proteins and the protoplast pellet were frozen in liquid nitrogen and stored at −80°C, but freezing was avoided for subcellular fractionation experiments.
For transient protein expression, protoplasts were isolated from small leaves of wild-type tobacco SR1 plants grown in axenic conditions and subjected to polyethylene glycol–mediated transfection as described (Pedrazzini et al., 1997) using 80 μg of plasmid. After overnight recovery, protoplasts were subjected to pulse–chase labeling as described above.
Protoplast Homogenation, Protein Immunoselection, and Protein Blotting
Homogenization of the protoplast incubation medium was performed by adding to frozen samples 2 volumes of ice-cold 1.5× protoplast homogenation buffer (150 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1.5 mM EDTA, 1.5% Triton X-100, and Complete protease inhibitor cocktail [Roche]) supplemented with 4% (v/v) 2-ME. Homogenation of protoplasts was performed similarly using homogenation buffer supplemented or not with 2-ME. Proteins were immunoselected as described (Pedrazzini et al., 1997) using rabbit polyclonal antisera against phaseolin purified from bean (Phaseolus vulgaris) seeds, recombinant tobacco BiP (Pedrazzini et al., 1997), or rabbit anti-mouse IgG (Sigma-Aldrich). For immunoprecipitation in reducing conditions, the immunoprecipitation buffer was supplemented with 4% 2-ME. Samples were analyzed by SDS-PAGE and fluorography using 15% acrylamide gels followed by fluorography. The sample denaturation buffer for SDS-PAGE always contained 4% 2-ME, except for electrophoresis in nonreducing conditions. Gels were treated with 2,5-diphenyloxazole dissolved in DMSO and dried. Rainbow 14C-methylated proteins (Sigma-Aldrich) were used as molecular mass markers.
For protein blotting, 500,000 protoplasts were incubated for 24 h in the presence or absence of 20 mM 2-ME, and the incubation medium was then removed and stored at −80°C. Protoplasts were washed with 500 μL of W5 medium, collected by centrifugation for 10 min at 60g and 4°C, and stored at −80°C. Incubation media and pelleted protoplasts were homogenated at 4°C with denaturation buffer for SDS-PAGE and analyzed by SDS-PAGE and protein blot using anti-phaseolin antiserum (1:5000 dilution) and the Super-Signal West Pico chemiluminescent substrate (Pierce Chemical).
Subcellular Fractionation and Endoglycosidase H Digestion
For subcellular fractionation, immediately after labeling, protoplast pellets were resuspended in 350 μL of buffer B (100 mM Tris-HCl, pH 7.6, 10 mM KCl, and 1 mM EDTA) supplemented with 12% (w/w) sucrose and homogenized by pipetting the resuspension 30 times through a Gilson 200-μL tip. The homogenate was loaded on top of 300 μL of buffer B supplemented with 17% (w/w) sucrose and centrifuged in a Beckman SW 55 Ti rotor (5- × 41-mm tubes) at 150,000g for 30 min at 4°C. Pellets (microsomes) and the 12% sucrose supernatants (soluble proteins) were diluted in protoplast homogenization buffer and immunoprecipitated as described above. Digestion with endoglycosidase H was performed using endo Hf (New England Biolabs; see the manufacturer's specifications for the compositions of storage, denaturing, and reaction buffers). After phaseolin immunoselection, the protein A–Sepharose beads were washed twice with ice-cold water and resuspended in 50 μL of endoglycosidase H denaturing buffer. The resuspension was denatured for 10 min at 90°C. Five microliters of 10× reaction buffer was added, and the solution was divided into two equal aliquots: one was treated with 200 IUB milliunits of endo Hf, and the other (control) was supplemented with an equal volume of endo Hf storage buffer. Incubation was for 1 h at 37°C. The samples were analyzed by SDS-PAGE and fluorography.
Mutagenesis of the Zeolin Coding Sequence
To change the six Cys codons of zeolin to Ser codons, the following pairs of primers were used (underlined letters indicate the mutated codons): forward 1, 5′-GGTGGATCCGGTGGGGGAGGGAGTGGTGGAGGCGGTTCTGGCGGTAGCGGATCTCAGCCACCTCCACCGGTCCATTTGCCCCCTCCAGTTCATTTACCGC-3′; reverse 1, 5′-GGTGCACAGGTGGAGGTAGGTGGACTGGAGGTGGAAGGTGAACAGGTGGGGGCAGATGAACGGGAGGCGGTAAATGAACTGGAGGG-3′; forward 2, 5′-CCACCTACCTCCACCTGTGCACCTCCCACCTCCCGTTCATGTACCGCCACCTGTGCATTTACCACCTCCACCTAGCCACTATCCTACTCAGCCTCCA-3′; reverse 2, 5′-CTGCAGCTATTGCGACGGACTAGGATGAGGCTGTTGTGACGGAGACGGGTGTGGTTGAGGATGCGGTTGTGGTCTTGGAGGCTGAGTAGGATAGT-3′. After annealing and filling in, using the zeolin coding sequence inserted into the pDHA plasmid (pDHA-zeolin) (Mainieri et al., 2004) as template, PCR amplification was performed using the primers 5′-GGTGGATCCGGTGGGGG-3′ (forward) and 5′-TTCCAATGCATTGGCTGCAGCTATTGCGACGG-3′ (reverse). The PCR product was restricted with BamHI and PstI and used to substitute for the corresponding fragment of wild-type zeolin in the HindIII/XbaI zeolin fragment cloned into pBluescript II SK−. The mutated HindIII/XbaI fragment was finally excised and used to substitute for the corresponding sequence in pDHA-zeolin, to produce pDHA-zeolin(Cys−).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The Polypeptide with Molecular Mass of <30 kD Is Not Released from BiP by ATP Treatment.
Supplemental Figure 2. The Different Forms of Zeolin Secreted by Protoplasts Are Soluble.
Supplementary Material
Acknowledgments
We thank Aniello Santoro for performing the IgA/G experiment and Julian K.-C. Ma for providing the IgA/G-expressing plants. This work was supported in part by Research Training Networks Contract HPRN-CT-2002-00262 (BioInteractions) of the European Union.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Alessandro Vitale (vitale@ibba.cnr.it).
Online version contains Web-only data.
References
- Andersson, H., Kappeler, F., and Hauri, H.P. (1999). Protein targeting to endoplasmic reticulum by dilysine signals involves direct retention in addition to retrieval. J. Biol. Chem. 274 15080–15084. [DOI] [PubMed] [Google Scholar]
- Anelli, T., Alessio, M., Bachi, A., Bergamelli, L., Bertoli, G., Camerini, S., Mezghrani, A., Ruffato, E., Simmen, T., and Sitia, R. (2003). Thiol-mediated protein retention in the endoplasmic reticulum: The role of ERp44. EMBO J. 22 5015–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan, P., Zhang, B.Y., Feng, L., Liu, M., and Kuliawat, R. (2002). Lumenal protein multimerization in the distal secretory pathway/secretory granules. Curr. Opin. Cell Biol. 14 448–453. [DOI] [PubMed] [Google Scholar]
- Bagga, S., Adams, H., Kemp, J.D., and Sengupta-Gopalan, C. (1995). Accumulation of 15-kilodalton zein in novel protein bodies in transgenic tobacco. Plant Physiol. 107 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga, S., Adams, H., Rodriquez, F.D., Kemp, J.D., and Sengupta-Gopalan, C. (1997). Co-expression of the maize δ- and β-zein genes results in stable accumulation of δ-zein in ER-derived protein bodies formed by β-zein. Plant Cell 9 1683–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zeev, O., Mao, H.Z., and Doolittle, M.H. (2002). Maturation of lipoprotein lipase in the endoplasmic reticulum. Concurrent formation of functional dimers and inactive aggregates. J. Biol. Chem. 277 10727–10738. [DOI] [PubMed] [Google Scholar]
- Braakman, I., Helenius, J., and Helenius, A. (1992). Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 11 1717–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanat, E., Weiss, U., Huttner, W.B., and Tooze, S.A. (1993). Reduction of the disulfide bond of chromogranin B (secretogranin I) in the trans-Golgi network causes its missorting to the constitutive secretory pathways. EMBO J. 12 2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, C.E., Herman, E.M., Takasaki, K., and Larkins, B.A. (1996). The maize γ-zein sequesters α-zein and stabilizes its accumulation in protein bodies of transgenic tobacco endosperm. Plant Cell 8 2335–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts, A.J., Leborgne-Castel, N., Pesca, M., Vitale, A., and Denecke, J. (1998). BiP and calreticulin form an abundant complex that is independent of endoplasmic reticulum stress. Plant Cell 10 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio, L., de Virgilio, M., Prada, A., Faoro, F., and Vitale, A. (1998). Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. Plant Cell 10 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio, L., Vine, N.D., Pedrazzini, E., Hein, M.B., Wang, F., Ma, J.K., and Vitale, A. (2000). Assembly, secretion, and vacuolar delivery of a hybrid immunoglobulin in plants. Plant Physiol. 123 1483–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli, M.I., Torrent, M., and Ludevid, D. (1994). Two structural domains mediate two sequential events in γ-zein targeting: Protein endoplasmic reticulum retention and protein body formation. Plant Cell 6 1911–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorr, S.U., Huang, X.F., Cowley, D.J., Kuliawat, R., and Arvan, P. (1999). Disruption of disulfide bonds exhibits differential effects on trafficking of regulated secretory proteins. Am. J. Physiol. 277 C121–C131. [DOI] [PubMed] [Google Scholar]
- Guenzi, S., Fra, A.M., Sparvoli, A., Bet, P., Rocco, M., and Sitia, R. (1994). The efficiency of cysteine-mediated intracellular retention determines the differential fate of secretory IgA and IgM in B and plasma cells. Eur. J. Immunol. 24 2477–2482. [DOI] [PubMed] [Google Scholar]
- Hanton, S.L., Bortolotti, L.E., Renna, L., Stefano, G., and Brandizzi, F. (2005). Crossing the divide—Transport between the endoplasmic reticulum and Golgi apparatus in plants. Traffic 6 267–277. [DOI] [PubMed] [Google Scholar]
- Huuskonen, J., Jauhiainen, M., Ehnholm, C., and Olkkonen, V.M. (1998). Biosynthesis and secretion of human plasma phospholipid transfer protein. J. Lipid Res. 39 2021–2030. [PubMed] [Google Scholar]
- Isidoro, C., Maggioni, C., Demoz, M., Pizzagalli, A., Fra, A.M., and Sitia, R. (1996). Exposed thiols confer localization in the endoplasmic reticulum by retention rather than retrieval. J. Biol. Chem. 271 26138–26142. [DOI] [PubMed] [Google Scholar]
- Jämsä, E., Simonen, M., and Makarow, M. (1994). Selective retention of secretory proteins in the yeast endoplasmic reticulum by treatment of cells with a reducing agent. Yeast 10 355–370. [DOI] [PubMed] [Google Scholar]
- Jürgens, G. (2004). Membrane trafficking in plants. Annu. Rev. Cell Dev. Biol. 20 481–504. [DOI] [PubMed] [Google Scholar]
- Kawagoe, Y., Suzuki, K., Tasaki, M., Yasuda, H., Akagi, K., Katoh, E., Nishizawa, N.K., Ogawa, M., and Takaiwa, F. (2005). The critical role of disulfide bond formation in protein sorting in the endosperm of rice. Plant Cell 17 1141–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, E.M., Mascheroni, L., Pompa, A., Ragni, L., Weimar, T., Lilley, K.S., Dupree, P., and Vitale, A. (2006). Plant endoplasmin supports the protein secretory pathway and has a role in proliferating tissues. Plant J., in press. [DOI] [PubMed]
- Kogan, M.J., Lopez, O., Cocera, M., Lopez-Iglesias, C., De La Maza, A., and Giralt, E. (2004). Exploring the interaction of the surfactant N-terminal domain of γ-zein with soybean phosphatidylcholine liposomes. Biopolymers 73 258–268. [DOI] [PubMed] [Google Scholar]
- Lee, M.C., Miller, E.A., Goldberg, J., Orci, L., and Schekman, R. (2004). Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 20 87–123. [DOI] [PubMed] [Google Scholar]
- Lending, C.R., and Larkins, B.A. (1989). Changes in the zein composition of protein bodies during maize endosperm development. Plant Cell 1 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Wu, Y., Zhang, D.Z., Gillikin, J.W., Boston, R.S., Franceschi, V.R., and Okita, T.W. (1993). Rice prolamine protein body biogenesis: A BiP-mediated process. Science 262 1054–1056. [DOI] [PubMed] [Google Scholar]
- Lodish, H.F., and Kong, N. (1993). The secretory pathway is normal in dithiothreitol-treated cells, but disulfide-bonded proteins are reduced and reversibly retained in the endoplasmic reticulum. J. Biol. Chem. 268 20598–20605. [PubMed] [Google Scholar]
- Ma, J.K.-C., Lehner, T., Stabila, P., Fux, C.I., and Hiatt, A. (1994). Assembly of monoclonal antibodies with IgG1 and IgA heavy chain domains in transgenic tobacco plants. Eur. J. Immunol. 24 131–138. [DOI] [PubMed] [Google Scholar]
- Mainieri, D., Rossi, M., Archinti, M., Bellucci, M., De Marchis, F., Vavassori, S., Pompa, A., Arcioni, S., and Vitale, A. (2004). Zeolin. A new recombinant storage protein constructed using maize γ-zein and bean phaseolin. Plant Physiol. 136 3447–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ, A., Ritzenthaler, C., and Robinson, D.G. (2002). Brefeldin A: Deciphering an enigmatic inhibitor of secretion. Plant Physiol. 130 1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzini, E., Giovinazzo, G., Bielli, A., de Virgilio, M., Frigerio, L., Pesca, M., Faoro, F., Bollini, R., Ceriotti, A., and Vitale, A. (1997). Protein quality control along the route to the plant vacuole. Plant Cell 9 1869–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzini, E., Giovinazzo, G., Bollini, R., Ceriotti, A., and Vitale, A. (1994). Binding of BiP to an assembly-defective protein in plant cells. Plant J. 5 103–110. [Google Scholar]
- Piñeiro, M., Garcia-Olmedo, F., and Diaz, I. (1994). Redox modulation of the expression of bacterial genes encoding cysteine-rich proteins in plant protoplasts. Proc. Natl. Acad. Sci. USA 91 3867–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, P., Sparvoli, A., Fagioli, C., Fassina, G., and Sitia, R. (1996). Formation of reversible disulfide bonds with the protein matrix of the endoplasmic reticulum correlates with the retention of unassembled Ig light chains. EMBO J. 15 2077–2085. [PMC free article] [PubMed] [Google Scholar]
- Rutkowski, D.T., and Kaufman, R.J. (2004). A trip to the ER: Coping with stress. Trends Cell Biol. 14 20–28. [DOI] [PubMed] [Google Scholar]
- Sawyer, J.T., Lukaczyk, T., and Yilla, M. (1994). Dithiothreitol treatment induces heterotypic aggregation of newly synthesized secretory proteins in HepG2 cells. J. Biol. Chem. 269 22440–22445. [PubMed] [Google Scholar]
- Shewry, P.R., and Halford, N.G. (2002). Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 53 947–958. [DOI] [PubMed] [Google Scholar]
- Shewry, P.R., Napier, J.A., and Tatham, A.S. (1995). Seed storage proteins: Structures and biosynthesis. Plant Cell 7 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia, R., and Braakman, I. (2003). Quality control in the endoplasmic reticulum protein factory. Nature 426 891–894. [DOI] [PubMed] [Google Scholar]
- Sonnichsen, B., Fullekrug, J., Nguyen Van, P., Diekmann, W., Robinson, D.G., and Mieskes, G. (1994). Retention and retrieval: Both mechanisms cooperate to maintain calreticulin in the endoplasmic reticulum. J. Cell Sci. 107 2705–2717. [DOI] [PubMed] [Google Scholar]
- Szczesna-Skorupa, E., and Kemper, B. (2000). Endoplasmic reticulum retention determinants in the transmembrane and linker domains of cytochrome P450 2C1. J. Biol. Chem. 275 19409–19415. [DOI] [PubMed] [Google Scholar]
- Tatu, U., Braakman, I., and Helenius, A. (1993). Membrane glycoprotein folding, oligomerization and intracellular transport: Effects of dithiothreitol in living cells. EMBO J. 12 2151–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta, E.S., Simons, J.F., and Helenius, A. (1996). Endoplasmic reticulum glucosidase II is composed of a catalytic subunit, conserved from yeast to mammals, and a tightly bound noncatalytic HDEL-containing subunit. J. Biol. Chem. 271 27509–27516. [DOI] [PubMed] [Google Scholar]
- Valetti, C., and Sitia, R. (1994). The differential effects of dithiothreitol and 2-mercaptoethanol on the secretion of partially and completely assembled immunoglobulins suggest that thiol-mediated retention does not take place in or beyond the Golgi. Mol. Biol. Cell 5 1311–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde, C., Pascale, M.C., Martire, G., Lotti, L.V., Torrisi, M.R., Helenius, A., and Bonatti, S. (1995). Effect of ATP depletion and DTT on the transport of membrane proteins from the endoplasmic reticulum and the intermediate compartment to the Golgi complex. Eur. J. Cell Biol. 67 267–274. [PubMed] [Google Scholar]
- Vitale, A., and Ceriotti, A. (2004). Protein quality control mechanisms and protein storage in the endoplasmic reticulum. A conflict of interests? Plant Physiol. 136 3420–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, A., and Hinz, G. (2005). Sorting of proteins to storage vacuoles: How many mechanisms? Trends Plant Sci. 10 316–323. [DOI] [PubMed] [Google Scholar]
- Vuori, K., Pihlajaniemi, T., Myllyla, R., and Kivirikko, K.I. (1992). Site-directed mutagenesis of human protein disulphide isomerase: Effect on the assembly, activity and endoplasmic reticulum retention of human prolyl 4-hydroxylase in Spodoptera frugiperda insect cells. EMBO J. 11 4213–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.