Abstract
The Tat protein is essential for HIV type 1 (HIV-1) replication and may be an important virulence factor in vivo. We studied the role of Tat in viral pathogenesis by immunizing rhesus macaques with chemically inactivated Tat toxoid and challenging these animals by intrarectal inoculation with the simian/human immunodeficiency virus 89.6PD. Immune animals had significantly attenuated disease with lowered viral RNA, interferon-α, and chemokine receptor expression (CXCR4 and CCR5) on CD4+ T cells; these features of infection have been linked to in vitro effects of Tat and respond similarly to extracellular Tat protein produced during infection. Immunization with Tat toxoid inhibits key steps in viral pathogenesis and should be included in therapeutic or preventive HIV-1 vaccines.
The Tat protein of HIV type 1 (HIV-1) is a critical component in the mechanisms for AIDS pathogenesis (reviewed in ref. 1). It is found both in the nucleus of infected cells where it serves a conventional role in virus transcription (reviewed in ref. 2) and as a secreted protein (3, 4) that can bind to the cell surface through electrostatic interactions, chemokine receptors (5), or cell surface integrins (6). Rapid uptake and importation of Tat into the nucleus occurs in many different cell types (7); however, some biological effects of Tat require only membrane binding because they occur below the concentrations needed for transactivation of nuclear gene expression (3, 4). Cells treated with Tat showed increased expression of chemokine receptors (8, 9), lower T-cell responses to antigenic stimulation (10), overproduction of interferon-α (IFN-α) (11), and enhanced HIV-1 replication (12). Extracellular Tat also promotes T cell destruction by increasing expression of CD95L/Fas ligand on monocyte/macrophages (13) and sensitizing cells to the effects of this molecule (14, 15). Thus, the role of Tat in HIV pathogenesis is not only as an essential protein for HIV replication in already infected cells, but also as an extracellular toxin (1) that increases the efficiency for virus dissemination and reduces antiviral immunity to promote HIV-1 disease.
The potential for therapeutic or preventive immunization with Tat protein has been addressed in both animal and human clinical studies. Macaques immunized with biologically active Tat (16) or recombinant vaccinia vectors expressing Tat and Rev proteins (17) showed lower virus burden after challenge. The presence of anti-Tat serum antibodies (18) or Tat-specific cytotoxic lymphocyte responses (19) were correlated with slow progression in HIV-infected individuals. Therapeutic immunization with chemically inactivated Tat toxoid elicited strong immune responses in human beings that may be associated with clinical improvement (20, 21). Our work tested the capacity for immunization with Tat toxoid to protect rhesus macaques against the virulent simian/HIV (SHIV) 89.6PD isolate (22), when virus is given by intrarectal inoculation as a model for sexually transmitted infection. We show that effective immunization with Tat toxoid failed to protect against mucosal transmission but did attenuate virus replication and disease. Further, we have been able to show changes in IFN-α production and chemokine receptor expression in immunized and challenged animals, as evidence that these effects of Tat protein that were known from in vitro studies are important parts of the viral pathogenesis mechanism in SHIV-challenged macaques.
Materials and Methods
Animal Immunization, Virus Stocks, and Challenges.
Healthy rhesus macaques (Macaca mulatta) were immunized (Table 1) with chemically inactivated Tat toxoid (23), recombinant vaccinia virus expressing gp160, soluble gp160 (MN strain from Pasteur Merieux Connaught, Paris), or biologically active Tat protein (Advanced Biosciences Laboratories, Rockville, MD), using either incomplete Freund's or polyphosphazene (Avant Immunotherapeutics, Cambridge, MA) adjuvant. Titered SHIV89.6PD stocks were kindly provided by Yichen Lu (Harvard School of Public Health) at a concentration of 25,000 TCID50 per ml of culture fluid. This stock had been used previously for pathogenesis and vaccine challenge studies (24, 25). Methods for mucosal inoculation were described previously (26). All protocols were approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Madison.
Table 1.
Group definitions and immunization schedules
| Day (relative to infection) | Immunogen | Adjuvant | Route |
|---|---|---|---|
| Group A1: Immunization with Tat toxoid alone, n = 8 | |||
| −42 | 20 μg of Tat Toxoid | Adjumer* | ID† |
| −35 | 40 μg of Tat Toxoid | Adjumer | ID |
| −28 | 60 μg of Tat Toxoid | Adjumer | ID |
| −21 | 60 μg of Tat Toxoid | Adjumer | IM† |
| +148 | 40 μg of Tat Toxoid | Adjumer | IM |
| Group A2: Immunization with Tat toxoid plus gp160, n = 4 | |||
| −68 | 20 μg of Tat Toxoid | Adjumer | ID |
| −62 | 40 μg of Tat Toxoid | Adjumer | ID |
| −56 | 40 μg of Tat Toxoid | Adjumer | ID |
| −48 | 80 μg of Tat Toxoid | IFA* | IM |
| −41 | vv-gp 160‡ | none | ID |
| −13 | 100 μg of gp 160 | IFA | IM |
| −13 | 40 μg of Tat Toxoid | IFA | IM |
| Group A3: Immunization with native Tat alone, n = 4 | |||
| −68 | 10 μg of native Tat | Adjumer | ID |
| −62 | 20 μg of native Tat | Adjumer | ID |
| −56 | 20 μg of native Tat | Adjumer | ID |
| −48 | 40 μg of native Tat | IFA | IM |
| −13 | 20 μg of native Tat | IFA | IM |
| Group B: Unimmunized control (4 animals) | |||
| Group C: Referential control (10 animals) | |||
Adjumer is the registered trademark for polyphosphazene (Avant Immunotherapeutics). IFA, incomplete Freund's adjuvant.
†ID, intradermal route for delivery; IM, intramuscular delivery.
‡vv-gp160 is a recombinant vaccinia virus expressing gp160 from the HIV-HXBc2 isolate.
Immune Responses and Flow Cytometry.
Anti-Tat serum antibodies were measured by standard ELISA assays (23), and Tat-neutralizing activities were measured with the Tat transactivation assay (4). Tat-specific lymphoproliferative responses were assessed in peripheral blood mononuclear cell cultures by using 2 μg of Tat protein per well with a concentration of 1 × 105 Ficoll-purified lymphocytes in 200 μl of culture volume. Proliferation was measured by 3H-thymidine incorporation, and the stimulation index was calculated according the formula (cpm incorporated in stimulated cultures/cpm incorporated with medium alone). Stimulation indices greater than or equal to 3 were considered positive. Purified peripheral blood mononuclear cells were stained with lymphocyte subset antibodies that were previously tested and verified crossreactive for rhesus macaques (27). Chemokine receptor levels were measured with CCR5-specific and CXCR4-specific monoclonal antibodies (R & D Systems). Total lymphocyte counts were determined by automated hematology.
Plasma Virus Levels and IFN-α Levels.
Plasma p27 antigen concentrations were measured with sandwich ELISA kits. Plasma vRNA levels were measured by the Viroquant reverse transcription–PCR assay (Viroquant, Paris). Plasma IFN-α levels were measured with a plaque reduction assay by using vesicular stomatitis virus infection of MDBK cells as described (11).
Results
Macaques were immunized against HIV-1 Tat (either the chemically inactivated Tat toxoid or biologically active Tat), then were challenged with a pathogenic SHIV to determine whether immune responses against Tat would prevent or slow disease. The immunized animals together with control macaques (Group B) were infected by using the same conditions (intrarectal inoculation with 2,500 TCID of SHIV89.6PD) as were macaques (Group C) previously challenged in 1996 and followed up as a referential control group for all SHIV89.6PD infection experiments conducted at our Center. The Tat protein from SHIV89.6P (a parallel isolate to the SHIV89.6PD stock) differed by only two amino acids from the sequence found in HIV-1 HXBc2 (28) that was used for production of the Tat protein. The HIV-1 HXBc2 envelope gene sequence used in the recombinant vaccinia virus and for production of soluble gp160 differed in several regions from the 89.6 envelope gene sequence used in the SHIV (29) with substantial variation in the V3 loop.
We tested whether immunization could protect against infection or attenuate disease progression. As our endpoints, we selected plasma viral RNA copies, p27 antigenemia, and CD4 T cell counts that are known to be correlated with progression (25). We also included circulating IFN-α levels and chemokine receptor expression; these markers are linked to disease progression and are triggered by Tat in vitro (8, 9, 11). Because the stable levels of plasma virus (or set-point) develop within 4 weeks after infection, we selected this time point for analyzing plasma vRNA burden.
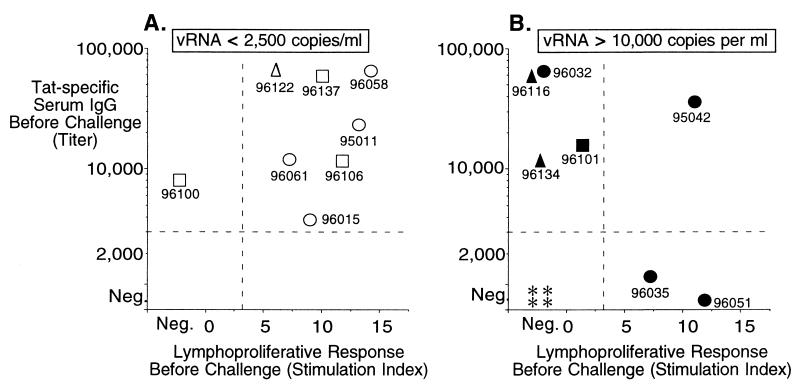
In 14 of 16 animals immunized with Tat toxoid or Tat, we observed antibody responses with titers ranging from 5,000 to over 64,000 (Fig. 1); the immune sera neutralized Tat in vitro (not shown). Tat-specific lymphoproliferative responses were seen in 10 of 15 immunized animals before challenge (Fig. 1). Antibody responses to gp160 immunization were variable because of the fact that animals received only a single recombinant vaccinia and a single soluble protein immunization (not shown). Although immune responses were variable, 8 of 15 macaques developed both humoral and cellular responses against Tat. Importantly, among these eight animals, seven had vRNA at or below the level of detection (Fig. 1A) by 8 weeks after inoculation. Among the seven animals that developed either a humoral or cellular response alone, only one (Fig. 1A) showed a similarly low level of vRNA at the set-point. These data show that, among animals that responded strongly to immunization (with both types of responses measured here), 88% were protected against high level virus replication and disease.
Figure 1.
Immune responses and virus burden at set-point (56 days after inoculation) for immunized and control animals challenged by intrarectal inoculation with SHIV89.6PD. Samples collected before challenge were characterized for anti-Tat serum antibody titers (reciprocal of greatest dilution that was positive by ELISA) and Tat-specific lymphoproliferative responses (stimulation indices). Data were separated according to the plasma viral burden at 56 days after infection (set-point); A shows low viral burden (open symbols), and B (closed symbols) shows high viral burden. Symbols indicate whether animals were immunized with Tat toxoid (circle), Tat toxoid plus gp160 (square), Tat (triangle), or Group B control (asterisk). Data points are labeled with the animal identification numbers.
Even though immunization was effective in protecting against disease, it did not provide sterilizing immunity against virus transmission. All control and immunized macaques, with the exception of one in Group A3 (native Tat), became infected after intrarectal SHIV89.6PD challenge. Macaque 079 (native Tat) showed no signs of productive or transient infection, including being negative for plasma viral RNA, and did not seroconvert. This individual case does not demonstrate protection against virus infection as a result of immunization. The efficiency of intrarectal SHIV89.6PD infection in rhesus macaques is ≈95%, and it was expected that one animal would fail to be infected in an experiment of this size.
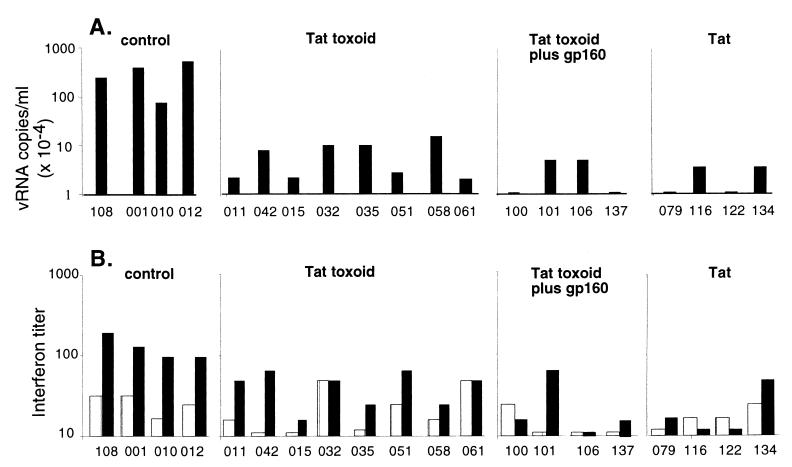
Plasma viral RNA levels were significantly lower in the immunized animals compared with Group B controls (P < 0.001) at 4 weeks after infection (Fig. 2A). By 8 weeks after infection, 4 of 4 control animals had plasma viral RNA levels above 104 copies per ml whereas 7 of 15 immunized and infected macaques declined below this critical threshold level (P < 0.001) and had set-point vRNA levels below the limit of detection (Fig. 1). Similar results were observed for plasma p27 antigenemia levels at the peak of responses: i.e., 2 weeks after infection. At this point, there were significant differences in plasma p27 between the immunized and referential control Group C (P = 0.03) or contemporary control Group B (P = 0.05), although there were no statistical differences between the referential and contemporary control groups (not shown). We also analyzed plasma IFN-α levels in unimmunized (Group B) and immunized (Groups A1, A2, and A3) animals within 4–8 weeks after infection (Fig. 2B). Tat toxoid immunization decreased IFN-α titers by up to 10-fold compared with controls (P < 0.001).
Figure 2.
Immunization decreases plasma virus burden and IFN-α accumulation. The upper four panels (A) show plasma viral RNA levels at 4 weeks after SHIV89.6PD infection in immunized or control macaques. Data are expressed on a semilogarithmic scale with a lower limit of sensitivity at 2,500 copies per ml. The lower four panels (B) show levels of plasma IFN-α on the day of challenge (open bars) and 8 weeks after challenge (solid bars). IFN-α levels were measured as described in the text. Results are expressed as reciprocal of the largest plasma dilution that produced a 50% reduction in the plaque count. Individual data point are coded with the last three digits of each animal identification number.
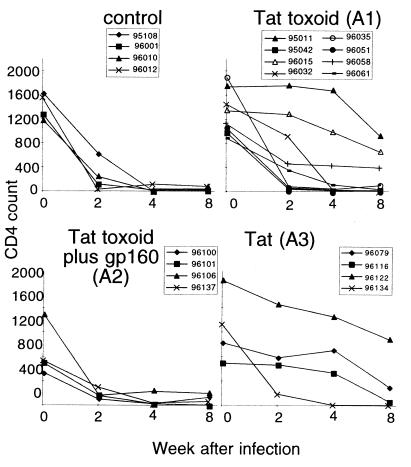
We did not observe statistically significant differences in the average rates of CD4+ T cell decline between the control and immunized groups (Fig. 3). However, relationships were apparent between the anti-Tat immune response before challenge and subsequent changes in CD4+ T cell counts. Animals 011, 015, and 058 (Tat toxoid alone) all had strong CMI and antibody responses to Tat before challenge, and their CD4 T cell counts remained in the normal range (averaging 48% of the starting CD4 T cell count) even by 8 weeks after infection. Animal 061 in this same group had moderate immune responses to Tat before challenge and still managed to maintain 14% of the starting CD4 T cell count by 8 weeks after infection, a level above the threshold for slow progressors, according to previously published criteria (25). Unimmunized control macaques all experienced sharp drops in the CD4+ T cell count (Fig. 3). By 8 weeks, all control animals had less than 10% of their starting CD4+ T cell population.
Figure 3.
Changes in CD4 T cell count for control and immunized animals through 8 weeks after virus challenge. CD4+ T cell counts were obtained from flow cytometry analysis (to determine CD4 T cell percentage) and the absolute lymphocyte count in blood (from automated cell counts). Data are provided for individual macaques (identified in each legend).
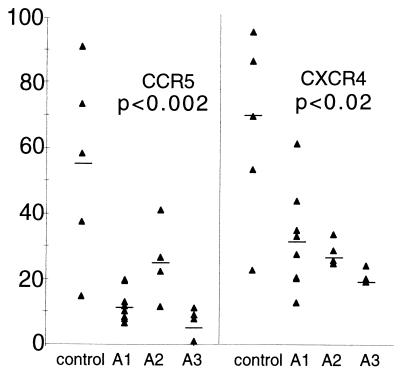
Last, we evaluated chemokine receptor expression levels on CD4+ T cells from control or immunized animals at 8 weeks after virus challenge. By this time after SHIV89.6PD infection, the chemokine receptors CCR5 and CXCR4 were up-regulated on CD4+ T cells from control macaques (Fig. 4). Immunization maintained chemokine receptor expression to near baseline levels in all groups (Fig. 4), and we did not observe changes in the kinetics of chemokine receptor expression (not shown).
Figure 4.
Levels of chemokine receptor expression on circulating CD4+ T cells at 28 days after virus inoculation in immunized and control macaques. The ordinate shows the percentage of CD4+ T cells that were positive for expression of cell surface CCR5 or CXCR4. The lower levels of CCR5 expression were statistically significant for all immunized groups compared with control (P ≤ 0.002), and the lower levels of CXCR4 were statistically significant for immunized groups compared with control (P ≤ 0.02).
Discussion
Immunization against Tat toxoid failed to produce sterilizing immunity against intrarectal challenge with SHIV89.6PD in rhesus macaques but did provide a significant level of attenuation judged by virus burden, IFN-α levels, and chemokine receptor expression. Animals with both cellular and humoral immune responses to Tat showed the highest level of virus attenuation with plasma vRNA levels at or below the level of detection by 8 weeks after inoculation. In this study, responses to immunization were variable, but, among animals with both cellular and humoral responses to Tat, 88% were protected against disease progression. Immunization that produces both high titer serum antibody (>3,000) and positive lymphoproliferative responses (stimulation index ≥3) to Tat, is the goal for therapeutic or preventive immunization in human clinical studies.
Our data also help to establish parameters for evaluating Tat toxoid therapeutic immunization studies in man. In addition to monitoring virus burden, CD4 T cell counts, and clinical markers of disease, our data validate the use of IFN-α and chemokine receptor levels as additional outcome measures. Results from these animal immunization studies show that at least two known in vitro activities of Tat (increased IFN-α and chemokine receptor expression) respond to Tat in vivo and are likely to be part of the viral pathogenesis mechanism. It is more difficult to discern direct effects of extracellular Tat on virus replication, but additional studies are in progress to show whether the transactivation mechanism that first established Tat as an important extracellular factor (4) might also be a component of disease progression.
A vaccine strategy that includes Tat toxoid (1, 30–32) and aims to reduce virus burden and AIDS pathogenesis is supported by our results. However, we do not support the idea that Tat vaccines and, especially, formulations containing native Tat will be effective as preventive, monovalent vaccines against HIV infection. Protection has been reported by others (16); however, these investigators used SHIV89.6P in the cynomolgus macaque, where peak viremia only reaches the levels of approximately 1 × 106 vRNA copies per ml compared with around 1 × 109 vRNA copies per ml that are observed after SHIV89.6P infection in rhesus macaques (33). In our study modeling sexual transmission with a virulent virus strain and a susceptible macaque species, immune responses against Tat did not prevent infection but did show significant disease attenuation.
We believe that Tat toxoid will be an important component in either preventive or therapeutic HIV vaccines but on its own will not be sufficient to block disease progression. Further, native Tat should not be used in human beings because of its demonstrated toxicity in murine models (13, 34–36) and continuing concerns about which tests are needed to establish safety for this type of biological response modifier. For mass vaccination, the use of Tat toxoid will avoid problems linked to toxicity of the native Tat protein, particularly for individuals with damaged immune systems because of chronic infections, parasitosis, malnutrition, or inherited disorders, yet the toxoid should elicit the range of immune responses necessary for controlling HIV-1 infection and disease.
Acknowledgments
We are grateful to Jacque Mitchen, Eva Rakasz, Maria Zayas, Jody Helgeland, Glen Hatfield, Leonard Acker, and Amy Usborne for help with macaque immunization and infection studies. We are grateful to Maria S. Salvato, Miroslav Malkovsky, Alessandro Gringieri, and Jean Francois Zagury for helpful discussions and critical comments on the manuscript. This work was supported by U.S. Public Health Service Awards AI38941 and AI/RR42534 (to C.D.P.), Primate Center Base Grant RR00167, and funds from Neovacs, (Paris) (to D.Z.).
Abbreviation
- SHIV
simian/HIV
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070049797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070049797
References
- 1.Gallo R C. Proc Natl Acad Sci USA. 1999;96:8324–8326. doi: 10.1073/pnas.96.15.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cullen B. J Virol. 1990;63:655–657. [Google Scholar]
- 3.Ensoli B, Barillari G, Salahuddin S Z, Gallo R C, Wong-Staal F. Nature (London) 1990;345:84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- 4.Frankel A D, Pabo C O. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 5.Albini A, Ferrini S, Benelli R, Sforzini S, Giunciuglio D, Aluigi M G, Proudfoot A E, Alouani S, Wells T N, Mariani G, et al. Proc Natl Acad Sci USA. 1998;95:13153–13158. doi: 10.1073/pnas.95.22.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barillari G, Gendelman R, Gallo R C, Ensoli B. Proc Natl Acad Sci USA. 1993;90:7941–7945. doi: 10.1073/pnas.90.17.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarze S R, Ho A, Vocero-Akbani A, Dowdy S F. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 8.Huang L, Bosch I, Hofmann W, Sodroski J, Pardee A B. J Virol. 1998;72:8952–8960. doi: 10.1128/jvi.72.11.8952-8960.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Secchiero P, Zella D, Capitani S, Gallo R, Zauli G. J Immunol. 1999;162:2427–2431. [PubMed] [Google Scholar]
- 10.Viscidi R P, Mayur K, Lederman H M, Frankel A D. Science. 1989;246:1606–1608. doi: 10.1126/science.2556795. [DOI] [PubMed] [Google Scholar]
- 11.Zagury D, Lachgar A, Chams V, Fall L S, Bernard J, Zagury J F, Bizzini B, Gringeri A, Santagostino E, Rappaport J, et al. Proc Natl Acad Sci USA. 1998;95:3851–3856. doi: 10.1073/pnas.95.7.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Re M C, Furlini G, Vignoli M, Ramazzotti E, Roderigo G, De Rosa V, Zauli G, Lolli S, Capitani S, La Placa M. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;10:408–416. doi: 10.1097/00042560-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S S, Li C, Ding L, Cao Y, Pardee A B, Shevach E M, Cohen D I. Proc Natl Acad Sci USA. 1999;96:10842–10847. doi: 10.1073/pnas.96.19.10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartz S R, Emerman M. J Virol. 1999;73:1956–1963. doi: 10.1128/jvi.73.3.1956-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K-M, Krammer P H. Nature (London) 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 16.Cafaro A, Caputo C, Fracasso C, Maggiorella M T, Goletti D, Baroncelli S, Pace M, Sernicola L, Koanga-Mogtomo M L, Betti M, et al. Nat Med. 1999;5:643–650. doi: 10.1038/9488. [DOI] [PubMed] [Google Scholar]
- 17.Osterhaus A D, van Baalen C A, Gruters R A, Schutten M, Siebelink C H, Hulskotte E G, Tijhaar E J, Randall R E, van Amerongen G, Fleuchaus A, et al. Vaccine. 1999;17:2713–2714. doi: 10.1016/s0264-410x(98)00498-8. [DOI] [PubMed] [Google Scholar]
- 18.Zagury J F, Sill A, Blattner W, Lachgar A, LeBuanec H, Richardson M, Rappaport J, Hendel H, Bizzini B, Gringeri A, et al. J Hum Virol. 1998;1:282–292. [PubMed] [Google Scholar]
- 19.van Baalen C A, Pontesilli O, Huisman R C, Geretti A M, Klein M R, de Wolf F, Miedema F, Gruters R A, Osterhaus A D. J Gen Virol. 1997;78:1913–1918. doi: 10.1099/0022-1317-78-8-1913. [DOI] [PubMed] [Google Scholar]
- 20.Gringeri A, Santagostino E, Muca-Perja M, Mannucci P M, Zagury J F, Bizzini B, Lachgar A, Carcagno M, Rappaport J, Criscuolo M, et al. J Hum Virol. 1998;1:293–298. [PubMed] [Google Scholar]
- 21.LeBuanec H, Lachgar A, Bizzini B, Zagury J F, Rappaport J, Santagostino E, Muca-Perja M, Gringeri A. Biomed Pharmacother. 1998;52:431–435. doi: 10.1016/s0753-3322(99)80020-1. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y C, Pauza C D, Lu X S, Montefiori D C, Miller C J. J Acquired Immune Defic Syndr Hum Retrovirol. 1998;19:6–18. doi: 10.1097/00042560-199809010-00002. [DOI] [PubMed] [Google Scholar]
- 23.Gringieri A. J Hum Virol. 1998;1:293–298. [PubMed] [Google Scholar]
- 24.Steger K K, Waterman P M, Pauza C D. J Virol. 1999;73:1853–1859. doi: 10.1128/jvi.73.3.1853-1859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steger K K, Dykhuizen M, Mitchen J, Hinds P W, Preuninger B L, Wallace M, Thomson J, Lu Y, Pauza C D. J Virol. 1998;72:1600–1605. doi: 10.1128/jvi.72.2.1600-1605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pauza C D, Emau P, Salvato M S, Trivedi P, MacKenzie D, Malkovsky M, Uno H, Schultz K T. J Med Primatol. 1993;22:154–161. [PubMed] [Google Scholar]
- 27.Dykhuizen, M., Ceman, J., Emerson, C., Mitchen, J., Zayas, M., MacDougall, A., Helgeland, J., Ruckwardt, T. J., Wallace, M., Rakasz, E. & Pauza, C. D. (2000) Cytometry, in press. [DOI] [PubMed]
- 28.Fisher A G, Collati E, Ratner L, Gallo R C, Wong-Staal F. Nature (London) 1985;316:262–265. doi: 10.1038/316262a0. [DOI] [PubMed] [Google Scholar]
- 29.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein G. Nat Med. 1996;2:960–964. doi: 10.1038/nm0996-960. [DOI] [PubMed] [Google Scholar]
- 31.Gringieri A, Musicco M, Hermans P, Bentwich Z, Cusini M, Bergamasco A, Santagostino E, Burny A, Bizzini B, Zagury D. J Acquired Immune Defic Syndr Hum Retrovirol. 1999;20:358–370. doi: 10.1097/00042560-199904010-00006. [DOI] [PubMed] [Google Scholar]
- 32.Gringeri A, Santagostino E, Muca-Perja M, LeBuanec H, Bizzini B, Lachgar A, Zagury J F, Rappaport J, Burny A, Gallo R C, Zagury D. J Acquired Immune Defic Syndr. 1999;20:371–375. doi: 10.1097/00042560-199904010-00007. [DOI] [PubMed] [Google Scholar]
- 33.Robinson H L, Montefiori D C, Johnson R P, Manson K H, Kalish M L, Lifson J D, Rizvi T A, Lu S, Hu S-L, Mazzara G P, et al. Nat Med. 1999;5:526–534. doi: 10.1038/8406. [DOI] [PubMed] [Google Scholar]
- 34.Rasty S, Thatikunta P, Gordon J, Khalili K, Amini S, Glorioso J C. Proc Natl Acad Sci USA. 1996;93:6073–6078. doi: 10.1073/pnas.93.12.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prakash O, Teng S, Ali M, Zhu X, Coleman R, Dabdoub R A, Chambers R, Aw T Y, Flores S C, Joshi B H. Arch Biochem Biophys. 1997;343:173–180. doi: 10.1006/abbi.1997.0168. [DOI] [PubMed] [Google Scholar]
- 36.Vogel J, Hinrichs S, Reynolds R, Luciw P, Jay G. Nature (London) 1988;335:606–611. doi: 10.1038/335606a0. [DOI] [PubMed] [Google Scholar]