Summary
Background
Most women receiving systemic therapy for breast cancer experience hot flashes. We undertook a randomised, double-blind, placebo-controlled, multi-institutional trial to assess the efficacy of gabapentin in controlling hot flashes in women with breast cancer.
Methods
420 women with breast cancer who were having two or more hot flashes per day were randomly assigned placebo, gabapentin 300 mg/day, or gabapentin 900 mg/day by mouth in three divided doses for 8 weeks. Each patient kept a 1-week, self-report diary on the frequency, severity, and duration of hot flashes before the start of the study and during weeks 4 and 8 of treatment. Analyses were by intention to treat.
Findings
Evaluable data were available on 371 participants at 4 weeks (119 placebo, 123 gabapentin 300 mg, and 129 gabapentin 900 mg) and 347 at 8 weeks (113 placebo, 114 gabapentin 300 mg, and 120 gabapentin 900 mg). The percentage decreases in hot-flash severity score between baseline and weeks 4 and 8, respectively were: 21% (95% CI 12 to 30) and 15% (1 to 29) in the placebo group; 33% (23 to 43) and 31% (16 to 46) in the group assigned gabapentin 300 mg; and 49% (42 to 56) and 46% (34 to 58) in the group assigned gabapentin 900 mg. The differences between the groups were significant (p=0.0001 at 4 weeks and p=0.007 at 8 weeks by ANCOVA for overall treatment effect, adjusted for baseline values); only the higher dose of gabapentin was associated with significant decreases in hot-flash frequency and severity.
Interpretation
Gabapentin is effective in the control of hot flashes at a dose of 900 mg/day, but not at a dose of 300 mg/day. This drug should be considered for treatment of hot flashes in women with breast cancer.
Introduction
Most women going through the menopause experience hot flashes, a symptom complex that includes a collection of vasomotor symptoms such as a sudden feeling of warmth and redness that begins in the chest and spreads to the neck and the face, accompanied by sweating, palpitations, and anxiety.1 Hot flashes are also among the most commonly reported symptoms in women receiving systemic therapy for breast cancer, adversely affecting quality of life.2
The pathophysiology of hot flashes is not entirely clear, but a working model has emerged, which hypothesises that physiological concentrations of oestrogen and progesterone maintain the concentrations of endorphin in the hypothalamus. At menopause, endorphin concentrations decrease with falling oestrogen concentrations, with the resulting release of the noradrenergic activity from its usual tonic inhibition, which culminates in increased hypothalamic release of norepinephrine and serotonin and leads to a lowering of the set point in the thermoregulatory nucleus. This process allows inappropriate heat-loss mechanisms to be triggered by subtle changes in core body temperature.3–8
Treatment with oestrogen and progestagen can ameliorate these symptoms, but there is controversy about their use in women with breast cancer.9–12 A trial of hormone replacement therapy in women with breast cancer was terminated early because of the finding that the treatment increased the risk of recurrence.13
Various non-hormonal agents have been tested. Clonidine, a centrally acting α-adrenergic agonist, was effective in a controlled trial with a transdermal patch14 and in a double-blind placebo-controlled trial given orally in women with breast cancer.15 Newer antidepressants, such as selective serotonin-reuptake inhibitors and inhibitors of serotonin and norepinephrine reuptake, are promising non-hormonal treatments for hot flashes. Randomised placebo-controlled trials have shown that venlafaxine,16 fluoxetine,17 and paroxetine18 are effective in control of hot flashes. Gabapentin is a GABA analogue used in the treatment of epilepsy, neurogenic pain, restless-leg syndrome, essential tremor, bipolar disorder, and migraine prophylaxis; it was first reported for its effects on hot flashes in five women and one man.19 A randomised double-blind, placebo-controlled trial has shown that gabapentin is effective in control of menopausal hot flashes,20 and a pilot study showed that it had promising effects in women with breast cancer.21
On the basis of these observations, we undertook a double-blind, placebo-controlled trial of gabapentin to assess its efficacy in the treatment of hot flashes in women with breast cancer. The most commonly used dose of gabapentin is 900 mg per day. However, we decided to study a lower dose (300 mg per day) also; if this dose could control hot flashes, the patients would benefit overall. The 8-week study duration was selected on the basis of our previous study of clonidine,15 to provide internal consistency.
Methods
Patients
The patients were participants in a multicentre clinical trial at 18 geographically diverse member sites of the University of Rochester Community Clinical Oncology Program. Women aged 18 years or older who had breast cancer and were having an average of two or more hot flashes per day were eligible to take part in the study. Acceptable non-steroidal contraceptive measures were required. Patients currently receiving chemotherapy were not eligible, although endocrine therapies were allowed. Most of the patients were taking adjuvant tamoxifen. Patients taking venlafaxine, clonidine, or anticonvulsants were not eligible for the study, but use of other antidepressants including selective serotonin-reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors was allowed. The other reasons for exclusion were: pregnancy; breastfeeding; use of steroidal contraception; coronary insufficiency; recent history of myocardial infarction, symptomatic cardiac disease, peripheral or cerebrovascular disease, stroke, syncope, or symptomatic hypotension; hepatic dysfunction (aspartate aminotransferase concentration above twice the upper limit of normal, or bilirubin concentration above the upper limit of normal, as defined at each institution); renal dysfunction (serum creatinine concentration above 1.25 times the upper limit of normal); and known allergy to gabapentin. The Institutional Review Boards of the University of Rochester and each participating site approved the protocol. Written informed consent was obtained from each participant.
Design and procedures
Patients were randomly assigned placebo, gabapentin 100 mg, or gabapentin 300 mg, each to be taken by mouth three times a day, for 8 weeks. There was a 3-day titration period for all patients because the study was double-blind. The study drugs were supplied as capsules of similar appearance in bottles. Treatment assignment was done by use of a randomisation table created in SAS computer program (version 8) and was stratified by the Community Clinical Oncology Program site and by the duration of hot flashes (<9 months or ≥9 months). A block size of three was used to ensure that the treatment assignment was balanced after every three participants within each stratum.
The primary objective of the study was to compare the efficacy and the side-effect profile of gabapentin 300 mg/day or 900 mg/day with that of placebo.
Each participant kept a 1-week self-report diary on hot flashes, originally developed by the North Central Cancer Treatment Group,22 before the start of the study and during weeks 4 and 8 of treatment. All hot flashes were recorded, and symptoms were assigned a grade of 1 (mild), 2 (moderate), 3 (severe), or 4 (very severe). In addition, a single question assessed the average duration in minutes of all hot flashes experienced that day.
A patient-report symptom inventory, modified from a measure created at the M D Anderson Cancer Center, Houston, TX, USA,23 was used to monitor other symptoms. The symptom inventory is a series of uniscales in which the severity of each of ten symptoms (fatigue, pain, nausea, sleep disturbance, shortness of breath, memory, appetite, drowsiness, vomiting, and distress) is indicated by filling in the appropriate circle on an 11-point scale, from 0 (not present) to 10 (as bad as you can imagine). The checklist was completed by patients three times: at the end of the pretreatment (baseline) week, at week 4, and at week 8.
Statistical analysis
In our previous research on clonidine, the SD of the percentage change from baseline in hot-flash frequency was about 35%. A sample of 114 evaluable participants per group would give 80% power to detect a 15% difference between any pair of groups. To allow for up to 16% dropout by 8 weeks, we planned to enrol 136 participants per group.
The method of analysis was decided prospectively and incorporated the intention-to-treat principle; data are included in the treatment group to which the participant was assigned, irrespective of any subsequent changes to the treatment. The statistical package SAS for Windows, version 8 was used for our analysis. Baseline characteristics, including age, marital status, education, current treatment, and baseline hot-flash measures were first compared between the three treatment groups by χ2 test or ANOVA as appropriate.
The efficacy of gabapentin was assessed by three measures of hot-flash symptoms: frequency (total number of mild, moderate, severe, and very severe hot flashes), severity score (calculated by assigning scores of 1, 2, 3, and 4, respectively, to mild, moderate, severe, and very severe hot flashes and adding the scores), and mean duration. Each of these measures for the three treatment cycles was obtained by averaging of data collected over 1 week.
Differences and percentage changes from baseline to week 4 or week 8 were calculated for each of the three measures. A mixed-effect model was used for comparison of the treatment effect on these measures. The fixed effects in the model included treatment group (three strata), time (two strata, 4 weeks and 8 weeks) and baseline measures. A random effect for participants was also included in the model. Further analyses included tests for interaction of treatment effects by time, age (less than 50 years vs 50 years or older), and current use of tamoxifen (yes/no). The assumptions made for mixed-effect models (normality of error terms, mean, and covariance structure) were checked thoroughly by use of residual plots. Outliers were identified and their influence on model estimation was examined. For purposes of comparison, simpler analyses were also done on change scores and percentage change scores at week 4 and week 8 separately, by ANCOVA.
Role of the funding source
The study was funded by the US National Cancer Institute (Public Health Service grant U10 CA37420). The drug and placebo were provided by Pfizer, Inc. The study was reviewed and approved by the National Cancer Institute, but not by Pfizer, and neither funding source had any role in study design; collection, analysis, or interpretation of data; or the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit the paper for publication.
Results
Between June, 2001, and July, 2003, 420 women with breast cancer were enrolled and randomly assigned placebo, gabapentin 300 mg/day, or gabapentin 900 mg/day by mouth in three divided doses, for 8 weeks (figure 1). The mean age of the participants was 55 years, and most were married (75%) and white (95%). Demographic, clinical, and hot-flash characteristics at baseline are given in table 1. The baseline hot-flash frequency ranged from 2 to 54 and the severity score from 2 to 191. Evaluable data were available for 371 (88%) of the 420 patients at week 4 and 347 (83%) at week 8. Diaries were obtained from all the patients, including those who discontinued the assigned treatment, unless the patient decided to withdraw from the study.
Figure 1.
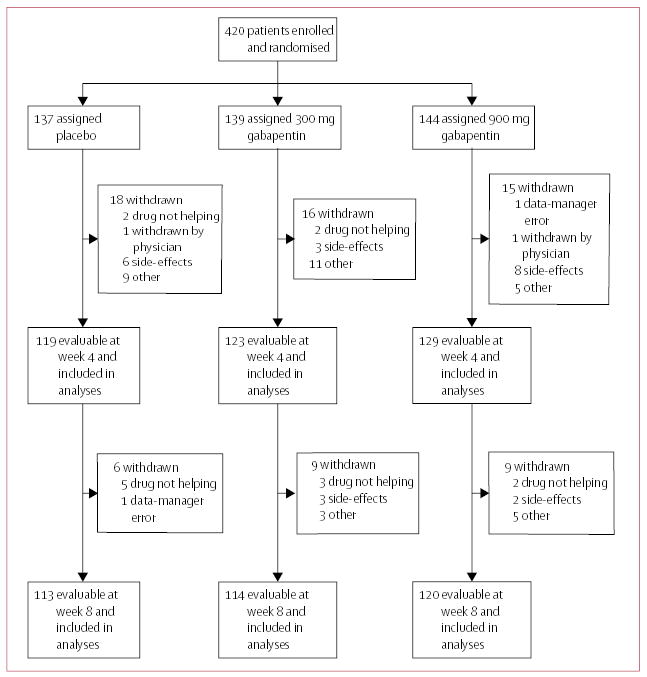
Trial profile
Table 1.
Demographic and baseline characteristics
| Characteristic | Placebo(n=137) | 300 mg gabapentin(n=139) | 900 mg gabapentin(n=144) |
|---|---|---|---|
| Demography | |||
| Mean (SD) age, years | 54 (7) | 55 (9) | 55 (9) |
| White | 131 (96%) | 131 (94%) | 138 (96%) |
| Married | 99 (72%) | 101 (73%) | 111 (77%) |
| Clinical | |||
| Currently taking tamoxifen | 103 (75%) | 95 (68%) | 100 (69%) |
| Previous chemotherapy | 12 (9%) | 13 (9%) | 13 (9%) |
| Previous radiotherapy | 9 (7%) | 11 (8%) | 12 (8%) |
| Hot flashes | |||
| Mean (SD) number per day | 8.8 (6.4) | 8.5 (5.9) | 8.7 (5.3) |
| Mean (SD) severity score | 19.9 (17.9) | 19.4 (19.6) | 18.7 (13.7) |
| Mean (SD) duration, min | 5.2 (6.4) | 5.0 (4.9) | 5.0 (4.3) |
Data are number of participants unless otherwise stated.
According to our preplanned analyses, we first examined between-group differences in changes in frequency, severity, and duration of hot flashes by a mixed-effects model. None of the interaction terms (time, age, and current use of tamoxifen) in these analyses was significant, so models including only main effects were used to describe our data. For hot-flash frequency, the estimated effect (the difference in the change scores between the gabapentin group and the placebo group at both week 4 and week 8) was −0.80 (95% CI −1.70 to 0.10) for the group assigned 300 mg gabapentin and −2.10 (−2.95 to −1.23) for the group assigned 900 mg gabapentin (table 2). For severity, the estimated effects were −1.79 (−4.38 to 0.80) for the 300 mg group and −4.88 (−7.23 to −2.53) for the 900 mg group. These findings show a clear benefit for the group assigned 900 mg gabapentin, but no evidence of benefit with 300 mg gabapentin in reducing hot-flash frequency and severity. Similar analyses provided no evidence of differences in the mean duration of hot flashes between the groups.
Table 2.
Estimated effect of gabapentin vs placebo
|
Gabapentin 300 mg |
Gabapentin 900 mg |
|||
|---|---|---|---|---|
| Outcome | Effect (95% CI)* | p | Effect (95% CI)* | p |
| Hot–flash frequency | ||||
| Mean change | −0.80 (−1.70 to 0.10) | 0.08 | −2.10 (−2.95 to −1.23) | <0.0001 |
| Percentage change | −12% (−23 to −1) | 0.04 | −26% (−37 to −15) | <0.0001 |
| Hot-flash severity | ||||
| Mean change | −1.79 (−4.38 to 0.80) | 0.18 | −4.88 (−7.23 to −2.53) | <0.0001 |
| Percentage change | −13% (−29 to 2) | 0.09 | −30% (−44 to −16) | <0.0001 |
Difference in change scores between gabapentin group and placebo group.
Similar analyses were done on the percentage change scores. Again, none of the interaction terms was significant, so models including only main effects were used. For hot-flash frequency, the estimated effects on percentage change scores were significant for both gabapentin groups (table 2). For severity, the difference in percentage change score was significant only for the 900 mg group.
After fitting each model, we did residual analyses to check for adequacy of the mixed-model assumptions, which include the normality of error terms, mean model, and covariance structures. Since there were only two repeated change scores for each individual, there was no need to check for correlation structure, so only the assumption of constant variance was checked. Since the residuals are correlated, we transformed the residuals by multiplying the residual vector from each participant by the inverse of the Cholesky decomposition of the estimated covariance matrix. The covariates and the predicted mean values were transformed in the same way. The Q-Q plot of the transformed residuals was used to check for outliers and normality assumptions of the error terms. The transformed-residual versus transformed-predicted mean values plot was used to check for the assumption of constant variance. The transformed-residual versus transformed-covariate plots were checked for adequacy of the mean models.
Outliers were identified for models involving different outcomes, and models were refitted without them. The parameter estimates were changed slightly when the outliers were excluded; however, none of these sensitivity analyses changed our main conclusion. The residual plots show that our mean models are adequate. In general, the distribution of the transformed residual for the models involving the change scores had some heavy tails, and models involving percentage change scores showed skewed distributions of the residuals. To accommodate these complications, we chose to use robust sandwich estimators for variance estimation. Large sample theory guarantees the consistency of the mean model parameters and the variance estimation no matter what distribution was present in the original data, as long as the model for the mean is correct.
Further confirmatory analyses were done by ANCOVA on the differences and percentage changes from baseline to week 4 and from baseline to week 8 for the three hot-flash indices, with adjustment for baseline scores. Table 3 shows the mean changes in the frequency, duration, and severity of hot flashes from baseline to weeks 4 and 8 for the three treatment groups. These results lend support to the conclusions obtained from the primary analyses. Figure 2 is a graphic representation of the mean change scores for the frequency and severity data.
Table 3.
Changes in hot-flash characteristics between baseline and week 4 or week 8
|
Change (95% CI) from baseline to week 4 |
Change (95% CI) from baseline to week 8 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Placebo(n=119) | Gabapentin 300 mg(n=123) | Gabapentin 900 mg(n=129) | p* | Placebo(n=113) | Gabapentin 300 mg(n=114) | Gabapentin 900 mg(n=120) | p* |
| Frequency | ||||||||
| Mean change | −1.98 (−2.79 to −1.17) | −2.64 (−3.47 to −1.81) | −4.00 (−4.83 to −3.17) | <0.0001 | −2.25 (−3.33 to −1.17) | −2.86 (−3.84 to −1.88) | −4.21 (−5.00 to −3.42) | 0.0002 |
| Percentage change | −18% (−25 to −11) | −28% (−36 to −20) | −41% (−48 to −34) | 0.0002 | −15% (−28 to −2) | −30% (−39 to −21) | −44% (−53 to −35) | 0.0006 |
| Duration | ||||||||
| Mean change | −1.06 (−1.90 to −0.22) | −1.57 (−2.24 to −0.90) | −1.56 (−1.99 to −1.13) | 0.206 | −1.20 (−2.01 to −0.39) | −1.80 (−2.59 to −1.01) | −1.17 (−1.74 to −0.60) | 0.215 |
| Percentage change | 9% (−16 to 34) | −19% (−27 to −11) | −22% (−31 to −13) | 0.009 | 1% (−13 to 15) | −12% (−30 to 6) | −9% (−26 to 8) | 0.589 |
| Severity | ||||||||
| Mean change | −5.45(−8.06 to −2.84) | −7.50 (−10.4 to −4.56) | −9.97 (−12.0 to −7.93) | 0.0001 | −6.61 (−9.68 to −3.54) | −7.75 (−11.2 to −4.35) | −9.94 (−12.0 to −7.85) | 0.002 |
| Percentage change | −21% (−30 to −12) | −33% (−43 to −23) | −49% (−56 to −42) | 0.0001 | −15% (−29 to −1) | −31% (−46 to −16) | −46% (−58 to −34) | 0.007 |
From ANCOVA tests of the overall treatment effect adjusted for baseline values.
Figure 2.
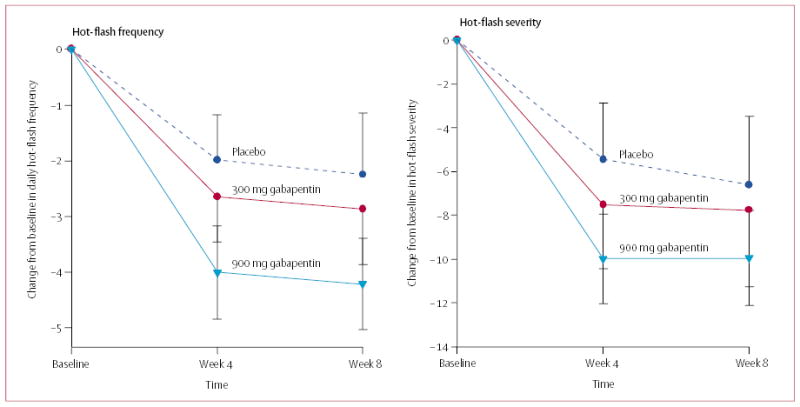
Mean change scores (and 95% CI) for hot-flash frequency and severity
The difference in change scores and the percentage changes at week 4 or week 8 in the frequency and severity of hot flashes between the groups assigned placebo and gabapentin 300 mg are small. By contrast, the differences in both measures between the group assigned gabapentin 900 mg and either of the other study groups were significant at both week 4 and week 8.
Table 4 shows the mean change in ten symptoms from baseline to weeks 4 and 8. To control partially for the multiple comparisons, a criterion significance of p<0.01 was set. None of the analyses met this criterion at week 4 or week 8. Only two symptoms, pain and appetite, met the less stringent criterion of p<0.05, and then only at week 4. In this less stringent analysis, pain was lessened, which is a known effect of gabapentin, and appetite worsened.
Table 4.
Changes in symptoms between baseline and week 4 or week 8
|
Change (95% CI) in symptoms from baseline to week 4 |
Change (95% CI) in symptoms from baseline to week 8 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Placebo (n=119) | Gabapentin 300 mg (n=123) | Gabapentin 900 mg (n=129) | p* | Placebo (n=113) | Gabapentin 300 mg (n=114) | Gabapentin 900 mg(n=120) | p* |
| Appetite | −0.27 (−0.49 to −0.05) | −0.36 (−0.55 to −0.17) | 0.05 (−0.14 to 0.24) | 0.012 | −0.12 (−0.34 to 0.10) | −0.23 (−0.40 to −0.06) | −0.03 (−0.28 to 0.22) | 0.413 |
| Distress | −0.08 (−0.57 to 0.41) | −0.23 (−0.71 to 0.25) | −0.54 (−1.04 to −0.04) | 0.239 | −0.33 (−0.85 to 0.19) | −0.43 (−0.85 to −0.01) | −0.49 (−0.99 to 0.01) | 0.313 |
| Drowsiness | −0.11 (−0.51 to 0.29) | −0.31 (−0.70 to 0.08) | −0.23 (−0.73 to 0.27) | 0.552 | −0.54 (−1.02 to −0.06) | −0.51 (−1.01 to −0.01) | −0.45 (−0.94 to 0.04) | 0.800 |
| Fatigue | −0.14 (−0.63 to 0.35) | −0.19 (−0.63 to 0.25) | −0.22 (−0.67 to 0.23) | 0.452 | −0.65 (−1.11 to −0.19) | −0.25 (−0.69 to 0.19) | −0.57 (−1.04 to −0.10) | 0.470 |
| Nausea | −0.17 (−0.42 to 0.08) | −0.11 (−0.32 to 0.10) | 0.08 (−0.14 to 0.30) | 0.775 | −0.30 (−0.54 to −0.06) | −0.19 (−0.43 to 0.05) | −0.03 (−0.23 to 0.17) | 0.721 |
| Pain | 0.20 (−0.23 to 0.63) | −0.30 (−0.69 to 0.09) | −0.09 (−0.47 to 0.29) | 0.039 | −0.15 (−0.56 to 0.26) | −0.21 (−0.65 to 0.23) | −0.28 (−0.68 to 0.12) | 0.549 |
| Memory | −0.33 (−0.73 to 0.07) | −0.38 (−0.70 to −0.06) | −0.31 (−0.62 to 0) | 0.209 | −0.73 (−1.12 to −0.34) | −0.04 (−0.36 to 0.44) | −0.20 (−0.56 to 0.16) | 0.386 |
| Shortness of breath | −0.07 (−0.43 to 0.29) | −0.22 (−0.50 to 0.06) | −0.22 (−0.59 to −0.07) | 0.165 | −022 (−0.53 to 0.09) | −0.22 (−0.59 to 0.15) | −0.30 (−0.59 to −0.01) | 0.404 |
| Sleep | −0.83 (−1.35 to −0.31) | −1.02 (−1.55 to −0.49) | −1.27 (−1.74 to −0.80) | 0.065 | −1.26 (−1.78 to −0.74) | −1.18 (−1.73 to −0.63) | −1.39 (−1.84 to −0.94) | 0.378 |
| Vomiting | −0.19 (−0.41 to 0.03) | −0.25 (−0.44 to −0.06) | 0.12 (−0.09 to 0.33) | 0.120 | −0.19 (−0.43 to 0.05) | −0.04 (−0.30 to 0.22) | −0.02 (−0.13 to 0.09) | 0.456 |
A negative value denotes an improvement in the symptom.
From ANCOVA tests of the overall treatment effect adjusted for baseline values.
Discussion
The results of this randomised, double-blind placebo-controlled trial accord with those of our pilot study of menopausal women;21 there was a 46% reduction in the hot-flash severity score with gabapentin 900 mg/day, compared with a 54% reduction versus placebo reported in postmenopausal women treated with gabapentin 900 mg/day for 12 weeks.20 We analysed our data in two different ways, and in each approach we observed a significant effect on hot flashes with gabapentin 900 mg/day, whereas gabapentin 300 mg/day was no better than placebo for any comparison. An even higher dose of gabapentin might be more effective. Evidence in support of that idea comes from the study by Guttuso and colleagues,20 in which an open-label dose escalation was allowed after the 12-week study period. 75% of patients who elected to continue requested an increase of their dose beyond 900 mg/day (maximum allowable dose 2700 mg/day); among these patients there was a 67% reduction in the hot-flash severity score, which suggests a strong dose effect in the control of hot flashes.
Short-term (<4 weeks) side-effects were not assessed in this study, because the first symptom inventory was obtained during week 4 of the study. We examined the reasons given for withdrawing from the study owing to side-effects and found that somnolence or fatigue was given as the reason by one, three, and six patients in the placebo, gabapentin 300 mg, and gabapentin 900 mg groups, respectively; the overall numbers withdrawing because of side-effects were six, six, and ten. The opposite pattern was noted in patients who withdrew because the study treatment was not helpful (seven, five, and two patients in the placebo, gabapentin 300 mg, and gabapentin 900 mg groups, respectively). Thus, although a slightly higher proportion of women withdrew owing to side-effects from gabapentin 900 mg/day, a very small proportion withdrew because the treatment was not helpful. The opposite was true for placebo, and gabapentin 300 mg/day was intermediate, which accords with our overall results.
We found a 46% reduction in hot flashes with gabapentin 900 mg. Previous studies have found reductions of 37% with clonidine in a placebo-controlled double-blind trial;15 61% with venlafaxine at 75 mg or 150 mg;16 62.2% and 64.6% with controlled-release paroxetine at 12.5 mg and 25.0 mg/day, respectively;18 and 50% with fluoxetine.17 Gabapentin 900 mg/day provides similar control of hot flashes in women with breast cancer. The side-effect profiles of these drugs differ. Oral clonidine was associated with sleeping difficulty,15 and transdermal clonidine was associated with dryness of mouth, constipation, drowsiness, and pruritus at the site of the patch.14 Side-effects of venlafaxine included dryness of mouth, decreased appetite, nausea, and constipation,16 and the most common side-effects of controlled-release paroxetine were headache, nausea, and insomnia.18 Without a randomised trial directly comparing not only the effect on hot flashes but also the side-effects of each of these drugs, the optimum non-hormonal drug for alleviation of hot flashes remains to be identified.
The selective serotonin-reuptake inhibitors are regarded as the most promising non-hormonal treatment for hot flashes in women with breast cancer, but a study by Stearns and colleagues has raised concern about the possible interaction between these agents and tamoxifen, a selective oestrogen-receptor modulator, because selective serotonin-reuptake inhibitors inhibit the cytochrome P450 enzymes that are important in converting tamoxifen to its active metabolites.24 Stearns and colleagues found that coadministration of paroxetine with tamoxifen decreased the plasma concentration of endoxifen, an active metabolite of tamoxifen, which is generated by N-demethylation mediated by cytochrome P450 3A4 and hydroxylation mediated by cytochrome P450 2D6 (CYP2D6), which suggests that CYP2D6 genotype and drug interactions should be considered in women treated with tamoxifen. A subsequent study25 found that paroxetine was the most potent inhibitor of CYP2D6, followed by fluoxetine, sertraline, and citalopram. Venlafaxine was the least potent inhibitor of CYP2D6.25 Gabapentin is not metabolised in human beings and is excreted unchanged, mainly in the urine. Concurrent administration of gabapentin has been studied extensively with respect to its interaction with other antiseizure drugs, and it does not affect plasma concentrations of phenytoin, carbamazepine, phenobarbital, or valproate, all of which are affected by the cytochrome P450 system.26 Therefore, even though gabapentin has not been specifically studied in terms of its interaction with tamoxifen, the assumption that no such interaction is to be expected is reasonable.
The mechanism of action of gabapentin remains unknown. A 37-year-old patient with known hypothalamic dysfunction who took gabapentin for 6 months for control of his seizure disorder had an increase in the frequency of hypothermic episodes of 100 times. The frequency of these episodes returned to baseline once gabapentin was discontinued.19 Gabapentin inhibits neuronal calcium currents in vitro,27 and its binding site is known to be on the α2 δ subunit of voltage-gated calcium channels.28 This binding site was upregulated by 17 times selectively in rat dorsal root ganglion in response to peripheral-nerve injury.28 This process could be one of the mechanisms of action of gabapentin in the treatment of neuropathic pain.29 We speculate that similar upregulation of the gabapentin binding site could be involved in the hypothalamus as a result of oestrogen withdrawal, leading to increased activity of the neurotransmitters in the hypothalamus. Gabapentin might exert its effect on hot flashes by this mechanism.
Our study was designed to test the intervention for 8 weeks; therefore, we cannot comment on long-term use of gabapentin. However, gabapentin is used for long durations for various other symptoms and certainly could be considered for hot flashes also. We did not obtain data on immediate side-effects of gabapentin, but we did examine the reasons for withdrawing from the study. We might have underestimated the adverse effects of gabapentin, and the withdrawal rate of 12% at 4 weeks and 17% at 8 weeks might be due entirely to the side-effects of the treatment. However, the withdrawal rate was much the same in all three study groups and thus cannot be attributed to side-effects of gabapentin. We cannot think of any systematic bias that can explain these results, and we believe that random errors have been kept to a minimum by means of the randomised, double-blind, placebo-controlled design.
We believe gabapentin can be added to the list of non-hormonal agents for the control of hot flashes in women with breast cancer, and the effects of doses higher than 900 mg/day merit further study.
Acknowledgments
We thank Maarten Hofman, Jacque Lindke, Shonda Ranson, Jennifer Yates, and Barbara Hartzog of the University of Rochester Cancer Center for their technical and writing assistance in the preparation of this article.
Footnotes
Conflict of interest statement We declare that we have no conflict of interest.
Contributors
Kishan J Pandya was Study Chair, had the idea for and designed the study, and wrote the protocol in collaboration with Gary R Morrow for study design, statistics, and behavioural science, and Joseph A Roscoe, Jane T Hickok, and Hongwei Zhao for study design and statistics; all of these authors also assisted in the review of the report and suggested changes. Gary R Morrow also oversaw the approval of the protocol at the National Cancer Institute in his capacity as the Principal Investigator of the University of Rochester Community Clinical Oncology Program. Eduardo Pajon, Thomas J Sweeney, Tarit K Banerjee, and Patrick J Flynn were Principal Investigators of the Community Clinical Oncology Programs that contributed patients to the study.
References
- 1.McKinlay SM, Jefferys M. The menopausal syndrome. Br J Prev Soc Med. 1974;28:108–15. doi: 10.1136/jech.28.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpenter JS, Andrykowski MA, Cordova M, et al. Hot flashes in postmenopausal women treated for breast carcinoma: prevalence, severity, correlates, management, and relation to quality of life. Cancer. 1998;82:1682–91. [PubMed] [Google Scholar]
- 3.Casper RF, Yen SS. Neuroendocrinology of menopausal flushes: an hypothesis of flush mechanism. Clin Endocrinol (Oxf ) 1985;22:293–312. doi: 10.1111/j.1365-2265.1985.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 4.Freedman RR, Norton D, Woodward S, Cornelissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab. 1995;80:2354–58. doi: 10.1210/jcem.80.8.7629229. [DOI] [PubMed] [Google Scholar]
- 5.Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181:66–70. doi: 10.1016/s0002-9378(99)70437-0. [DOI] [PubMed] [Google Scholar]
- 6.Kronenberg F, Downey JA. Thermoregulatory physiology of menopausal hot flashes: a review. Can J Physiol Pharmacol. 1987;65:1312–24. doi: 10.1139/y87-208. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg J, Larsen SH. Hypothesis: pathogenesis of postmenopausal hot flush. Med Hypotheses. 1991;35:349–50. doi: 10.1016/0306-9877(91)90282-4. [DOI] [PubMed] [Google Scholar]
- 8.Shanafelt TD, Barton DL, Adjei AA, Loprinzi CL. Pathophysiology and treatment of hot flashes. Mayo Clin Proc. 2002;77:1207–18. doi: 10.4065/77.11.1207. [DOI] [PubMed] [Google Scholar]
- 9.Creasman WT. Estrogen and cancer. Gynecol Oncol. 2002;86:1–9. doi: 10.1006/gyno.2001.6499. [DOI] [PubMed] [Google Scholar]
- 10.O’Meara ES, Rossing MA, Daling JR, Elmore JG, Barlow WE, Weiss NS. Hormone replacement therapy after a diagnosis of breast cancer in relation to recurrence and mortality. J Natl Cancer Inst. 2004;93:754–62. doi: 10.1093/jnci/93.10.754. [DOI] [PubMed] [Google Scholar]
- 11.Quella SK, Loprinzi CL, Sloan JA. Long term use of megestrol acetate by cancer survivors for the treatment of hot flashes. Cancer. 1998;82:1784–88. [PubMed] [Google Scholar]
- 12.Leonetti HB, Longo S, Anasti JN. Transdermal progesterone cream for vasomotor symptoms and postmenopausal bone loss. Obstet Gynecol. 1999;94:225–28. doi: 10.1016/s0029-7844(99)00266-5. [DOI] [PubMed] [Google Scholar]
- 13.Holmberg L, Anderson H for the HABITS steering and data monitoring committees. HABITS (hormonal replacement therapy after breast cancer–is it safe?), a randomised comparision: trial stopped. Lancet. 2004;363:453–55. doi: 10.1016/S0140-6736(04)15493-7. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg RM, Loprinzi CL, O’Fallon JR, et al. Transdermal clonidine for ameliorating tamoxifen-induced hot flashes [published erratum appears in J Clin Oncol 1996; 14: 2411] J Clin Oncol. 1994;12:155–58. doi: 10.1200/JCO.1994.12.1.155. [DOI] [PubMed] [Google Scholar]
- 15.Pandya KJ, Raubertas RF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Intern Med. 2000;132:788–89. doi: 10.7326/0003-4819-132-10-200005160-00004. [DOI] [PubMed] [Google Scholar]
- 16.Loprinzi CL, Kugler JW, Sloan JA. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:307–11. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 17.Loprinzi CL, Sloan JA, Perez EA. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20:1578–83. doi: 10.1200/JCO.2002.20.6.1578. [DOI] [PubMed] [Google Scholar]
- 18.Stearns V, Beebe KL, Iyenger M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA. 2003;289:2827–34. doi: 10.1001/jama.289.21.2827. [DOI] [PubMed] [Google Scholar]
- 19.Guttuso TJ., Jr Gabapentin’s effects on hot flashes and hypothermia. Neurology. 2000;54:2161–63. doi: 10.1212/wnl.54.11.2161. [DOI] [PubMed] [Google Scholar]
- 20.Guttuso T, Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2003;101:337–45. doi: 10.1016/s0029-7844(02)02712-6. [DOI] [PubMed] [Google Scholar]
- 21.Pandya KJ, Thummala AR, Griggs JJ, et al. Pilot study using gabapentin for tamoxifen-induced hot flashes in women with breast cancer. Breast Cancer Res Treat. 2004;83:87–89. doi: 10.1023/B:BREA.0000010676.54597.22. [DOI] [PubMed] [Google Scholar]
- 22.Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–90. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 23.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the MD Anderson Symptom Inventory. Cancer. 2000;89:1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 24.Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–64. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 25.Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 26.McNamara JO. Drugs effective in the therapy of the epilepsies. In: Hardman JG, Limberd LE, eds. Goodman and Gilman’s the pharmacological basis of therapeutics. New York: McGraw-Hill, 2001: 521–47.
- 27.Stefani A, Spadoni F, Bernardi G. Gabapentin inhibits calcium currents in isolated rat brain neurons. Neuropharmacology. 1998;37:83–91. doi: 10.1016/s0028-3908(97)00189-5. [DOI] [PubMed] [Google Scholar]
- 28.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–76. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 29.Luo ZD, Chaplan SR, Higuera ES, et al. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci. 2001;21:1868–75. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


