Abstract
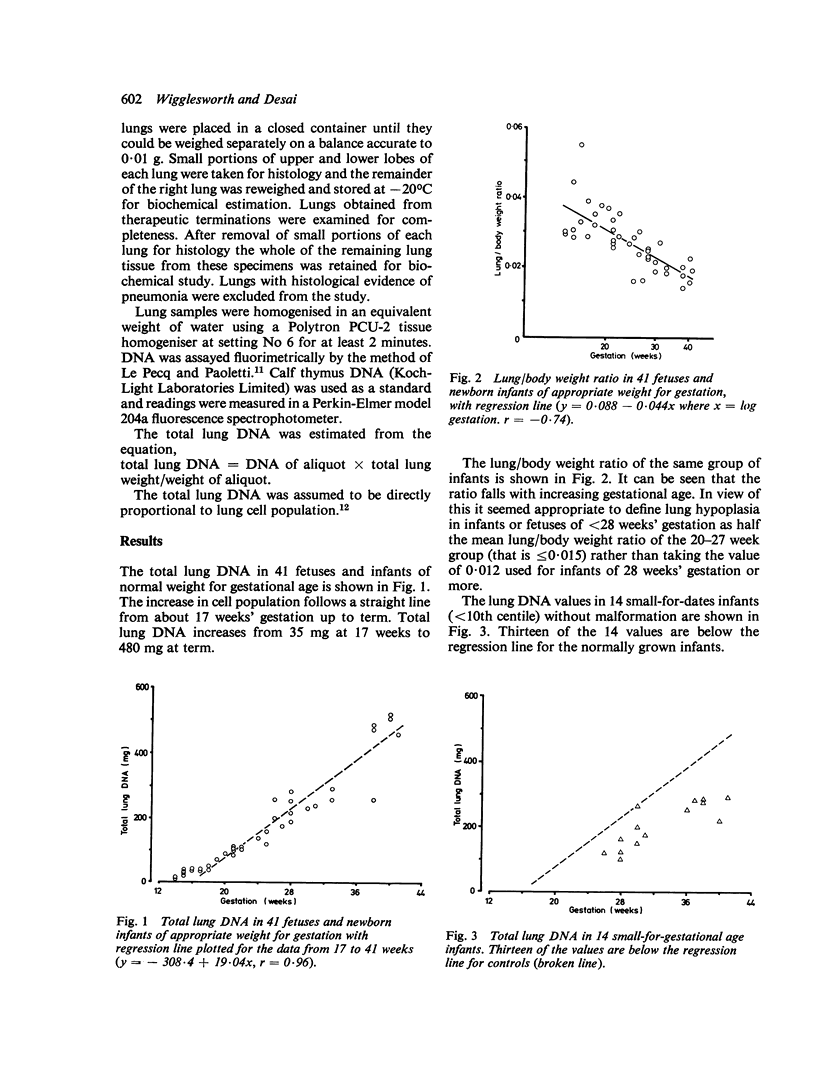
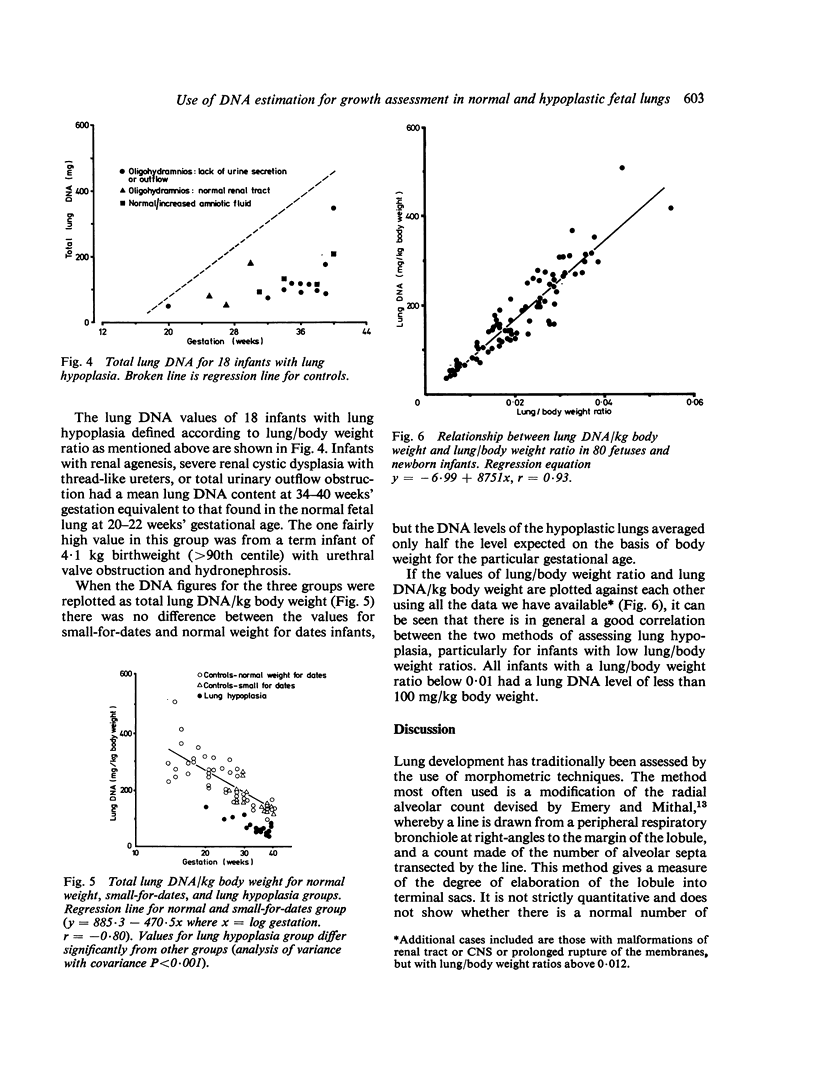
Total DNA was estimated in the lungs of 80 fetuses and newborn infants varying in gestation from 14 weeks to term. In fetuses of appropriate weight for gestational age total lung DNA increased at a constant rate from about 35 mg at 17 weeks' gestation to 480 mg at term. The lungs of immature fetuses were heavier and contained more DNA relative to body weight than did those of mature infants. Small-for-dates infants had lower lung DNA levels for gestation than infants with weights appropriate for gestational age, but there was no difference when lung DNA was corrected for body weight. Lung hypoplasia defined in terms of lung/body weight ratio was associated with low lung DNA content for gestation, even when corrected for body weight. The total lung DNA at 34-40 weeks' gestation in infants with lung hypoplasia associated with fetal anuria or urinary outflow obstruction was equivalent to that seen in normal fetuses at 20-22 weeks' gestation. We conclude that the early second trimester is a critical period for human fetal lung growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcorn D., Adamson T. M., Lambert T. F., Maloney J. E., Ritchie B. C., Robinson P. M. Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. J Anat. 1977 Jul;123(Pt 3):649–660. [PMC free article] [PubMed] [Google Scholar]
- Askenazi S. S., Perlman M. Pulmonary hypoplasia: lung weight and radial alveolar count as criteria of diagnosis. Arch Dis Child. 1979 Aug;54(8):614–618. doi: 10.1136/adc.54.8.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAIN A. D., SMITH I. I., GAULD I. K. NEWBORN AFTER PROLONGED LEAKAGE OF LIQUOR AMNII. Br Med J. 1964 Sep 5;2(5409):598–599. doi: 10.1136/bmj.2.5409.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy K., Dawes G. S. Fetal breathing. Br Med Bull. 1975 Jan;31(1):3–7. doi: 10.1093/oxfordjournals.bmb.a071237. [DOI] [PubMed] [Google Scholar]
- Dobbing J., Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973 Oct;48(10):757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorovini-Zis K., Dolman C. L. Gestational development of brain. Arch Pathol Lab Med. 1977 Apr;101(4):192–195. [PubMed] [Google Scholar]
- ELLIOTT P. M., INMAN W. H. Volume of liquor amnii in normal and abnormal pregnancy. Lancet. 1961 Oct 14;2(7207):835–840. doi: 10.1016/s0140-6736(61)90737-1. [DOI] [PubMed] [Google Scholar]
- EMERY J. L., MITHAL A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch Dis Child. 1960 Dec;35:544–547. doi: 10.1136/adc.35.184.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold M. J., Katzew H., Genieser N. B., Becker M. H. Lung structure in thoracic dystrophy. Am J Dis Child. 1971 Aug;122(2):153–159. doi: 10.1001/archpedi.1971.02110020087013. [DOI] [PubMed] [Google Scholar]
- Hislop A., Hey E., Reid L. The lungs in congenital bilateral renal agenesis and dysplasia. Arch Dis Child. 1979 Jan;54(1):32–38. doi: 10.1136/adc.54.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pecq J. B., Paoletti C. A new fluorometric method for RNA and DNA determination. Anal Biochem. 1966 Oct;17(1):100–107. doi: 10.1016/0003-2697(66)90012-1. [DOI] [PubMed] [Google Scholar]
- POTTER E. L. BILATERAL ABSENCE OF URETERS AND KIDNEYS: A REPORT OF 50 CASES. Obstet Gynecol. 1965 Jan;25:3–12. [PubMed] [Google Scholar]
- Perlman M., Williams J., Hirsch M. Neonatal pulmonary hypoplasia after prolonged leakage of amniotic fluid. Arch Dis Child. 1976 May;51(5):349–353. doi: 10.1136/adc.51.5.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swischuk L. E., Richardson C. J., Nichols M. M., Ingman M. J. Primary pulmonary hypoplasia in the neonate. J Pediatr. 1979 Oct;95(4):573–577. doi: 10.1016/s0022-3476(79)80773-8. [DOI] [PubMed] [Google Scholar]
- Wigglesworth J. S. Aetiology of hyaline membrane disease. Arch Dis Child. 1979 Nov;54(11):835–837. doi: 10.1136/adc.54.11.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigglesworth J. S., Desai R. Effect on lung growth of cervical cord section in the rabbit fetus. Early Hum Dev. 1979 Mar;3(1):51–65. doi: 10.1016/0378-3782(79)90020-3. [DOI] [PubMed] [Google Scholar]
- Wigglesworth J. S., Desai R., Guerrini P. Fetal lung hypoplasia: biochemical and structural variations and their possible significance. Arch Dis Child. 1981 Aug;56(8):606–615. doi: 10.1136/adc.56.8.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigglesworth J. S., Winston R. M., Bartlett K. Influence of the central nervous system on fetal lung development. Experimental study. Arch Dis Child. 1977 Dec;52(12):965–967. doi: 10.1136/adc.52.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winick M., Rosso P., Waterlow J. Cellular growth of cerebrum, cerebellum, and brain stem in normal and marasmic children. Exp Neurol. 1970 Feb;26(2):393–400. doi: 10.1016/0014-4886(70)90134-2. [DOI] [PubMed] [Google Scholar]


