Abstract
The subjective experience of allocating one's attentional resources among competing tasks is nearly universal, and most current models of cognition include a mechanism that performs this allocation; examples include the central executive system and the supervisory attentional system. Yet, the exact form that an executive system might take and even its necessity for cognition are controversial. Dual-task paradigms have commonly been used to investigate executive function. The few neuroimaging studies of these paradigms have yielded contradictory findings. Using functional MRI, we imaged brain function during two dual-task paradigms, each with a common auditory component task (NOUN task) but varying with respect to a visual component task (SPACE or FACE tasks). In each of the two dual-task paradigms, the results showed that the activated areas varied with the component tasks, that all of the areas activated during dual task performance were also activated during the component tasks, and that surplus activation within activated areas during DUAL conditions was parsimoniously accounted for by the addition of the second task. These findings suggest that executive processes may be mediated by interactions between anatomically and functionally distinct systems engaged in performance of component tasks, as opposed to an area or areas dedicated to a generic executive system.
Working memory refers to the cognitive capacity for maintenance and manipulation of information. The widely influential model of working memory proposed by Baddeley and Hitch (1) postulated that working memory was subdivided into content-specific slave systems. The interplay of these slave systems, the visuo-spatial sketchpad and the articulatory loop, was thought to be regulated by a general-purpose, supramodal mechanism for resource allocation, the central executive, based on the supervisory attentional system originally proposed by Shallice (reviewed in ref. 2). Other authors have questioned whether such supervisory systems are necessary components of cognitive models (e.g., refs. 3 and 4). Because the central executive system (CES) is thought to be crucial for coordination of concurrent processing, it has commonly been investigated by using dual-task paradigms in which two behavioral tasks, often with disparate sensory and cognitive processing, are performed concurrently (see, for example refs. 5–7). The few brain mapping studies of these paradigms have yielded contradictory findings (8–11).
In the present study, we have used functional MRI to investigate brain activation during two dual-task paradigms. To test the hypothesis that the locations of activations during dual-task performance depended on the specific component tasks performed, we studied two dual-task paradigms. The first combined an auditory verbal categorization task (NOUN) and a visual mental rotation task (SPACE). The second combined the same auditory task with a different visual task involving object identification (FACE) rather than mental rotation. A demonstration that the same brain regions were activated in both paradigms during concurrent performance only would lend support to the idea that some general coordinating function, not required for the component tasks per se, was evoked by their concurrent performance. Conversely, the relocation of such “surplus” activations when component tasks were changed would suggest instead that that they reflected task-specific processes such as an increased demand for maintenance of task-specific information in the presence of distraction.
Methods
Sixteen subjects (8 female, 8 male, 14 right-handed) were recruited from the medical campus of Yale University and participated after giving informed consent. No subject had a history of psychiatric or neurological illness. None were medicated at the time of testing.
Experimental Tasks.
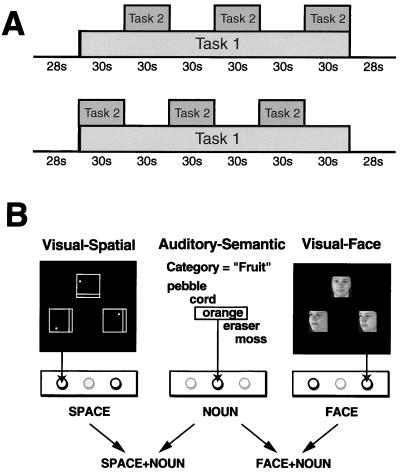
Each subject was scanned during two block-design dual-task paradigms, SPACE-NOUN and FACE-NOUN. Each dual-task paradigm incorporated auditory presentation of the same verbal categorization task, NOUN (12), and one of two visual matching tasks. The visual task involved either spatial rotation (SPACE task) or face identification (FACE task) (13). These visual tasks have previously been shown to evoke dissociable domain-specific patterns of activity in posterior cortical areas when compared with a delayed-alternation control task (13). For each paradigm, scans were obtained during both SINGLE conditions (“SINGLE” refers to separate performance of the component tasks NOUN and SPACE or FACE) and during the DUAL condition (simultaneous performance of the tasks SPACE + NOUN or FACE + NOUN) for that paradigm. An experimental session consisted of three anatomical scans followed by 10 functional scans of ≈4 min each. Functional scans included 3 min of task performance, shown schematically in Fig. 1. One task (Task 1 in Fig. 1A) was administered continuously throughout the scan whereas the other component task (Task 2 in Fig. 1A) was administered only in every other block, so that subjects alternately performed 30-s blocks of a DUAL condition and one of the SINGLE component tasks for that condition in each scan. Runs were counterbalanced for starting block (DUAL and SINGLE, as shown in Fig. 1A) and for Task 1 (NOUN and SPACE or FACE, as appropriate). The final two scans were alternations between the DUAL paradigms, accomplished by switching the visual task (SPACE to FACE and back) in alternate blocks while the NOUN task ran continuously (not illustrated in Fig. 1). Experimental tasks were practiced both singly and concurrently before scanning and during acquisition of anatomical images.
Figure 1.
Behavioral tasks. Block-design paradigms were used, with blocks alternating between isolated (SINGLE) and concurrent (DUAL) performance of the component tasks. (A) Graphic representation of the experimental task runs. Task 1 is performed continuously whereas Task 2 is performed only in alternate blocks. Tasks 1 and 2 were counterbalanced for component task (auditory or visual) and for starting block (SINGLE or DUAL) for each paradigm. (B) Component tasks and sample stimuli for each paradigm. The NOUN task used new categories and word lists for each run of data acquisition.
Responses were indicated by manually depressing one of three buttons on a fiber optic response box, as represented below the stimuli in Fig. 1B. The auditory task responses were indicated on a different button from those used for the visual tasks. Stimuli were presented at different rates and staggered by 500 ms, so that motor responses never exactly coincided for the two tasks in DUAL conditions. Subjects were instructed to attend to both tasks equally.
In NOUN, subjects listened to lists of concrete nouns (presented every 2 s) and responded to exemplars of the target semantic category for each run (e.g., “fruit,” “vehicles”) by pressing the center button of a three-button fiber-optic response box. A different target category was used for each run, along with new word lists.
For SPACE and FACE, visual stimuli were back projected every 3 s onto a frosted Plexiglas screen visible through a mirror. Stimuli in both visual tasks subtended equal visual angles (≈4°), and the entire visual display covered ≈12°. In SPACE, subjects indicated which of two squares (left or right), each containing a straight line and a dot, was identical to a rotated target square (upper) by pressing the button on the same side as the correct stimulus. In FACE, subjects indicated which of two 45° rotated faces was the same as a full-face target picture by pressing the button on the same side as the correct stimulus. Sample visual stimuli are shown in Fig. 1B. The SPACE and NOUN tasks followed the procedure previously used in ref. 8.
Data Acquisition.
Activation data were acquired by using BOLD-contrast functional MRI on a GE Signa 1.5 Tesla magnet equipped with echoplanar (EPI) hardware (Advanced NMR Systems, Wilmington, MA). Subjects were immobilized by using vacuum cushions and forehead and chin straps. T1-weighted (TR = 500, TE = 13) and echo-planar (TR = 3,000, TE = 80) anatomical images were acquired for coregistration of activation images while subjects practiced the tasks. Six slices were acquired in the plane parallel to the AC-PC line with the most inferior slice centered at +8 mm above the AC-PC line and the most superior slice centered at +58 mm above the AC-PC line. Activation runs consisted of 168 EPI single shot gradient-echo images per slice (TR = 1.406, TE = 45 ms, a = 60, FOV = 40 × 20 cm, 128 × 64 data acquisition matrix, slice thickness 8 mm, skip 2 mm). Each data acquisition run began with 28-s resting baseline, included six 30-s blocks of task performance, and ended with a second 28-s baseline for a total of 3:56.
Data Analyses.
Data quality criteria.
Activation images were screened for movement occurring during the scan before statistical analysis by plotting center of mass changes for each individual across each acquisition and across the entire session. Only acquisitions with movement less than 1 mm within-run or 1.5 mm across counterbalanced pairs were analyzed further. For two subjects, fewer than five runs met these criteria; no data from these subjects were analyzed further, leaving n = 14. All hypothesis testing was performed on individual subject data. The nine subjects included in group-level analyses (logical analyses, spatial normalization, and averaging) were those who performed at 80% or better on all component tasks and fell within the permitted motion criteria on all 10 runs.
Generation of individual and group activation maps.
Statistical parametric maps of contrasts between DUAL relative to SINGLE conditions and of contrasts between all tasks and resting baseline were generated by using voxel-wise t statistics, averaged over all runs in which a given condition occurred. Intersubject statistical averaging was performed by three-dimensional spatial normalization in Talairach (14) coordinate space and calculation of voxel-wise median t values across subjects for each behavioral condition.
Identification of candidate areas for a CES.
To dissociate putative CES function from component task processes, we planned our analyses to include the identification of dual-task activations greater than the sum of their parts with respect to areas activated, which we refer to here as “surplus” activations, using logical analyses analogous to the “and” rule used by split t tests. Subtraction methods of comparing SINGLE to DUAL conditions are less useful for testing hypotheses regarding activations specific to dual-task performance because only one of the two component tasks can be subtracted out of the DUAL condition image, and because the degree to which overlapping activations will be strictly additive in magnitude is unknown. However, it could be argued that CES function might also be manifest in greater activation during dual-task performance of those areas already activated by component tasks. Conversely, areas activated by component tasks might show relative deactivations during simultaneous performance, so that the magnitude of activation was less than in SINGLE conditions. Finally, it is also possible that CES-related activations would only be observed when performance costs were observed in DUAL conditions relative to SINGLE conditions. To address these issues, we included analyses of levels of activation for regions-of-interest (ROIs) in key regions.
Logical analyses comparing anatomical patterns of activation in DUAL and SINGLE conditions.
To identify areas activated only by DUAL conditions and to determine the extent to which the particular component tasks used affected the localization of these activations, progressively restrictive decision rules were sequentially applied to the individual maps. The first two rules were applied to individual-subject data; the third looked for anatomical areas activated across subjects. These rules isolated (i) for each paradigm (SPACE-NOUN and FACE-NOUN), within each subject, areas activated during a DUAL condition but NOT activated by either SINGLE component; (ii) within each subject, areas satisfying criterion 1 in both SPACE-NOUN and FACE-NOUN paradigms; and (iii) across subjects, anatomical locations containing voxels that consistently satisfied criteria 2 (and thus also 1). To estimate noise level variation in anatomical localization of activations for each individual, we counted voxels active in only one run vs. voxels active in all runs of the same task. Results are reported for analyses with thresholds set at t = 1.96 (uncorrected P < 0.00125, one-tailed; P < 0.0025, two-tailed). This level was chosen to minimize threshold effects. We also performed the analyses with varying thresholds (higher and lower) with orthogonal t tests, as the original comparisons shared common baselines.
ROI analyses.
Two separate methods of defining ROIs were used. Before spatial normalization, ROIs were defined for each subject by that subject's activations in the current study (functionally defined ROIs). ROIs were also defined for each subject over anatomical areas (anatomically defined ROIs) implicated in CES function in a prior neuroimaging study (8): inferior anterior cingulate and anterior middle frontal gyrus (MFG), illustrated for a representative subject in Fig. 4 (published as supplemental data on the PNAS web site, www.pnas.org).
Unthresholded mean t values, percent signal change relative to baseline, and average signal intensity levels were used to compare activations in all ROIs (functionally and anatomically defined) for each DUAL task and its SINGLE components, for each subject. As stated above, these comparisons would be expected to identify changes in activation attributable to the second component task as well as any concurrent performance effect. Results are reported for percent signal change; other measures gave identical findings. Sign tests on the direction of change for individual subjects were also performed to detect small effects that were consistent across subjects. Finally, correlational analyses were performed between strength of activation in prefrontal ROIs and behavioral measures, as outlined below.
Results
Behavioral Data.
Details of individual subjects' performance are given in Table 1. All subjects reported that the DUAL conditions were subjectively more demanding than the SINGLE conditions. Some subjects, but not all, showed significant performance decrements on one or both tasks during DUAL conditions relative to SINGLE, consistent with decrements reported in prior studies using dual task paradigms to study CES function in normal subjects (5, 6, 8).
Table 1.
Individual subject performance data, with change in accuracy from SINGLE to DUAL conditions and P value of Fisher's exact test for that difference
| Subject | SPACE SPACE
|
FACE FACE
|
NOUN NOUN
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single | Dual | Change | P | Single | Dual | Change | P | Single | Dual | Change | P | |
| 1 | 93.3% | 94.4% | 1.1% | 0.550 | 95.0% | 93.9% | −1.1% | 0.494 | 91.9% | 94.5% | 2.6% | 0.245 |
| 2 | 95.0% | 91.1% | −3.9% | 0.017 | 93.3% | 91.7% | −1.7% | 0.370 | 88.0% | 89.5% | 1.5% | 0.578 |
| 3 | 98.3% | 97.2% | −1.1% | 0.244 | 98.3% | 97.2% | −1.1% | 0.244 | 92.0% | 91.4% | −0.6% | 0.803 |
| 4 | 95.0% | 97.2% | 2.2% | 0.171 | 93.3% | 97.2% | 3.9% | 0.036 | 91.9% | 96.6% | 4.7% | 0.038 |
| 5 | 81.7% | 73.9% | −7.8% | 0.011 | 88.3% | 92.2% | 3.9% | 0.104 | 80.0% | 78.3% | −1.7% | 0.616 |
| 6 | 90.0% | 92.8% | 2.8% | 0.214 | 91.7% | 93.3% | 1.7% | 0.418 | 81.1% | 84.9% | 3.9% | 0.235 |
| 7 | 96.7% | 92.8% | −3.9% | 0.004 | 83.3% | 82.2% | −1.1% | 0.689 | 86.0% | 85.3% | −0.7% | 0.813 |
| 8 | 93.3% | 92.8% | −0.6% | 0.765 | 91.7% | 91.7% | 0.0% | 1.000 | 96.0% | 90.2% | −5.8% | 0.000 |
| 9 | 90.0% | 84.4% | −5.6% | 0.013 | 95.0% | 97.2% | 2.2% | 0.171 | 94.7% | 90.3% | −4.4% | 0.018 |
| 10 | 96.7% | 91.7% | −5.0% | 0.000 | 98.3% | 93.9% | −4.4% | 0.000 | 91.9% | 89.5% | −2.4% | 0.001 |
| 11 | 91.7% | 88.3% | −3.3% | 0.106 | 95.0% | 90.6% | −4.4% | 0.006 | 86.0% | 84.9% | −1.1% | 0.710 |
| 12 | 96.7% | 97.2% | 0.6% | 0.678 | 95.0% | 93.3% | −1.7% | 0.305 | 91.9% | 89.5% | −2.4% | 0.297 |
| 13 | 95.0% | 87.8% | −7.2% | 0.000 | 91.7% | 80.6% | −11.1% | 0.000 | 82.0% | 71.3% | −10.7% | 0.002 |
| 14 | 98.3% | 97.2% | −1.1% | 0.244 | 95.0% | 95.0% | 0.0% | 1.000 | 78.0% | 67.1% | −10.9% | 0.002 |
| 15 | 90.0% | 91.7% | 1.7% | 0.456 | 91.7% | 86.7% | −5.0% | 0.015 | 80.0% | 79.0% | −1.0% | 0.770 |
| 16 | 71.7% | 70.6% | 1.1% | 0.456 | 76.7% | 75.0% | 1.7% | 0.520 | 89.5% | 84.9% | 4.6% | 0.030 |
Two subjects were excluded from group-averaged data analyses because performance on a component task was below 80%. Performance on the SPACE visual task for the subjects included in group-averaged data ranged from 90 to 98%, with changes ranging from +3 to −7% during the SPACE + NOUN dual task. Performance on the FACE visual task ranged from 92 to 98%, with changes during FACE + NOUN dual task ranging from + 4 to −11%. Performance for included subjects on the NOUN task ranged from 80 to 95%, with changes from + 1 to −11% during the DUAL conditions. As new categories were used for each scan, and as some subjects showed list-specific impairments (e.g., for insects or tools), these summary performance data for the NOUN task were calculated by dropping the score from each subject's worst list; these scores were, however, included in correlations of activation level with behavior. Changes in performance were not statistically significant at the group level for any component task.
Imaging Results.
Description of anatomical patterns of activation by component tasks.
t tests relative to baseline revealed significant task-related activations for all three component tasks (SPACE, FACE, and NOUN) and for their respective DUAL conditions (SPACE + NOUN and FACE + NOUN) in bilateral frontal and parietal association areas, as well as predicted activations in auditory and visual association cortices. Frontal lobe activations are described here; see supplemental material for details of posterior activations. These descriptions refer to general areas; details are given in Table 2 (published as supplemental data) and are illustrated in Fig. 5 (published as supplemental data).
The areas activated by SPACE and FACE tasks show considerable overlap, as can be seen by inspection of the group and individual subject data in Fig. 2 and 3, but locations of peaks and degree of activation differed for the two visual tasks. Both tasks activated bilateral middle frontal gyrus [primarily Brodmann's areas (BAs) 10/46], bilateral inferior frontal/inferior precentral sulcus, and regions near the junction of the superior frontal sulcus and the precentral sulcus. The SPACE visual task additionally activated the right anterior insula. In accord with other data for object and spatial memory processing tasks (15–18), the most significant frontal peak for the FACE task was dissociated from the SPACE task peak. In our data, this separation measured 27.7mm, with the FACE peak more lateral, anterior, and ventral in the right inferior precentral/inferior frontal sulcus (at Talairach coordinates 45, 6, 41 for composite maps) than the SPACE task peak (at 26, −12, 50), which was centered more on the superior frontal sulcus. SPACE task activations in this region (precentral sulcus/superior frontal junction) were also more lateralized toward the right for the SPACE task than for the FACE task. Despite equivalent levels of behavioral performance and equivalent degrees of interference as defined by NOUN performance decrements during DUAL conditions, the SPACE task tended to produce stronger activations than the FACE task in all areas of the brain except at the frontal FACE task peak (45, 6, 41) and in occipital cortex near the right fusiform gyrus (20, −94, 5). In these areas, FACE task activations were more significant (see Table 2). In frontal lobes, the NOUN task activated left inferior frontal gyrus, bilateral middle frontal gyrus (primarily BA 9/45/46), left inferior precentral sulcus, and left anterior insula.
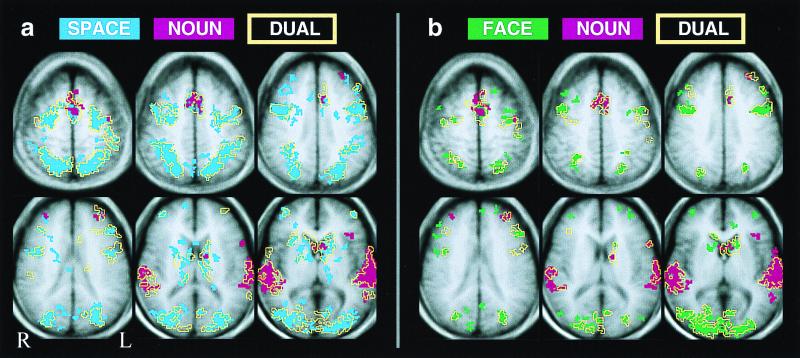
Figure 2.
Activation map overlays for composite data, showing anatomical relationship of dual-task activations (yellow outline masks) to component tasks (solid colors: red, NOUN; blue, SPACE; green, FACE). For clarity of display, maps are cluster-filtered at three contiguous voxels; however, the logical analysis used to identify activations attributable to CES function did not use a cluster filter. Changing thresholds changes size and scatter of activations but does not affect ratio of SINGLE/DUAL overlap of activations.
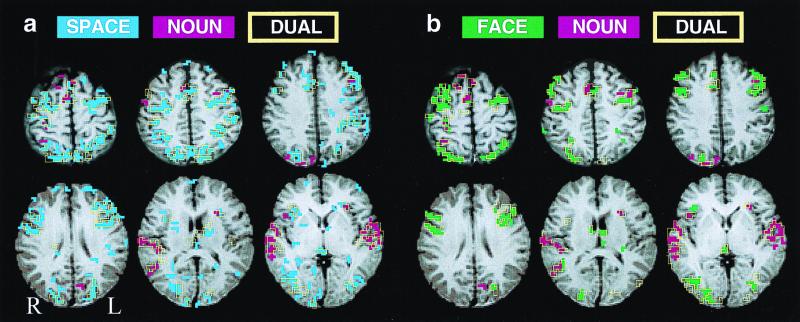
Figure 3.
Activation map overlays for Subject 3. Color conventions are as in Fig. 2. (a) SPACE, NOUN, and SPACE + NOUN. (b) FACE, NOUN, and FACE + NOUN. Note absence of (MFG) activations across DUAL and SINGLE conditions in this subject. All activations above t = 1.96 (for two-run averages P < 0.00125 one-tailed, P < 0.0025 two-tailed, uncorrected for multiple comparisons) are represented for each task. Here too, maps for DUAL conditions are predictable from their SINGLE component task maps. Just as for individual data, changing map thresholds changes spread of activations but does not change the amount of overlap between DUAL and SINGLE conditions.
Dorsomedial frontal cortex, near supplementary motor area (SMA) and anterior cingulate (BA 6, 32, 24), was activated in every subject by all tasks. Anterior MFG was activated bilaterally across all tasks in some subjects, although peaks and extents of activation differed for the visual (BA 10/46) and auditory (BA 9/46) tasks. Critically, every subject who activated MFG in a DUAL condition also demonstrated MFG activation in at least one of the component tasks for that condition. It should be noted that these large activations are orders of magnitude larger than functional units in cortex (19–21), and that the resolution of functional MRI is insufficient for the differentiation of possible task-specific patterns within these large activations.
Comparison of Activations in SINGLE and DUAL Conditions.
Anatomical patterns of activation: Are there areas activated by DUAL but not SINGLE conditions across paradigms?
In both paradigms, qualitative and quantitative analyses revealed virtually identical anatomical distribution of activations by DUAL conditions and by SINGLE component tasks in both paradigms. Qualitative illustrations of these anatomical relationships are given in Fig. 2 for the group-averaged data and in Fig. 3 for a representative subject. The quantitative test of the hypothesis that DUAL conditions activate a common area not activated by the component tasks provided results consistent with the qualitative illustrations: voxels surviving the first statistical decision rule, activation by DUAL condition but not SINGLE components, were indistinguishable from noise-level fluctuations in component task activation maps for both SPACE-NOUN and FACE-NOUN paradigms. Surviving voxels tended to occur singly or in small clusters on the edges of or bridging gaps between SINGLE component task activations. The second- and third-tier analyses gave the following results: Across all subjects, the seven clusters of three to four voxels that survived the second decision rule were located in auditory areas (two clusters), intraparietal sulcus (two clusters), middle frontal gyrus (two clusters), inferior frontal sulcus (one cluster), and callosal white matter (one cluster). Thus, no predisposition for these voxels to occur in any particular area was apparent (third rule).
Degree of activation in DUAL relative to SINGLE tasks.
Analyses of strength of activation during DUAL and SINGLE tasks were performed in all ROIs. The results obtained were generally consistent with the findings from anatomical comparisons, as follows: DUAL activations in a given ROI were generally significantly stronger in comparison to one SINGLE task but not the other, consistent with an interpretation of this increase as attributable to the specific second component task. For example, in left MFG, percent signal change was significantly increased for DUAL relative to SINGLE only when SPACE + NOUN was compared with SPACE (P = 0.002), suggesting that the increase in activation was caused by NOUN task performance. In the functionally defined MFG ROI, a similar but more general effect was detected by a sign test performed on the direction of change in individual subjects. For all comparisons in left but none in right MFG, DUAL was greater than SINGLE (P = 0.04 relative to NOUN to P = 0.007 relative to SPACE). In cingulate cortex ROIs, the SPACE + NOUN evoked significantly less activation than NOUN alone (P = 0.02 by t test on percent difference).
In visual cortex, comparisons of DUAL to SINGLE conditions yielded expected significant differences when comparisons were to the NOUN task (least significant comparison P = 0.0065) but also revealed increases relative to visual SINGLE conditions in the left lateral occipital sulcus. Given the variability in level, these increases were not significant despite an average increase in mean t value of 1.72 in SPACE and 1.55 in FACE, but sign tests on individual direction of change were significant at P = 0.0004 for SPACE (P = 0.008 for FACE). In auditory cortex ROIs (BA 21/22 and 41/42, functionally defined by activations during NOUN), comparisons of DUAL to SINGLE conditions yielded expected significant differences when comparisons were to visual single tasks (least significant comparison P = 0.001), but also revealed that activations in DUAL conditions were smaller than NOUN task activations in both hemispheres (change in mean t value P = 0.05 for left; P = 0.008 by sign test for both right and left).
An additional, unanticipated result revealed by ROI analyses in sensory areas was that small but consistent absolute deactivations were seen in these areas during performance of the cross-modal task. In visual cortex (BA 17/18/19), medial to the lateral occipital sulcus, data from every subject showed small deactivations for cross-modal task blocks (NOUN), which were significant by sign tests on the direction of the effect in the left hemisphere (P = 0.009; P = 0.09 in the right hemisphere). In right auditory cortex, similar small deactivations were found, during visual SINGLE tasks, in all but one subject (sign test P = 0.002 for right, not significant for left).
In sum, the following three ROIs showed some evidence of stronger activation in DUAL tasks in all comparisons: by t test on percent change, dorsomedial frontal cortex, near supplementary motor area (SMA)/anterior cingulate (BA 6, 32, 24); by sign test, left MFG, and the left lateral occipital sulcus.
Correlation of prefrontal activation with performance measures: Does middle frontal gyrus activation depend on performance costs or other behavioral measures?
Percent signal change in MFG ROIs was correlated with performance variables to further characterize MFG activations. For each component task, percent correct in SINGLE condition, percent correct in DUAL condition, and change in performance (measured both as raw difference scores and as Fisher's exact scores) in DUAL relative to SINGLE conditions were analyzed. Correlations were performed for both the anatomically defined and the functionally defined ROIs.
Interestingly, activations in anterior MFG were far more variable across subjects than activations elsewhere in the brain; most subjects showed suprathreshold activations in one or two conditions. However, despite observed correlations with task performance, no significant correlations were seen between signal increases in MFG and decreases in performance from SINGLE to DUAL conditions, as would be expected if these activations arose from increased load on the CES reflected in performance costs. Significant correlations with NOUN task performance in both SINGLE (right, P < 0.001; left, P < 0.01) and DUAL (right, P < 0.02; left, P < 0.01) conditions were seen in anatomically defined MFG ROIs in both hemispheres. Relationships with NOUN performance were not as significant in functionally defined ROIs, (right, DUAL only P < 0.05; all others not significant), as might be expected given that the functional ROIs were defined by DUAL activations, which corresponded more closely to visual activations. Activations in functionally defined ROIs in MFG were significantly correlated with FACE task performance on the left (P < 0.01, for SINGLE condition only) and approached significant correlation with SPACE task performance on the right (P = 0.06, for SINGLE condition only).
Discussion
In each subject, in both paradigms tested, all areas activated by concurrent performance were also activated by one or both component tasks. The main finding is thus that, despite other interesting changes in activation patterns during concurrent performance, no evidence of an activated locus (or loci) for a possible central executive was seen. Increases in activation, detected within regions-of-interest defined by component task activations or by previous literature, were more parsimoniously accounted for by the additive effects of component task activation, except in left lateral occipital sulcus. As this sensory area is an unlikely candidate locus for central executive function, we hypothesize that this increase is attributable to use of visual mental imagery during the NOUN task.
Two classes of question arise regarding these results and their relevance to the issue of a neural basis for executive function. The first class concerns the suitability of these particular behavioral paradigms for examination of executive function whereas the second concerns the interpretation of the data obtained in any paradigm.
It could be argued that the central executive is continuously active. If so, the current methodology would not detect a difference between the DUAL and SINGLE tasks, but neither would any physiological method that compares one state to another, including single-cell recording, and the idea of a central executive would not be testable. Conversely, it could be argued that the current paradigms did not tax executive function sufficiently because, for the group, no significant decreases in accuracy during DUAL relative to SINGLE conditions were seen. However, hypothesis testing was performed at the level of individual subjects. Individual scores showed significant decrements of up to 11%, and for these subjects, for whom the same results held, that argument does not apply. Furthermore, this argument implies that, across subjects, performance costs would predict central executive-related activation. Correlational analyses failed to demonstrate any such relationship in prefrontal areas, despite modest task-specific correlations between prefrontal activation and successful performance.
Finally, it could be argued that even our SINGLE conditions were demanding enough to engage the central executive, so that an area like the preSMA, which was activated relative to baseline in all subjects, in all conditions, might reflect executive function. However, the left occipitotemporal sulcus, an unlikely locus for executive function, shows a similarly suggestive profile of stronger activation in DUAL conditions relative to all SINGLE conditions as that seen in preSMA. On what basis can we ascribe one activation, but not the other, to executive function? The interpretation of the results of any study as supportive or nonsupportive of a neural machinery for executive function is complicated by the lack of a clear independent measure. In the case of memory, for example, behavioral errors might result from mishaps at many neural levels, but correct responses indicate that memory has occurred. Such a measure is not available for the study of executive function; the use of performance costs to gauge central executive “load” (1, 6) rests on the assumptions that a central processor exists, is of limited capacity, and that no other sources of interference between tasks exist. Importantly, however, the current data can speak to the issue of a locus (or loci) activated by dual-task performance, and to changes in brain function during multitasking.
The current results are at odds with one prior study, in which activations were not seen during isolated performance of tasks almost identical to the current SPACE-NOUN paradigm (8). The previous authors thus concluded that the activations seen in middle frontal gyrus and anterior cingulate during DUAL conditions represented executive function. Of the few differences in details of task administration between the two studies, including new word lists for each run in the NOUN task (here), more and longer runs per subject (here), responses indicated with fingers (here) rather than toes, none offers a clear explanation for why D'Esposito et al. (8) did not see the prefrontal activations observed here for all three SINGLE component tasks. The present results are, however, consonant with many prior reports of similar middle frontal gyrus activations in settings like those of the component tasks (18, 22–30). In particular, one recent study using a spatial mental rotation task much like the SPACE component task reported activations (Talairach coordinates 34, 36, 5) close to those described here (31). Finally, a few other neuroimaging studies have incorporated dual-task performance of various types (9–11), with results similar to the current report.
In sum, these data provide no evidence for a neural locus for executive function, in the prefrontal cortex or elsewhere, selectively activated by dual-task performance, or for appropriate additional activation not adequately and parsimoniously accounted for by the summation of component task processes. The data do reveal subtle alterations in activation patterns, including relative and absolute deactivations in sensory and parietal cortex, which invite further study into the role of inhibitory processes in multitasking and attention. These findings are consistent with our view that the various specialized information-processing systems in the human brain may, by their interplay, accomplish the regulation of complex operations such as multitasking. Further, we suggest that similar bottom-up mechanisms may underlie other operations sometimes characterized as executive processes.
Supplementary Material
Acknowledgments
This work was supported by grants awarded to P.S.G.-R. (National Institutes of Health Grants K05 MH00298 and P50 MH44866).
Abbreviations
- CES
central executive system
- ROI
region-of-interest
- MFG
middle frontal gyrus
- SMA
supplementary motor area
- BA
Brodmann's area
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.060588897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.060588897
References
- 1.Baddeley A D, Hitch G J. In: The Psychology of Learning and Motivation. Bower G, editor. Vol. 8. New York: Academic; 1974. pp. 47–90. [Google Scholar]
- 2.Norman D A, Shallice T. In: Consciousness and Self-Regulation. Davidson R J, Schwartz G E, Shapiro D, editors. Vol. 4. New York: Plenum; 1986. [Google Scholar]
- 3.Allport . In: Cognitive Psychology: New Directions. Bower G, editor. London: Routledge & Kegan Paul; 1980. pp. 112–153. [Google Scholar]
- 4.Spelke E, Hirst W, Neisser U. Cognition. 1976;4:215–230. [Google Scholar]
- 5.Baddeley A D. Working Memory. Oxford: Clarendon; 1986. [Google Scholar]
- 6.Della Sala S, Baddeley A, Papagno C, Spinnler H. Ann NY Acad Sci. 1995;769:161–171. doi: 10.1111/j.1749-6632.1995.tb38137.x. [DOI] [PubMed] [Google Scholar]
- 7.Miyake A, Shah P. In: Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Miyake A, Shah P, editors. Cambridge, U.K.: Cambridge Univ. Press; 1999. pp. 1–27. [Google Scholar]
- 8.D'Esposito M, Detre J A, Alsop D C, Shin R K, Atlas S, Grossman M. Nature (London) 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg T E, Berman K F, Fleming K, Ostrem J, Van Horn J D, Esposito G, Mattay V S, Gold J M, Weinberger D R. Neuroimage. 1998;7:296–303. doi: 10.1006/nimg.1998.0338. [DOI] [PubMed] [Google Scholar]
- 10.Klingberg T, Roland P E. Brain Res Cognit Brain Res. 1997;6:1–8. doi: 10.1016/s0926-6410(97)00010-4. [DOI] [PubMed] [Google Scholar]
- 11.Klingberg T. Cereb Cortex. 1998;8:593–601. doi: 10.1093/cercor/8.7.593. [DOI] [PubMed] [Google Scholar]
- 12.Demonet J F, Chollet F, Ramsay S, Cardebat D, Nespoulous J L, Wise R, Rascol A, Frackowiak R. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- 13.Haxby J V, Grady C L, Horwitz B, Ungerleider L G, Mishkin M, Carson R E, Herscovitch P, Schapiro M B, Rapoport S I. Proc Natl Acad Sci USA. 1991;88:1621–1625. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 15.Courtney S M, Ungerleider L G, Keil K, Haxby J V. Cereb Cortex. 1996;6:39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- 16.Baker S C, Frith C D, Frackowiak R S, Dolan R J. Cereb Cortex. 1996;6:612–619. doi: 10.1093/cercor/6.4.612. [DOI] [PubMed] [Google Scholar]
- 17.Courtney S M, Petit L, Maisog J M, Ungerleider L G, Haxby J V. Science. 1998;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- 18.Belger A, Puce A, Krystal J H, Gore J C, Goldmanrakic P, McCarthy G. Hum Brain Mapp. 1998;6:14–32. doi: 10.1002/(SICI)1097-0193(1998)6:1<14::AID-HBM2>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman-Rakic P S, Schwartz M L. Science. 1982;216:755–757. doi: 10.1126/science.6177037. [DOI] [PubMed] [Google Scholar]
- 20.Friedman H R, Bruce C J, Goldman-Rakic P S. J Neurosci. 1989;9:4111–4121. doi: 10.1523/JNEUROSCI.09-12-04111.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adcock R A, Friedman H R, Goldman-Rakic P S. Soc Neurosci Annu Meeting Abstr. 1992;18:387. [Google Scholar]
- 22.Frith C D, Friston K, Liddle P F, Frackowiak R S. Proc R Soc London Ser B. 1991;244:241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- 23.Petrides M, Alivisatos B, Meyer E, Evans A C. Proc Natl Acad Sci USA. 1993;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J D, Forman S D, Braver T D, Casey B J, Servan-Schreiber D, Noll D C. Hum Brain Mapp. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- 25.Smith E E, Jonides J, Koeppe R A. Cereb Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- 26.Jahanshahi M, Jenkins H, Brown R, Marsden C D, Passingham R E, Brooks D J. Brain. 1995;118:913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- 27.Schacter D L, Alpert N M, Savage C R, Rauch S L, Albert M S. Proc Natl Acad Sci USA. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tagaris G A, Kim S G, Strupp J P, Andersen P, Ugurbil K, Georgopoulos A P. J Cognit Neurosci. 1997;9:419–432. doi: 10.1162/jocn.1997.9.4.419. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy G, Blamire A M, Puce A, Nobre A C, Bloch G, Hyder F, Goldman-Rakic P, Shulman R G. Proc Natl Acad Sci USA. 1994;91:8690–8694. doi: 10.1073/pnas.91.18.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy G, Puce A, Constable R T, Krystal J H, Gore J C, Goldmanrakic P. Cereb Cortex. 1996;6:600–611. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- 31.Alivisatos B, Petrides M. Neuropsychologia. 1997;35:111–118. doi: 10.1016/s0028-3932(96)00083-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.