Abstract
Working memory (WM) refers to the temporary storage and processing of goal-relevant information. WM is thought to include domain-specific short-term memory stores and executive processes, such as coordination, that operate on the contents of WM. To examine the neural substrates of coordination, we acquired functional magnetic resonance imaging data while subjects performed a WM span test designed specifically to measure executive WM. Subjects performed two tasks (sentence reading and short-term memory for five words) either separately or concurrently. Dual-task performance activated frontal-lobe areas to a greater extent than performance of either task in isolation, but no new area was activated beyond those activated by either component task. These findings support a resource theory of WM executive processes in the frontal lobes.
The process whereby information is temporarily maintained in memory for use in ongoing mental operations is referred to as working memory (WM). The WM system is thought to consist of verbal and spatial short-term stores and executive processes that operate on the contents of these stores (1–3). Executive WM processes such as multiple task coordination, set shifting, interference resolution, and memory updating are thought to be essential for high-level thought processes and to be subserved by prefrontal cortex (PFC) (2–6). Executive WM is likely to be comprised of a number of distinct processes, and functional neuroimaging may be useful in dissociating those processes to the extent that these processes rely on distinct neural substrates (7–18). The present experiments focus on the neural substrates of dual-task coordination during the concurrent performance of two tasks.
Psychologists have developed test paradigms with the goal of measuring executive WM capacity. These WM span tests, which include reading span (19–20), listening span (21), operation span (22), and counting span (23), share the common property that they require concurrent processing (such as reading sentences for comprehension) and short-term maintenance (such as remembering the last word in each sentence). Unlike single-task short-term memory measures such as digit span or word span, WM span tests are powerful predictors of performance on a wide variety of verbal (e.g., verbal Scholastic Aptitude Test, text comprehension) and nonverbal measures (e.g., mathematical and reasoning problems) (19–20, 24–28). WM span tests are also sensitive to changes in cognitive ability throughout development (23, 29) and in old age (26), as well as in neurological diseases that compromise frontal-lobe functioning (30, 31). WM span tests yield a psychometrically robust measure of executive WM capacity, but the neural substrates of performance of such a task have not been yet been thoroughly explored (32).
In the present experiments, we used functional magnetic resonance imaging to measure brain activity associated with performance of a WM span test. Performing two tasks concurrently instead of one requires additional mental resources. There are two ways in which the recruitment of additional resources could be instantiated in the brain. Resources may be recruited from new areas specialized for dual task-specific processes, such as task coordination, that are not invoked by either component task (7). Alternatively, the recruitment of additional resources may be manifested as enhanced activation in the same brain regions that subserve performance of the component tasks (8, 9, 32, 33). The goal of these experiments was to ask whether performing two tasks instead of one recruits novel dual task-specific regions or leads to increased activation in the regions recruited by the component tasks.
Experiment 1
Methods.
Subjects.
Eight healthy right-handed volunteers (aged 18–28; 3 males, 5 females) participated after providing informed consent.
Tasks.
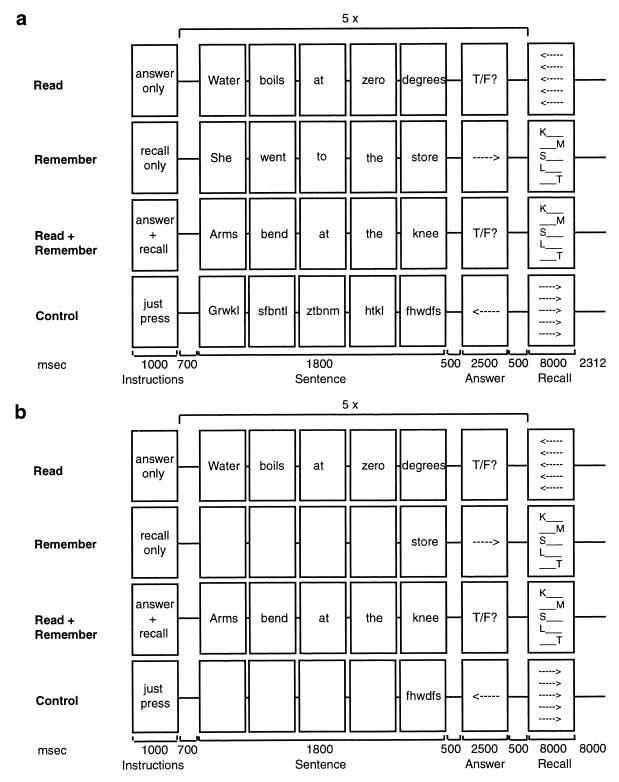
Subjects performed four different types of trials in the scanner: the WM span test, its two component tasks, and a baseline condition (Fig. 1a). Each trial was 41.3 sec in length and had the same basic structure, with Instruction, Sentence Processing, and Recall phases. Words in the Sentence Processing phase appeared one at a time at the center of the screen. On Read Trials, subjects evaluated five consecutive statements as true or false by pressing one of two buttons and then viewed an array of arrows. Subjects pressed the left button if the arrows pointed to the left and the right button if they pointed to the right. This left/right button press served to equate visual and motor processing demands across trial types. On Remember Trials, subjects viewed five consecutive narrative sentences and were instructed to simply remember the final word of each sentence. At the end of the trial, an array containing the first or last letter of the final word of each sentence appeared on the screen. Subjects pressed one of two buttons to indicate whether these letters correctly represented the order in which the five final words had appeared. The purpose of the recall cue was to ensure that subjects actively maintained the final words in memory throughout the trial. On Read+Remember Trials, subjects evaluated five sentences and remembered the final word of each sentence. The Sentence Processing phase of the Read+Remember Trials required concurrent processing of information (evaluating the content of each sentence) and maintenance of separate information (remembering the last word of each sentence). A set size of five sentences was chosen based on preliminary behavioral data suggesting that subjects would find this task challenging but would perform significantly above chance. On Control Trials, subjects viewed meaningless consonant strings and made left/right button presses.
Figure 1.
Schematic representation of the four Trial types in (a) Experiment 1, and (b) Experiment 2.
Testing procedure.
A scan session consisted of 2 anatomical scans and 5 functional scans of 5.6 min each. Two of each of the four trial types were interleaved within a scan, and the order of trials was counterbalanced across subjects. Participants responded by pressing one of two buttons on a button box with different fingers of their right hand. psyscope (34) was used to generate stimuli as well as to collect responses.
Data acquisition.
Whole-brain imaging data were acquired on a GE Signa 1.5 Tesla MRI scanner (General Electric Medical Systems Signa, Rev. 5.3). T1-weighted flow-compensated spin-echo anatomical images (TR = 500 ms; minimum TE) were acquired in 16 contiguous 7-mm axial slices parallel to a line passing through the anterior and posterior commissures. Functional images were acquired in the same set of slices by using a T2*-sensitive gradient echo spiral pulse sequence (35) (1 interleave, 40-ms TE, 105-ms TR/slice, 85° flip angle, 20-cm field of view, 64 × 64 data acquisition matrix).
Data analysis.
Functional images were motion corrected and normalized using spm96 (36), interpolated to 2 × 2 × 4 mm voxels, and spatially smoothed with a Gaussian filter (8-mm full width–half maximum). Low-frequency noise and differences in global signal were removed. Single subject data were analyzed with a fixed-effects model (36). Group data were analyzed by using a random effects model (37). For the group analysis, images were averaged to create one image of mean activity per Trial type and subject. t tests were performed on these average images to create a series of spm{z} maps depicting differences in brain activity between Trial types.
Conjunction analyses (38) were performed with spm96 by summing all the effects exhibited in either of two contrasts (Read+Remember vs. Remember and Read+Remember vs. Read) and excluding those voxels for which there was a significant interaction between contrasts. Activations common to the three Trial types were identified by overlaying on one another the activation maps for each Trial type relative to baseline.
Results.
Subjects were accurate in judging whether sentences were true or false for Read Trials (92 ± 2%; mean ± SEM) and Read+Remember Trials (95 ± 2%). They were also accurate in remembering the final words for both Remember Trials (86 ± 7%) and Read+Remember Trials (86 ± 5%). Dual-task performance did not affect accuracy on either component task. Subjects were, however, slower to evaluate sentences on Read+Remember Trials (725 ± 81 ms) than on Read Trials (618 ± 111) (P < 0.05; two-tailed t test). Subjects were also slower to verify the order of final words on Read+Remember Trials (3,761 ± 160 ms) than on Remember Trials (3,382 ± 124 ms) (P < 0.03; two-tailed t test). The slowed responses reflected the increased difficulty of performing both tasks concurrently rather than separately.
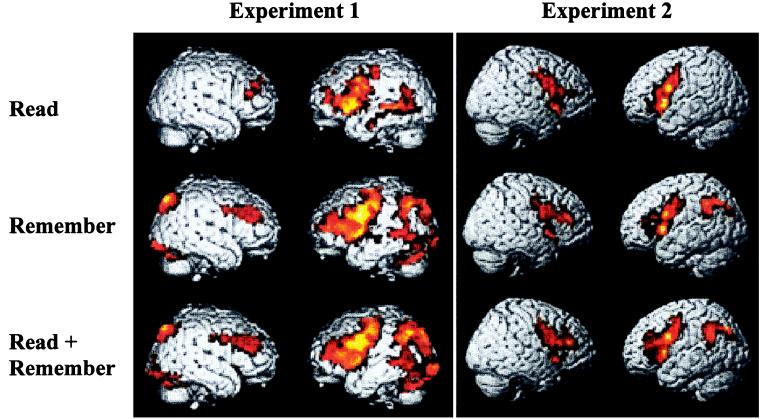
Activations were similar across the three Trial types (Fig. 2; Table 1), with prominent activations in left PFC and, to a lesser extent, right PFC. Also activated in all three Trial types were the left middle temporal gyrus and bilateral anterior cingulate. Remember and Read+Remember Trials additionally resulted in activations of bilateral parietal, occipital, and cerebellar regions. The prefrontal, parietal, and occipital/cerebellar activations were all more extensive and/or higher in amplitude in Read+Remember than in the Remember Trials (Table 1). These activations resemble those reported previously for sentence processing (33, 39, 40) and for demanding verbal WM tasks (41–45).
Figure 2.
Whole-brain renderings of group-averaged activations for the three Trial types relative to Control. A lenient threshold was chosen to display all trends toward activation (P < 0.05 corrected for multiple comparisons). Lighter colors indicate higher Z values.
Table 1.
Activations for each condition relative to control—Experiment 1
| Brain region (Brodman area) | Read
|
Remember
|
Read + Remember
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z* | Z score† | Volume‡ | x | y | z | Z score | Volume | x | y | z | Z score | Volume | |
| L inferior frontal gyrus (44,45,47), precentral gyrus (6) | −52 | 8 | 0 | 3.93 | 58672 | −48 | 6 | 28 | 4.99 | 71456 | −48 | 6 | 28 | 5.68 | 150960 |
| R middle frontal gyrus (9/46) | 32 | 36 | 16 | 2.94 | 8736 | 38 | 28 | 36 | 4.06 | 17952 | 34 | 38 | 20 | 4.31 | |
| L middle frontal gyrus (9/46) | −34 | 40 | 12 | 2.87 | −36 | 42 | 12 | 3.91 | −36 | 42 | 12 | 4.27 | |||
| L superior frontal Gyrus/sulcus (10) | −26 | 42 | 12 | 2.50 | −24 | 50 | 8 | 3.62 | |||||||
| R superior frontal Gyrus/sulcus (10) | 20 | 50 | 36 | 2.26 | 34 | 42 | 32 | 3.52 | 34 | 38 | 20 | 4.31 | |||
| R anterior cingulate (24) | 8 | 22 | 36 | 3.21 | 9840 | 8 | 16 | 36 | 3.74 | 8 | 16 | 36 | 4.43 | ||
| L anterior cingulate (24) | −8 | 2 | 60 | 3.02 | −6 | 6 | 52 | 4.47 | −6 | 6 | 52 | 5.17 | |||
| R precuneus (19) | 24 | −68 | 32 | 2.53 | 26 | −80 | 48 | 4.44 | 13088 | 30 | −66 | 48 | 4.63 | 16736 | |
| L precuneus (19) | −30 | −82 | 44 | 4.06 | 31280 | −30 | −82 | 44 | 5.13 | 79616 | |||||
| L middle temporal gyrus (21) | −52 | −14 | −24 | 3.26 | 8768 | −58 | −64 | 0 | 2.71 | −52 | −52 | 0 | 3.29 | ||
| L visual association areas (19)/cerebellum | −42 | −80 | −16 | 2.69 | −42 | −80 | −16 | 3.68 | |||||||
| R visual association areas (19)/cerebellum | 30 | −74 | −24 | 3.11 | 17952 | 30 | −58 | −36 | 4.40 | 31728 | |||||
Global maxima are in boldface; local maxima are in the regular face.
Coordinates for significant activations (P < 0.05 corrected for multiple comparisons).
†Z score for local maximum.
‡Volume of activation in mm3.
The critical question was whether Read+Remember Trials would elicit activity in any regions that were not recruited by performance of either component task. Examination of the data suggested that all areas activated by the Read+Remember Trials were also activated by either or both of the component Read and Remember Trials (Table 1). Averaged across subjects, no area—in PFC or elsewhere—appeared to be active exclusively during dual-task performance. A conjunction analysis failed to find areas that were more active for Read+Remember Trials relative to both Read Trials and Remember Trials (P > 0.05 corrected for multiple comparisons). The area of maximal activation in the conjunction analysis was in left PFC, but this area was also activated above baseline for Remember Trials in seven of eight subjects. When performed on individual subjects' data, the same type of conjunction analysis revealed activations in only four of eight subjects. These activations occurred in no consistent region of the brain (P > 0.05 uncorrected) and were specific to PFC in only one subject.
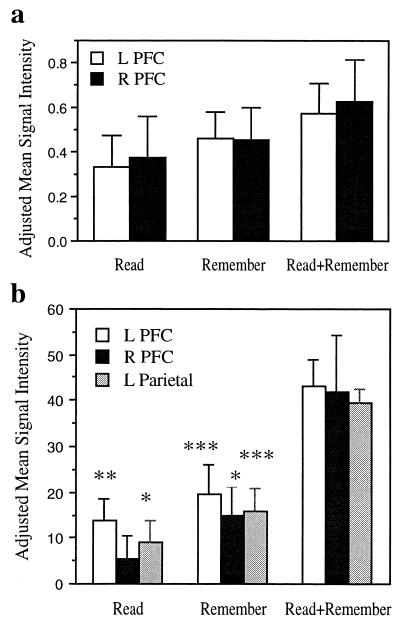
A volume-of-interest (VOI) analysis was performed to examine the possibility that dual-task performance yielded greater magnitudes of activation in the areas commonly activated across Trial types. Creation of an overlap map identified common activations across the three Trial types in left PFC, right PFC (middle frontal gyrus), and left temporo-parietal junction (Fig. 3a). Read+Remember yielded greater activation relative to Read in the left and right prefrontal VOIs (P < 0.001 and P < 0.02, respectively, two-tailed paired t tests) and relative to Remember in the right prefrontal VOI, with a tendency toward greater activity in the left prefrontal VOI (P < 0.017 and P < 0.166 respectively; two-tailed paired t tests). There was no difference in magnitude of activation in the temporo-parietal VOI between Read+Remember and either Read (P > 0.05) or Remember (P > 0.05).
Figure 3.
VOI analyses. (a) Experiment 1. Changes in mean signal intensity in the prefrontal activations common to all three trial types relative to baseline. (b) Experiment 2. Changes in mean signal intensity in the regions identified by a conjunction analysis as being more active for the dual task than for component tasks. Changes in signal intensity were determined from parameter estimates of the fit between each contrast and the estimated hemodynamic response, as calculated by the general linear model implemented in SPM99. Statistical significance of t tests for each condition relative to baseline. *, P < 0.05; **, P < 0.025; ****, P < 0.01.
Dual-task performance did not recruit additional brain regions relative to the component tasks but was associated with increased activation in regions activated by one or both component tasks. Two aspects of the task design in Experiment 1, however, limit interpretation of these results. First, although the Remember condition was not designed as a dual task, subjects may have treated it as such by reading the sentences for comprehension as well as retaining the final words. Second, because it is specifically during the Sentence Processing phase that one might expect to see differences in activation between dual-task and component-task performance, the demanding Recall phase may have obscured differences between Remember and Read+Remember by invoking executive processes related to recall in both trial types. The trials were modified in Experiment 2 so as to eliminate these concerns. To have a well-matched baseline for the critical Remember condition, the Control trials were modified so as to be visually matched to the Remember trials.
Experiment 2
Methods.
Subjects.
Eight right-handed volunteers (aged 19–31; 4 males, 4 females) participated after providing informed consent.
Tasks.
The same four Trial types were used as in Experiment 1, with several modifications (Fig. 1b). In Experiment 2, only the five to-be-remembered words on the Remember Trials appeared on the screen in the Sentence Processing phase. Similarly, subjects viewed only five consonant strings on Control Trials. Additionally, each trial ended with an intertrial (ITI) interval of 8 sec. Based on the average response time to verify the recall cue in Experiment 1, it was estimated that this length of ITI would allow roughly 12 sec for the recall-related hemodynamic response to subside before the start of the next trial.
Testing procedure.
The testing procedure was identical to that in Experiment 1, except that each functional scan was 6.3 min in length.
Data acquisition.
Whole-brain imaging data were acquired on a 3 Tesla MRI Signa LX Horizon Echospeed (General Electric Medical Systems, 8.2.5 systems revision). T2-weighted flow-compensated spin-echo anatomical images (2,000 ms TR; 85 ms TE) were acquired in 17 contiguous 7-mm axial slices. Functional images were acquired in the same set of slices by using a T2*-sensitive gradient echo spiral pulse sequence (46) (1 interleave, 30-ms TE, 1,680-ms TR, 72° flip angle, 24-cm field of view, 64 × 64 data acquisition matrix).
Data analysis.
Functional images were motion corrected and normalized using spm99 (Wellcome Department of Cognitive Neurology, University College, London), interpolated to 2 × 2 × 4 mm voxels, and spatially smoothed with a Gaussian filter (6 mm full width–half maximum). Low-frequency noise and differences in global signal were removed. Data were analyzed in a similar manner to Experiment 1, except that only the functional images corresponding to the Sentence Processing phase were submitted to analyses. Conjunction analyses (47) were performed in spm99 to identify activations common to two contrasts.
Results.
Subjects were equally accurate in judging whether sentences were true or false for Read Trials (90 ± 3%; mean ± SEM) and Read+Remember Trials (90 ± 3%) but responded more slowly on Read+Remember Trials (724 ± 103 ms) than on Read Trials (602 ± 111 ms) (P < 0.05 two-tailed t test). On the other hand, subjects tended toward more accurately remembering the final words for Remember Trials (84 ± 6%) than for Read+Remember Trials (70 ± 6%) (P = 0.07, two-tailed t test) but did not take significantly longer to verify the order of the final words on Read+Remember (4,609 ± 566 ms) than Remember Trials (4,158 ± 693 ms) (P > 0.05, two-tailed t test). Thus, in this experiment, the costs of dual-task performance were manifested in accuracy for remembering final words and in response times for answering questions.
The Sentence Processing phase of all three Trial types yielded prominent activations in bilateral frontal cortex (Fig. 2, Table 2). Both Remember and Read+Remember Trials were additionally associated with robust left parietal activation.
Table 2.
Activations for each condition relative to control—Experiment 2
| Brain region (Brodman area) | Read
|
Remember
|
Read + Remember
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z* | Z score† | Volume‡ | x | y | z | Z score | Volume | x | y | z | Z score | Volume | |
| L inferior frontal gyrus (44/45) and L precentral gyrus (6) | −44 | 6 | 28 | 4.02 | 56944 | −50 | 12 | 4 | 4.76 | 18800 | −44 | 6 | 32 | 5.12 | 90944 |
| L middle frontal gyrus (46) | −42 | 36 | 16 | 2.39 | −28 | 2 | 52 | 3.53 | |||||||
| L insula | −36 | 20 | 0 | 3.32 | −34 | 18 | 4 | 2.65 | |||||||
| R inferior frontal gyrus (44/45) and R precentral gyrus (6) | 40 | 4 | 36 | 3.14 | 50 | 2 | 28 | 3.11 | 50 | 20 | 20 | 3.34 | |||
| R insula | 36 | 26 | −4 | 3.95 | 32 | 24 | −8 | 3.24 | |||||||
| R middle frontal gyrus (9/10/46) | 36 | 40 | 20 | 3.72 | 23904 | 40 | 36 | 20 | 4.18 | ||||||
| L parietal cortex (40) | −46 | −48 | 40 | 3.83 | 12640 | −40 | −52 | 40 | 3.22 | ||||||
| L precuneus (19) | −34 | −66 | 40 | 3.72 | −36 | −72 | 40 | 4.9 | 13808 | ||||||
| L superior temporal gyrus (22) | −56 | −54 | 28 | 2.74 | −58 | −52 | 20 | 2.94 | |||||||
| L putamen | −22 | 4 | −4 | 3.2 | |||||||||||
| R putamen | 20 | 2 | −4 | 3.2 | 30 | 12 | 4 | 2.55 | Included in prefrontal cluster | ||||||
| R visual association nucleus of thalamus | Included in prefrontal cluster | 14 | −6 | 12 | 3.49 | ||||||||||
Global maxima are in boldface; local maxima are in the regular face.
Coordinates for significant activations (P < 0.05 corrected for multiple comparisons).
†Z score for local maximum.
‡Volume of activation in mm3.
A conjunction analysis identified three regions that were more active for Read+Remember Trials relative to both Read Trials and Remember Trials (P < 0.05 corrected for multiple comparisons): left PFC, right PFC, and left parietal cortex. All three areas were significantly active above baseline in one or both component tasks, confirming that there were no dual task-specific activations in Experiment 2 (Fig. 3b).
Discussion.
Dual-task performance on a WM span test was associated with performance decrements relative to performing either component task. Dual-task performance did not activate any novel prefrontal (or any other) region that was not activated in one or both component tasks. This finding was replicated across two experiments despite differences in task design, data acquisition, and analysis. Dual-task performance was, on the other hand, associated with an increased magnitude of activation in prefrontal areas—and, in Experiment 2, left parietal cortex—invoked by the component tasks. These findings provide support for the resource model, whereby the demands of dual-task performance are met by increased activation in brain regions that subserve performance of the component tasks.
The executive WM demands of dual-task performance enhanced activation not only in prefrontal areas but also in other regions. In this and other studies, executive task conditions have consistently been associated with activation in PFC, parietal cortex, cerebellum, and occasionally the striatum (8, 9, 12, 13, 48), all considered components of WM circuitry. These multifocal activations suggest that executive WM processes involve interactions between these different brain regions (49).
The finding that dual-task performance results in greater activation of regions activated by component tasks, rather than recruitment of a novel region, is consistent with two other neuroimaging experiments. One experiment involved concurrent delayed matching of pitch and luminance (8); the other consisted of concurrent performance of an auditory verbal task with either a visual spatial rotation or face identification task (9). Both studies differed from the present study in two important ways: (i) they used relatively simple component tasks, and (ii) they used component tasks in different modalities. Despite these differences, all three studies came to the same conclusion. Dual-task processing failed to elicit activation of any new areas relative to the component tasks but rather elicited stronger and/or more extensive activation in areas recruited by each of the component tasks.
In an earlier study (7), dual-task, but not component-task, performance was associated with activations in bilateral dorsolateral PFC (DLPFC) and anterior cingulate cortex. The finding that only dual-task performance resulted in prefrontal activation appears to differ from the above studies. All the findings would be reconciled, however, by consideration of the statistical thresholding of activations and sensitivity of measurement. In the present Experiments, a higher threshold would have made prefrontal activation appear to be present only for the dual-task condition, because activation in that condition was much greater in magnitude than for either component task. Indeed, the verbal and spatial component tasks used in the earlier study (7) resulted in prefrontal activation in a subsequent study involving more than double the number of subjects (9), and similar tasks of spatial rotation and verbal classification have also resulted in prefrontal activation (e.g., refs. 50, 51). Measurement of prefrontal activation for component tasks may depend on sensitivity and statistical thresholds, but all four studies are in agreement that dual-task performance enhances prefrontal activation relative to component tasks.
In stark contrast, parietal activation in Experiments 1 and 2 did provide an example of the recruitment of a novel area. For sentence-reading trials, when subjects did not have to remember any words, there was no left parietal activation. When subjects had to maintain five words in WM for the Remember or Read+Remember Trials, there was robust left parietal activation. These findings are consistent with the view that parietal regions are involved specifically in the storage or maintenance aspects of WM (43, 52).
A number of studies have suggested that prefrontal activation related to short-term active maintenance of information is restricted to ventrolateral PFC (15, 43), whereas tasks involving manipulation of information additionally recruit DLPFC (14, 18, 53–56). In Experiment 2 of the present study, however, simply remembering five words for a short period of time resulted in prominent DLPFC activation. This finding is consistent with the observation that maintaining a large memory load (six letters) over a delay was sufficient to recruit DLPFC (57). These results suggest that DLPFC may be recruited in WM tasks involving only maintenance when the WM load exceeds the processing limits of ventrolateral PFC.
Performance decrements in WM span tests and other dual-performance tasks may arise when the component tasks compete for common resources. In the present experiments, both component tasks recruited similar brain regions, especially left PFC, and these regions were recruited to an even greater extent during dual-task performance. As in other dual-task studies (8, 9), these results demonstrate the greater demands placed on a brain region when each of two components tasks tax that region and the mental resources supported by it. The WM span test may be a good predictor of a range of cognitive abilities in both normal and patient populations (19, 20, 24, 25–31) because it effectively taxes WM circuitry to its limits.
Acknowledgments
We thank Russell Poldrack, Zuo Zhao, John Desmond, Amy Shelton, and Moriah Thomason. The authors were supported by grants from the National Institutes of Health, the Wenner–Gren Foundation, and the Baxter Foundation.
Abbreviations
- WM
working memory
- VOI
volume of interest
- PFC
prefrontal cortex
- DLPFC
dorsolateral prefrontal cortex
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050583797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050583797
References
- 1.Baddeley A, editor. Working Memory. New York: Academic; 1974. [Google Scholar]
- 2.Baddeley A. Working Memory. Oxford: Clarendon; 1986. [Google Scholar]
- 3.Shallice T. Philos Trans R Soc London B. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 4.Stuss D T, Benson D F. Psychol Bull. 1984;95:3–28. [PubMed] [Google Scholar]
- 5.Luria A R. Higher Cortical Functions in Man. New York: Basic; 1966. [Google Scholar]
- 6.Fuster J M. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobes. New York: Raven; 1997. [Google Scholar]
- 7.D'Esposito M, Detre J A, Alsop D C, Shin R K, Atlas S, Grossman M. Nature (London) 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 8.Klingberg T. Cereb Cortex. 1998;8:593–601. doi: 10.1093/cercor/8.7.593. [DOI] [PubMed] [Google Scholar]
- 9.Adcock, R. A., Constable, R. T., Gore, J. C. & Goldman-Rakic, P. S. (March 21, 2000) Proc. Natl. Acad. Sci. USA, 10.1073/pnas.060588897. http://www.pnas.org/cgi/doi/10.1073/pnas.060588897
- 10.Goldberg T E, Berman K F, Fleming K, Ostrem J, Van Horn J D, Esposito G, Mattay V S, Gold J M, Weinberger D R. NeuroImage. 1998;7:296–303. doi: 10.1006/nimg.1998.0338. [DOI] [PubMed] [Google Scholar]
- 11.Braver T S, Cohen J D, Nystrom L E, Jonides J, Smith E E, Noll D C. NeuroImage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 12.Evans J C, Lauber E J, Meyer D E, Rubenstein J, Gmeindl L, Junck L, Koeppe R A. Soc. Neurosci. Abstr. Vol. 22. 1996. p. 7. [Google Scholar]
- 13.Collette F, Salmon E, Van der Linden M, Chicherio C, Belleville S, Degueldre C, Delfiore G, Franck G. Cogn Brain Res. 1999;7:411–417. doi: 10.1016/s0926-6410(98)00045-7. [DOI] [PubMed] [Google Scholar]
- 14.Konishi S, Nakajima K, Uchida I, Kameyama M, Nakahara K, Sekihara K, Miyashita Y. Nat Neurosci. 1998;1:8–84. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- 15.Jonides J, Smith E E, Marshuetz C, Koeppe R A. Proc Natl Acad Sci USA. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. Eur J Neurosci. 1998;10:1209–1213. doi: 10.1046/j.1460-9568.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- 17.Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. Nature (London) 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- 18.Smith E E, Jonides J. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 19.Daneman M, Carpenter P A. J Verb Learn Verb Behav. 1980;19:450–466. [Google Scholar]
- 20.Daneman M, Carpenter P A. J Exp Psychol Learn Mem Cogn. 1983;9:561–584. [Google Scholar]
- 21.Salthouse T A, Babcock R L. Dev Psychol. 1991;27:763–776. [Google Scholar]
- 22.Turner M L, Engle R W. J Mem Lang. 1989;28:127–154. [Google Scholar]
- 23.Case R, Kurland M D, Goldberg J. J Exp Child Psych. 1982;33:386–404. [Google Scholar]
- 24.Just M A, Carpenter P A. Psychol Rev. 1992;99:122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- 25.Daneman M, Merikle P M. Psych Bull Rev. 1996;3:422–433. doi: 10.3758/BF03214546. [DOI] [PubMed] [Google Scholar]
- 26.Salthouse T A. Acta Psychol. 1991;79:155–170. doi: 10.1016/0001-6918(92)90030-h. [DOI] [PubMed] [Google Scholar]
- 27.LaPointe L B, Engle R W. J Exp Psychol Learn Mem Cogn. 1990;16:1118–1133. [Google Scholar]
- 28.Kyllonen P C, Christal R E. Intelligence. 1990;14:389–433. [Google Scholar]
- 29.Fry A, Hale S. Psych Sci. 1996;7:237–241. [Google Scholar]
- 30.Gabrieli J D E, Singh J, Stebbins G T, Goetz C G. Neuropsychology. 1996;10:322–332. [Google Scholar]
- 31.Stebbins G T, Singh J, Weiner J, Goetz C G, Gabrieli J D E. Neuropsychology. 1995;9:329–337. [Google Scholar]
- 32.Just M A, Carpenter P A, Keller T A. Psych Rev. 1996;4:773–780. doi: 10.1037/0033-295x.103.4.773. [DOI] [PubMed] [Google Scholar]
- 33.Just M A, Carpenter P A, Keller T A, Eddy W F, Thulborn K R. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J, MacWhinney B, Flatt M, Provost J. Behav Res Methods Instr Comput. 1993;25:257–271. [Google Scholar]
- 35.Glover G H, Lai S. Magn Reson Med. 1995;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- 36.Friston K J, Holmes A P, Worsley K J, Poline J-P, Frith C D, Frackowiak R S J. Hum Brain Map. 1995;2:189–210. [Google Scholar]
- 37.Holmes A P, Friston K J. NeuroImage. 1998;7:S754. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- 38.Price C J, Friston K J. NeuroImage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- 39.Demonet J F, Chollet F, Ramsay S, Cardebat D, Nespoulos J L, Wise R, Rascol A, Frackowiak R. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- 40.Binder J R, Frost J A, Hammeke T A, Cox R W, Rao S M, Prieto T. J Neurosci. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petrides M, Alivisatos B, Meyer E, Evans A C. Proc Natl Acad Sci USA. 1993;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jonides J, Smith E E, Koeppe R A, Awh E, Minoshima S, Mintun M A. Nature (London) 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 43.Paulesu E, Frith C D, Frackowiak R S J. Nature (London) 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- 44.Fiez J A, Raife E A, Balota D A, Schwarz J P, Raichle M E, Petersen S E. J Neurosci. 1996;16:808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Courtney S M, Ungerleider L G, Keil K, Haxby J V. Nature (London) 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- 46.Glover G H, Lai S. Magn Reson Med. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- 47.Friston K J, Holmes A P, Price C J, Buchel C, Worsley K J. NeuroImage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- 48.Salmon E, Van der Linden M, Collette F, Delfiore G, Maquet P, Degueldre C, Luxen A, Franck G. Brain. 1996;119:1617–1625. doi: 10.1093/brain/119.5.1617. [DOI] [PubMed] [Google Scholar]
- 49.Baddeley A D. J Int Neuropsychol Soc. 1998;4:523–526. doi: 10.1017/s135561779800513x. [DOI] [PubMed] [Google Scholar]
- 50.Cohen M S, Kosslyn S M, Breiter H C, DiGirolamo G J, Thompson W L, Anderson A K, Brookheimer S Y, Rosen B R, Belliveau J W. Brain. 1996;119:89–100. doi: 10.1093/brain/119.1.89. [DOI] [PubMed] [Google Scholar]
- 51.Poldrack R A, Wagner A D, Prull M W, Desmond J E, Glover G H, Gabrieli J D E. NeuroImage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- 52.Jonides J, Schumacher E H, Smith E E, Koeppe R A, Awh E, Reuter-Lorenz P A, Marshuetz C, Willis C R. J Neurosci. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prabhakaran V, Smith J A L, Desmond J E, Glover G H, Gabrieli J D E. Cogn Psychol. 1997;33:43–63. doi: 10.1006/cogp.1997.0659. [DOI] [PubMed] [Google Scholar]
- 54.Berman K F, Ostrem J L, Randolph C, Gold J, Goldberg T E, Coppola R, Carson R E, Herscovitch P, Weinberger D R. Neuropsychologia. 1995;33:1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- 55.Owen A M. Eur J Neurosci. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 56.D'Esposito M, Aguirre G K, Zarahn E, Ballard D, Shin R K, Lease J. Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 57.Rypma B, Prabhakaran V, Desmond J E, Glover G H, Gabrieli J D E. NeuroImage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]