Abstract
Fetal or adult rat-brain cytosol and fetal rat-brain microtubules contain a high-affinity, low-capacity pregnenolone-binding protein. The equilibrium dissociation constant is in the 30–50 nM range. The best competitors (in decreasing order) are pregnenolone sulfate, progesterone, Δ5-pregnene-3β,20α-diol, and 3β-hydroxy-5α-pregnan-20-one. It was hypothesized that the pregnenolone-binding protein pertained to microtubule-associated proteins (MAPs). Indeed, partial purification of fetal brain cytosol by fast pressure liquid chromatography with sequential ion-exchange and gel-filtration columns yielded two fractions, one of very high molecular mass, >200 kDa, and the other of 40–60 kDa, enriched in [3H]pregnenolone-binding activity and in proteins immunolabeled with monoclonal anti-tubulin and anti-MAP2 antibodies. Because many proteins are associated with microtubules, binding assays were repeated with purified calf-brain tubulin, MAP2, and Tau protein. Only the MAP2 fraction showed saturable [3H]pregnenolone binding with an affinity very close to that of rat-brain microtubules, but with a much larger concentration of binding sites (16 pmol/mg MAP2), which was increased more than 8-fold after copolymerization of MAP2 with tubulin. Finally, steroid effects on microtubule-assembly kinetics were assayed. Pregnenolone induced a large, dose-related increase of both the rate and extent of MAP2-induced tubulin assembly, whereas progesterone, inactive per se, counteracted the stimulatory effect of pregnenolone. Electron microscopic analysis confirmed that pregnenolone-increased assembly of microtubules produced a completely normal structure. The stimulatory effect on MAP2–tubulin interaction was also observed in fetal rat-brain neuron cultures. Therefore, we propose a mechanism of neurosteroid action, the control of microtubule or, more generally, of neural cytoskeleton dynamics, with potential roles in brain development, plasticity, and aging.
The characterization of pregnenolone (PREG) and of dehydroepiandrosterone (DHEA) in the rat brain as nonconjugated steroids and their sulfate and fatty acid esters has led to the discovery of a steroid biosynthetic pathway in the central nervous system. Nongenomic, membrane receptor-mediated activities have been documented for PREG sulfate (PREGS) and DHEA sulfate (DHEAS). They are prototypic, naturally excitatory neurosteroids, because at low-micromolar concentrations, they antagonize γ-aminobutyric acid type A receptors or potentiate glutamate receptors of the N-methyl-d-aspartate type, and they may modulate other neurotransmitter receptors (1–3).
However, no intracellular binding protein has been reported so far in the nervous system for DHEA or PREG. In this paper, we have investigated PREG binding sites in rat-brain cytosol. The search for potential binding proteins in cytosol preparations led to the discovery of a PREG binding site on microtubule-associated protein 2 (MAP2), which has important roles in the nervous system.
Neurons have a characteristic morphology of neuritic processes densely filled with parallel arrays of microtubules, which play an essential role in the growth and maintenance of neurites during neuronal differentiation (4, 5). Neuronal microtubules are composed of tubulin and of several nontubulin proteins that purify with tubulin throughout several courses of polymerization–depolymerization (6). The proteins present in in vitro-polymerized microtubules from brain extracts have been characterized. Tubulin is a protein with a quaternary structure composed of two subunits, α and β. There are about six isotypes for α- and β-tubulin, respectively (7). The MAPs regulate microtubule lattice formation and dynamics; hence, they determine neuronal shape and control the balance between rigidity and plasticity in neuronal processes (8, 9). They are distributed either in a group of high molecular mass (200–300 kDa) proteins, MAP1A, MAP1B, MAP1C, MAP2A, and MAP2B, or in a group of low molecular mass (50–70 kDa) proteins, MAP2C, MAP2D, and the Tau family. MAP2 is expressed in neurons and is limited to dendrites, whereas Tau is detected mainly in axons.
We propose that neurosteroids are involved physiologically in the formation and stabilization of microtubules, and that they could be used to reverse the instability of microtubules involved in several pathological states.
Materials and Methods
Animals.
Sprague–Dawley rats were from Janvier Animal Suppliers (Le Genest St. Isle, France). They were housed under a 12-h light–dark cycle (lights on at 8:00 a.m.) at 20°C and fed ad libitum. Pregnant females (the morning after mating is designated as embryonic day 0) were housed at least 7 days before sacrifice at embryonic day 18. Adult males, 60–70 days old, were also used. Animal care was in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC).
Calf brains were kindly provided by the animal farm of Institut National de la Recherche Agronomique (Jouy-en-Josas, France). The brains were stripped of meninges and large blood vessels and brought to the laboratory in chilled isotonic saline.
Steroids.
[7-3H]PREG [22.5 Ci/mmol (1 Ci = 37 GBq)] was obtained from NEN. The nonradioactive reference steroids progesterone, PREGS, testosterone, corticosterone, triamcinolone acetonide, 17α-OH-PREG, 17α-OH-progesterone, and cholesterol were purchased from Sigma-Aldrich (St. Quentin Fallavier, France), and Roussel Uclaf (Romainville, France) generously donated other steroids.
Enzymes.
Proteinase K (Merck) and Pronase (Roche Molecular Biochemicals) were dissolved at 1 mg/ml in 10 mM Tris/0.1 M NaCl/3 mM MgCl2, pH 7.4 (TNM buffer). DNase I and ribonuclease A were obtained from Sigma-Aldrich and adjusted to 100 units/ml and 5 units/ml, respectively. Phospholipase A2 (Roche Molecular Biochemicals) was diluted to 20 units/ml in TNM buffer.
Antibodies.
Monoclonal anti-α-tubulin antibody N-356 was purchased from Amersham Pharmacia. Monoclonal antibody 152 (10) was shown to react specifically with high molecular weight MAP2. Recombinant doublecortin N-terminal glutathione S-transferase fusion protein and specific polyclonal antiserum were kindly provided by F. Francis (Institut Cochin de Génétique Moléculaire, Paris) (11). All of the different antibodies were diluted in PBS containing 3% BSA.
Preparation of Cytosol.
Fetal rat brains were removed, stripped from meninges, and rinsed with TEDG homogenization buffer (10 mM Tris⋅HCl, pH 8.5/1.5 mM EDTA/1 mM DTT/10% glycerol) and protease inhibitor mixture Complete (Roche Molecular Biochemicals) at 4°C. Brains of male adult rats were removed after perfusion with PBS containing 2 units/ml heparin. The brain tissues were weighed and homogenized with 4 vol of homogenization buffer in glass/Teflon Potter homogenizers (two strokes) and centrifuged at 800 × g for 10 min. The supernatant was then centrifuged at 100,000 × g for 1 h at 4°C. The samples were assayed immediately or stored in liquid nitrogen until analysis.
Protein concentrations were determined by the Bio-Rad protein assay kit with BSA as the reference standard.
Equilibrium Binding Constants.
Just before the binding assay, each sample was stripped with charcoal-dextran suspension, and 0.2-ml aliquots of the supernatant (stripped cytosol) were added to a series of tubes containing [3H]PREG (specific activity, 22.5 Ci/mmol) in the 1–500 nM range with or without 1,000-fold excess unlabeled steroid. The tubes were incubated at 45°C for 30 min. Separation of bound and free steroid was performed with a Sephadex LH-20 minicolumn (Amersham Pharmacia). The radioactivity in the vials was counted in a liquid scintillation spectrometer with quench correction (Tri-Carb 2100 TR, Packard).
Nature of PREG-Binding Component.
Aliquots (1 ml) of brain cytosol were incubated with 0.2 ml of enzyme solution (see Enzymes) at 37°C for 30 min. Enzyme-treated or buffer-diluted control sample (0.2 ml) was examined for binding activity.
Competitive Binding Assay.
For competition of PREG binding by different steroids, 0.2 ml of stripped cytosol was incubated with 100 nM [3H]PREG and 1-, 10-, 100-, and 1,000-fold excesses of nonradioactive steroid.
Anion-Exchange Chromatography.
The FPLC system (Amersham Pharmacia) was equipped with an anion-exchange column (5-ml Econo-Pac High Q; Bio-Rad). The column was equilibrated with TEDG buffer containing 0.03% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) and 100 nM PREG (TEDGCP buffer) at 4°C. Cytosol (20 ml, 60 mg of protein) was applied and eluted at a flow rate of 1 ml/min with TEDGCP buffer for 20 min. Fractions (2 ml) were collected, then a linear gradient of NaCl in TEDGCP buffer was applied to reach 1 M NaCl after 30 min, followed by the application of isocratic 1 M NaCl in TEDGCP buffer. Appropriate fractions were pooled and desalted by passage through Hi-Trap desalting minicolumns (Amersham Pharmacia).
Gel Filtration.
The Superose 12 column (1 × 30 cm; Amersham Pharmacia) was equilibrated with TEDGCP buffer containing 0.15 M NaCl at 4°C. The flow rate was adjusted to 0.25 ml/min, and 0.4-ml fractions were collected.
Control of Binding Protein Enrichment.
At each purification step, collected fractions were incubated with 100 nM [3H]PREG with or without a 100-fold excess of nonradioactive PREG, and saturable binding was determined.
Western Blots.
Crude cytosol and partially purified samples (10 μg of protein) were separated on 10% SDS/PAGE gels, then transferred to nitrocellulose membranes. The membranes were blocked by incubation in 5% skim milk/0.1% Tween 20/Tris-buffered saline (10 mM Tris/50 mM NaCl/2 mM EDTA, pH 8.8) for 1 h at room temperature. All washes were in 0.1% Tween 20/Tris-buffered saline (TNT buffer). The membranes were then incubated with the anti-tubulin (1:2,000) or the monoclonal anti-MAP2 (1:1,000) antibodies at 4°C overnight. Incubation with the secondary anti-mouse Ig-peroxidase-conjugated IgG, F(ab′)2 fragment (1:10,000) (Pierce) was performed in TNT buffer containing 5% milk for 45 min at room temperature. The signal was detected by the enhanced chemiluminescence system (Amersham Pharmacia), according to manufacturer's instructions, with Kodak X-Omat film.
Preparation of Microtubule Proteins.
Calf brains were washed twice in buffer L (0.1 M Mes/1 mM EGTA/0.1 mM EDTA/1 mM MgCl2/1 mM DTT/1 mM PMSF, pH 6.4). Microtubules were prepared from calf brain or from fetal and adult rat brain by a temperature-dependent in vitro assembly–disassembly procedure (12). Calf tubulin was purified by the method of Weingarten et al. (13), except that the phosphocellulose step was replaced by chromatography on a cation-exchange column (Fractogel; Merck) (14). Microtubules and tubulin were prepared in buffer L supplemented with 1 mM GTP (buffer A). MAP2 and Tau were purified by the microtubule heat-denaturation and column gel-filtration protocols of Fellous et al. (12), except that Sephacryl 300 was used instead of Ultrogel ACA34. Tau proteins were further purified according to the procedure of Lindwall and Cole (15).
Assay of Microtubule Assembly.
Microtubule assembly was measured by monitoring the turbidity increases with an UVICON spectrophotometer (Kontron, Montigny-le-Bretonneux, France) equipped with an automatic thermostated six-sample changer connected to a circulating water bath set at 37°C.
Culture of Neurons.
Primary neuronal cultures of embryonic day 17 fetuses were carried out as described by El-Etr et al. (16). In brief, dissociated cells were plated (200,000 cells per cm2) on glass coverslips coated with poly(l-ornithine) and cultured in a completely defined medium in 5% CO2 at 37°C. The culture conditions were such that an almost pure neuronal population was present.
Twenty-four hours after seeding, 1 μM PREG was added (final ethanol concentration was 0.01%), and the cultures were continued for 2 days. Cells were fixed in 4% paraformaldehyde then frozen at −80°C. When needed, they were rehydrated by 5-min passages through graded ethanol concentrations (100%, 90%, and 70%). After two washes in PBS for 5 min, cells were incubated in PBS containing 3% BSA and 0.1% Triton X-100 at room temperature for 1 h. After four washes in PBS, cells were incubated at 37°C for 1 h, then at 4°C overnight in the presence of either anti-α-tubulin monoclonal antibody diluted 1:2000, anti-MAP2 monoclonal antibody diluted 1:1000, or anti-doublecortin antiserum diluted 1:300. After three washes in PBS, the secondary antibody was added at room temperature for 45 min. The secondary antibody was either rabbit anti-mouse Ig-biotin-conjugated IgG, F(ab′)2 fragment, diluted 1:350 (Roche Molecular Biochemicals) or anti-rabbit biotinylated IgG diluted 1:300 (Boehringer Mannheim). After three washes, the streptavidin-peroxidase amplification complex (Dako) was added for 30 min at room temperature. The cells were then washed and the aminoethylcarbazole substrate (Sigma-Aldrich) was deposited on the cells for 15 min. The reaction was stopped with distilled water, and cells were counterstained with hematoxylin and mounted in Glycergel (Dako).
Electron Microscopy.
Once microtubule polymerization was completed, a small drop of each experimental sample was deposited on the surface of a hydrophilic grid (300 mesh) coated with Formvar carbon and glow-discharged shortly before use. The grids were stained with 1% aqueous uranyl acetate and observed with a Philips CM 12 electron microscope.
Statistical Analyses.
Equilibrium binding data were analyzed by the Macintosh version of the ligand program, adapted from Munson and Rodbard (17). Curves were drawn with the kaleidograph tm 3.0 software (Abelbeck Software, Ritme Informatique, Paris). The logit-log transformation and curve fitting were used to calculate the relative competition ratios of tested steroids. Comparisons between variables were made with the unpaired Student's t test. Results are presented as mean ± SE or SD; P < 0.05 is taken as the limit of statistical significance.
Results
PREG Binding Sites in Rat Brain.
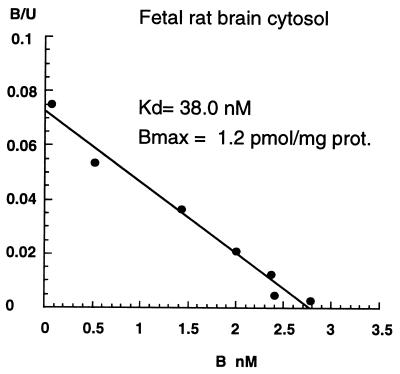
High-affinity saturable binding sites were found in fetal rat-brain cytosol prepared in TEDG buffer. The binding of [3H]PREG was optimal under the conditions of pH 8.5, 45°C, and 30 min (data not shown). Analysis of equilibrium binding constants by the ligand program indicated that the best fit was for one category of specific sites plus nonsaturable binding. A representative Scatchard analysis plot of specific binding is given in Fig. 1. The dissociation constant Kd was 31.1 ± 0.8 nM, and the maximum concentration of binding sites was 1.2 ± 0.1 pmol/mg of protein (mean ± SEM, n = 3). Proteinase K and Pronase destroyed [3H]PREG binding sites by ≈60%, whereas DNase, RNase, and phospholipase A2 had no significant effect (Table 1).
Figure 1.
Equilibrium binding of [3H]PREG to fetal brain cytosol. Charcoal-stripped cytosol samples (0.2 ml, 2.5 mg of protein per ml) were incubated with increasing concentrations of [3H]PREG in TEDG buffer, pH 8.5, at 40°C for 30 min. Bound steroid was separated by gel filtration on Sephadex LH20 at 0–4°C. Binding constants were determined with the Macintosh ligand program. B/U, bound/unbound; B, bound.
Table 1.
Effects of hydrolytic enzymes on [3H]PREG binding
| Enzyme | Concentration | % of control |
|---|---|---|
| Proteinase K | 0.17 mg/ml | 42.2 ± 7.5 |
| Pronase | 0.17 mg/ml | 40.5 ± 5.7 |
| Deoxyribonuclease 1 | 17 units/ml | 99.4 ± 12.7 |
| Ribonuclease A | 0.8 units/ml | 82.4 ± 9.2 |
| Phospholipase A2 | 33 units/ml | 85.0 ± 7.3 |
Samples (1 ml) of cytosol were incubated with 0.2 ml of enzyme solution at 37°C for 30 min. Results are expressed as percent of control incubation (buffer-diluted cytosol), mean ± SD; n = 3.
The best competitors of [3H]PREG binding were PREGS, progesterone, and Δ5-pregnene-3β,20α-diol (20α-dihydro-PREG) (Table 2). Another steroid with structural similarity to PREG, 3β-hydroxy-5α-pregnan-20-one (5α-dihydro-PREG), was also an efficient competitor, although 5-fold less active than PREG. Among other steroids tested, DHEA was a weak competitor and DHEAS was a negligible one. The preferred structures seemed to be C-21 steroids with a ketone or an alcohol group in positions 3 and 20 and with an unsaturated A or B ring providing a more planar structure to the steroid skeleton.
Table 2.
Steroid specificity of [3H]PREG binding
| Steroid | RCR50 |
|---|---|
| PREG | 100 |
| PREGS | 78.8 ± 7.0 |
| Progesterone | 72.1 ± 17.7 |
| Δ5-Pregnene-3β,20α-diol | 68.4 ± 15.3 |
| 3β-Hydroxy-5α-pregnan-20-one | 18.4 ± 4.0 |
| Testosterone | 5.6 ± 2.8 |
| DHEA | 4.3 ± 2.6 |
| Estrone | 4.4 |
| Corticosterone | 2.8 |
| Androstenediol | 1.7 |
| Estradiol | 1.3 |
| Triamcinolone acetonide | 1 |
| 17α-Hydroxypregnenolone | 0.85 |
| 17α-Hydroxyprogesterone | 0.79 |
| DHEAS | 0.045 |
| Cholesterol | 0.02 |
A relative competition ratio (RCR) is expressed as (concentration of PREG over concentration of competitor) × 100, each of which produces a 50% decrease of specific [3H]PREG binding (mean ± SD; n = 3).
The adult rat brain also contains a PREG-binding component with binding constants similar to those of fetal brain (Kd = 50.7 nM; Bmax = 0.75 pmol/mg of protein; data not shown).
Attempts to Purify the Cytosolic Binding Protein.
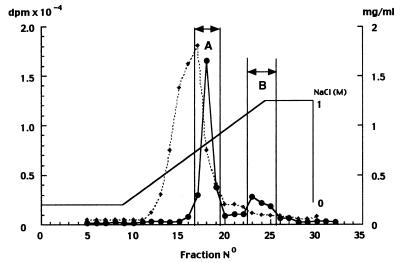
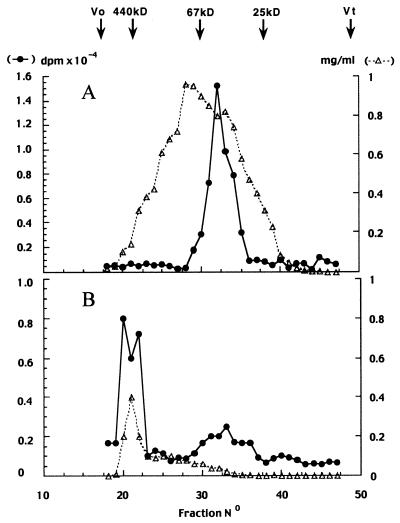
PREG binding sites were eluted from the Mono Q ion-exchange column in two fractions at 0.5–0.6 M NaCl (fraction A) and 0.9–1.0 M NaCl (fraction B), respectively (Fig. 2). Both fractions were desalted, concentrated, and submitted to gel filtration on a Superose 12 column (Fig. 3). Specific [3H]PREG binding was determined again on each column fraction and was found in one peak of 40–60 kDa (chromatography of fraction A) and one peak of >440 kDa (chromatography of fraction B), respectively.
Figure 2.
Chromatography of fetal brain cytosol by FPLC on a Mono Q ion-exchange column. Cytosol protein (60 mg) in TEDGCP buffer was loaded and eluted with a gradient of NaCl, and 2-ml fractions were collected. Saturable binding of [3H]PREG (●) and protein concentration (♦) were measured in each fraction. Peaks A (fractions 17–19) and B (fractions 23–25) were pooled separately, desalted, lyophilized, and redissolved in TEDGCP buffer containing 0.15 M NaCl.
Figure 3.
Chromatography of Mono Q peaks (see Fig. 2) by FPLC on a Superose 12 gel-filtration column. Fractions A (4.5 mg of protein) and B (1.1 mg of protein) were deposited on the column and eluted with TEDGCP buffer, 0.4-ml fractions were collected, and saturable [3H]PREG binding was measured. The column was calibrated with molecular-mass markers. Distinct low and high molecular mass components corresponding to peaks A and B, respectively, were determined. ●, [3H]PREG bound in dpm; ▵, protein concentration in mg/ml.
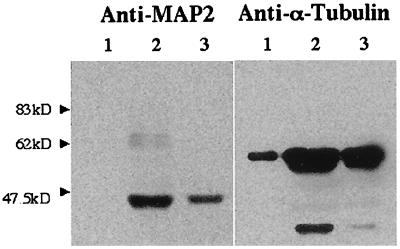
The presence of a very high molecular weight component suggested to us that the cytosolic binding protein might correspond to high molecular weight proteins associated with microtubules, which are major components of neuronal cytoplasm. To gain further support for this hypothesis, we attempted to check whether fraction A also contained components of the microtubular system. For that purpose, the peaks of PREG binding were submitted to SDS/PAGE analysis followed by immunoblotting (Fig. 4). Indeed, proteins immunoreactive with anti-MAP2 and anti-α- and β-tubulin monoclonal antibodies were enriched in those partially purified preparations in amounts compatible with the increase of specific binding activity. The low molecular mass of the polypeptides recognized by anti-MAP2 strongly suggests that some breakdown of the 300 (or 70) kDa MAP2 occurred during cytosol protein purification.
Figure 4.
Immunoblotting of cytosol and of peak-A fractions eluted from ion-exchange and gel-filtration columns. Lane 1, crude cytosol; lane 2, Mono Q fraction A; lane 3, fraction A rechromatographed on Superose 12. Protein (10 μg) was deposited in lanes 1 and 2. The amount of protein in lane 3 was below the limit of detection.
Binding of [3H]PREG to Purified Rat-Brain Microtubules.
Because the amount of tissue available did not allow for us to prepare purified tubulin and MAP2, binding of [3H]PREG to microtubules prepared by repeated cycles of polymerization–depolymerization was assayed.
Fetal microtubules had a Kd for [3H]PREG of 42.9 nM and a Bmax of 3.7 pmol/mg of protein, representing a 3-fold enrichment as compared with fetal brain cytosol. The ligand specificity was unchanged [relative competition ratio: PREG (100) > PREGS (78.8) > progesterone (72.1) ≫ testosterone (5.6) > DHEA (4.3) > estradiol (1.3)]. Adult microtubules had a Kd for [3H]PREG of 54.8 nM and a Bmax of 3.4 pmol/mg of protein, representing a 4.5-fold enrichment as compared with adult brain cytosol.
PREG-Binding Activities of Purified MAP2, Tubulin, and Tau.
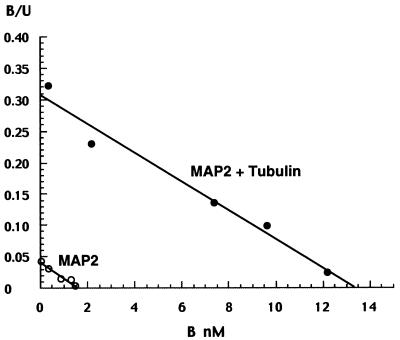
Purified calf-brain tubulin (90 μg/ml), MAP2 (45 μg/ml), or Tau (25 μg/ml) was incubated with 100 nM [3H]PREG with or without a 1,000-fold excess of nonradioactive PREG. Neither tubulin, Tau, nor rabbit IgG bound [3H]PREG in a saturable manner. On the other hand, the MAP2 fraction showed specific binding sites, which were increased by coincubation with tubulin.
The equilibrium binding constants of purified calf MAP2 were Kd = 39.2 nM, Bmax = 16 pmol/mg of MAP2, whereas those of MAP2 + tubulin copolymers were Kd = 44.0 nM, Bmax = 135 pmol/mg of MAP2 (Fig. 5). Hence, binding of MAP2 to tubulin did not modify the affinity of PREG, but increased the concentration of binding sites >8-fold. Expressed in molar concentrations, the concentration of binding sites was 14 nM, whereas the concentration of MAP2 (assuming an average molecular weight of 200,000) was about 200 nM. Therefore, <10% of MAP2 molecules could bind [3H]PREG. The substitution of buffer A for TEDG buffer did not influence PREG equilibrium binding constants (data not shown). The ligand specificity of binding to MAP2 + tubulin copolymers was very close to that of fetal microtubules [relative competition ratio: PREG (100) > PREGS (91.5) > progesterone (81.3) ≫ estradiol (3.2) > testosterone and DHEA (2.3). No PREG binding to recombinant doublecortin could be detected (data not shown).
Figure 5.
Equilibrium binding of [3H]PREG to purified MAP2 complexed or not complexed with purified tubulin. ○, Equilibrium binding of purified calf-brain MAP2; ●, equilibrium binding of purified calf-brain MAP2 associated with purified calf-brain tubulin. See legend to Fig. 1 for experimental details.
Steroid Effects on Microtubule Assembly Kinetics.
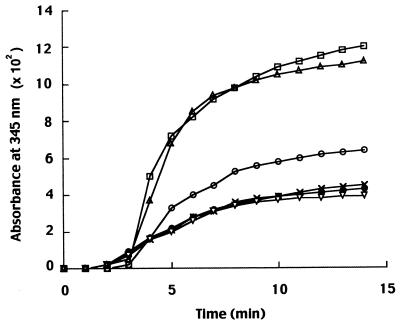
PREG induced a large increase of both the rate and the extent of tubulin assembly, as indicated by increased absorbance at 345 nm (Fig. 6). Estradiol, which does not bind to MAP2, was inactive. However progesterone, which has an affinity for MAP2 in the same range as that of PREG, was also ineffective, but contrary to estradiol, progesterone counteracted the stimulatory effect of PREG on microtubule assembly. PREGS was also ineffective but, like progesterone, counteracted the stimulatory effect of PREG (data not shown).
Figure 6.
Kinetics of microtubule assembly. Microtubules were reconstituted in vitro with purified tubulin (1 mg/ml) and MAP2 (0.05 mg/ml) in buffer A. The increase of absorbance was monitored at 345 nm at 37°C and recorded every 30 sec for 15 min. Microtubule assembly was monitored either in the absence of steroid (●) or in the presence of 500 nM steroid [PREG (□), progesterone (×), or estradiol (▿)], or in the presence of both PREG and progesterone (○) or PREG and estradiol (▵), each at 500 nM.
Electron Microscopic Analysis.
It was important to investigate whether the large amount of microtubules polymerized in the presence of PREG were of normal appearance, or whether they were structurally altered as observed, for example, when tubulin is assembled in the presence of Taxol (18). In the presence of calf-brain MAP2, calf-brain tubulin assembles in apparently normal microtubules characterized by a remarkably constant conformation and a uniform diameter. When MAP2-induced tubulin polymerization was performed in the presence of 500 nM PREG, microtubule structure remained completely normal (data not shown).
Effects of PREG on Neuronal Cultures.
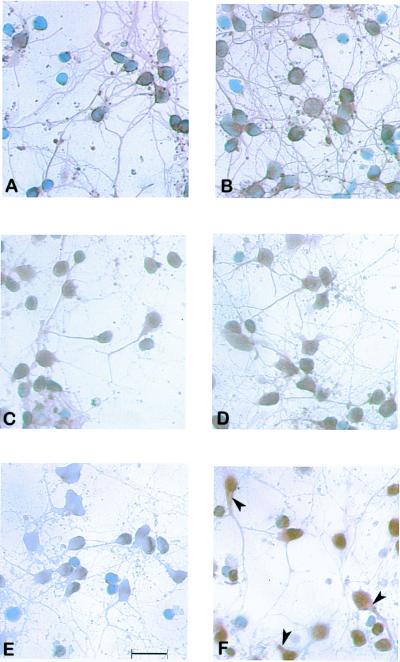
After 3 days of culture, neurons were processed for immunocytochemistry. Staining with monoclonal antibodies to α-tubulin or to doublecortin did not show any difference between control cultures and cultures exposed to 1 μM PREG for 2 days (Fig. 7). On the contrary, exposure to PREG increased MAP2 immunostaining of cell bodies and produced its appearance in proximal neurites.
Figure 7.
Immunocytochemistry of fetal rat-brain neurons. (A–C) Results of a 3-day control culture. (D–F) PREG (1 μM) added the last 2 days of culture. The antibodies used were monoclonal anti-α-tubulin antibody (A and D), polyclonal anti-doublecortin antibody (B and E), and monoclonal anti-MAP2 antibody (C and F). PREG increased the immunostaining of cell bodies and induced the appearance of immunostaining in proximal neurites (arrows). (Scale bar = 0.2 nm.)
Discussion
Previous reports on steroid–microtubule interactions were focused on the estradiol metabolite 2-methoxyestradiol (19). At high concentrations it can inhibit microtubule polymerization and act as a weak inhibitor of colchicine binding to tubulin. At lower concentrations, already effective in blocking mitoses, microtubules are not depolymerized but have an abnormal morphology, similar to that seen after Taxol treatment. Moreover, 2-methoxyestradiol binds directly to purified tubulin either unpolymerized or polymerized by glutamate in the absence of MAPs.
No intracellular binding component has been documented so far for the neurosteroids DHEA and PREG in rodent brain. Initial attempts to observe a specific binding component for DHEA in rat-brain cytosol provided irreproducible results (K.M., unpublished results). On the contrary, PREG binding sites could be demonstrated in rat-brain cytosol, particularly in fetal brain. The affinity of PREG for rat fetal cytosol, rat adult cytosol, purified rat-brain microtubules, purified calf MAP2, calf MAP2, or MAP2 + tubulin copolymers was determined repeatedly. The affinities and ligand specificities of all active preparations were remarkably similar, thus indicating that the binding protein in rat-brain cytosol indeed belongs to the MAP2 family.
The concentration of PREG binding sites in high molecular weight calf MAP2 was definitely much smaller than the molar concentration of MAP2 (at least 1 order of magnitude). It is well known that MAP2 is a mixture of several components. Although they are heat stable, their drastic method of purification might produce massive denaturation of the proteins. It is also known that MAP2 undergoes posttranslational modifications, in particular phosphorylations. Phosphorylation determines the binding of MAP2 to microtubules and the regulatory role of MAP2 in microtubule dynamics (20–22). Phosphorylation of MAP2C and MAP4 also affects the stability of microtubules in cells (23). Phosphorylation may destabilize the active conformation of MAPs. Unfolding of the Tau protein is responsible for its loss of activity, and refolding the protein with an organic osmolyte can restore its tubulin-binding activity and promote tubulin assembly (24). The fact that the concentration of MAP2 binding sites was increased 8-fold after copolymerization with tubulin is compatible with a refolding (chaperon) effect of tubulin on MAP2 conformation. Binding of PREG was enriched in a fraction of embryonic brain cytosol containing low molecular weight protein(s) recognized by anti-MAP2 antibody. This component was smaller than MAP2C, which, in addition, is not recognized by the monoclonal antibody that was used. Breakdown fragments of MAP2 can interact with tubulin and promote its polymerization (25, 26). Further studies will need to be performed to determine whether such fragments also bear the PREG binding site. Finally, it cannot formally be excluded that this binding site is borne by another protein, which is tightly associated with and copurifies with high molecular weight MAP2.
PREG was strongly active on MAP2-induced microtubule assembly. It dose-relatedly accelerated microtubule polymerization and increased the amount of microtubules formed. This effect was more spectacular when relatively low MAP2/tubulin ratios were used (data not shown). The pattern of microtubule assembly induced by PREG was characterized by a sudden increase of absorbance at 345 nm, suggesting a selective effect of the steroid on the nucleation step. Progesterone and PREGS, which have affinities for MAP2 slightly smaller than that of PREG, were inactive, per se, but antagonized the effect of PREG. Electron microscopic observation indicated that the microtubules formed under the influence of PREG retained a strictly normal appearance. PREG effects on microtubule formation are therefore completely different from those produced by Taxol, Taxotere, or 2-methoxyestradiol.
DHEA and DHEAS are both active on cultures of neocortical neurons of embryonic day 16.5 (27). DHEA selectively increases the length of neurites containing the axonal marker Tau, whereas DHEAS selectively increases the length of neurites containing the dendrite marker MAP2. These effects are observed at subnanomolar concentrations of either DHEA or DHEAS and seem to involve glutamate receptors of the N-methyl-d-aspartate type. The mechanism of action of neurosteroids demonstrated in this paper obviously is completely different from the one suggested by Compagnone and Mellon (27). Although a direct effect of DHEA on microtubule polymerization could not be excluded, PREG was by far the most active neurosteroid, and its action was mediated by MAP2 protein, either intact MAP2 or eventually a fragment thereof.
Further studies will need to be done to envision the role of PREG in brain development and brain plasticity and the interest of steroid therapy in conditions of deficient neuronal microtubule assembly.
Acknowledgments
We thank D. Zucman and K.-I. Kang for their preliminary experiments; B. Eychenne, M. El-Etr, N. Gago, and R. Veitia for fruitful discussions; J. Mazie and F. Francis for the gift of anti-MAP2 and anti-doublecortin monoclonal antibodies; P. Berda and S. David for technical assistance; and S. Delhaye and L. Outin for the preparation of the manuscript. This work has been supported in part by the Florence Gould and the G. Harold and Leila Mathers Charitable Foundations (New York).
Abbreviations
- MAP
microtubule-associated protein
- PREG
pregnenolone
- DHEA
dehydroepiandrosterone
- PREGS
PREG sulfate
- DHEAS
DHEA sulfate
References
- 1.Robel P, Schumacher M, Baulieu E-E. In: Neurosteroids: A New Regulatory Function in the Nervous System. Baulieu E-E, Robel P, Schumacher M, editors. Totowa, NJ: Humana; 1999. pp. 1–25. [Google Scholar]
- 2.Baulieu E-E. Recent Prog Horm Res. 1997;52:1–32. [PubMed] [Google Scholar]
- 3.Monnet F-P, Mahe V, Robel P, Baulieu E-E. Proc Natl Acad Sci USA. 1995;92:3774–3777. doi: 10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniels M. Ann NY Acad Sci. 1975;253:535–544. doi: 10.1111/j.1749-6632.1975.tb19227.x. [DOI] [PubMed] [Google Scholar]
- 5.Yamada K-M, Spooner B-S, Wessels N-K. Proc Natl Acad Sci USA. 1970;66:1206–1212. doi: 10.1073/pnas.66.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shelanski M-L, Gaskin F, Cantor C-R. Proc Natl Acad Sci USA. 1973;70:765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan K-F. Annu Rev Cell Biol. 1988;4:687–716. doi: 10.1146/annurev.cb.04.110188.003351. [DOI] [PubMed] [Google Scholar]
- 8.Matus A. Annu Rev Neurosci. 1988;11:29–44. doi: 10.1146/annurev.ne.11.030188.000333. [DOI] [PubMed] [Google Scholar]
- 9.Tucker R P. Brain Res Rev. 1990;15:101–120. doi: 10.1016/0165-0173(90)90013-e. [DOI] [PubMed] [Google Scholar]
- 10.Kalil J, Fellous A, Fellous M. In: Culture de Cellules Animales, Méthodologies, Applications. Adolphe M, Barlovatz-Meimon G, editors. Vol. 199. Paris: Editions INSERM; 1988. pp. 101–130. [Google Scholar]
- 11.Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet M-C, Friocourt G, McDonnell N, Reiner O, Kahn A, et al. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- 12.Fellous A, Francon J. Eur J Biochem. 1977;78:167–174. doi: 10.1111/j.1432-1033.1977.tb11726.x. [DOI] [PubMed] [Google Scholar]
- 13.Weingarten M D, Lockwood A H, Hwo S Y. Proc Natl Acad Sci USA. 1975;72:1848–1852. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fromes Y, Gounon P, Veitia R, Bissery M C, Fellous A. J Protein Chem. 1996;15:377–388. doi: 10.1007/BF01886864. [DOI] [PubMed] [Google Scholar]
- 15.Lindwall G, Cole R D. J Biol Chem. 1984;259:12241–12245. [PubMed] [Google Scholar]
- 16.El-Etr M, Cordier J, Glowinski J, Premont J. J Neurosci. 1989;9:1473–1480. doi: 10.1523/JNEUROSCI.09-05-01473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munson P J, Rodbard D. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 18.D'Amato R J, Lin C M, Flynn E, Folkman J, Hamel E. Proc Natl Acad Sci USA. 1994;91:3964–3968. doi: 10.1073/pnas.91.9.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamel E, Lin C M, Flynn E, D'Amato R J. Biochemistry. 1996;35:1304–1310. doi: 10.1021/bi951559s. [DOI] [PubMed] [Google Scholar]
- 20.Hoshi M, Ohta K, Gotoh Y, Mori A, Murofushi H, Sakai H, Nishida E. Eur J Biochem. 1992;203:43–52. doi: 10.1111/j.1432-1033.1992.tb19825.x. [DOI] [PubMed] [Google Scholar]
- 21.Brugg B, Matus A. J Cell Biol. 1991;114:735–743. doi: 10.1083/jcb.114.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoh T J, Hisanaga S, Hosoi T, Kishimoto T, Hotani H. Biochemistry. 1997;36:12574–12582. doi: 10.1021/bi962606z. [DOI] [PubMed] [Google Scholar]
- 23.Ebneth A, Drewes G, Mandelkow E. Cell Motil Cytoskeleton. 1999;44:209–224. doi: 10.1002/(SICI)1097-0169(199911)44:3<209::AID-CM6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Tseng H-C, Lu Q, Henderson E, Graves D J. Proc Natl Acad Sci USA. 1999;96:9503–9508. doi: 10.1073/pnas.96.17.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vallée R B. Proc Natl Acad Sci USA. 1980;77:3206–3210. doi: 10.1073/pnas.77.6.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fellous A, Prasad V, Ohayon R, Jordan M A, Luduena R F. J Protein Chem. 1994;13:381–391. doi: 10.1007/BF01901694. [DOI] [PubMed] [Google Scholar]
- 27.Compagnone N A, Mellon S H. Proc Natl Acad Sci USA. 1998;95:4678–4683. doi: 10.1073/pnas.95.8.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]