Abstract
Hunger/satiation state interacts with appetitive and noxious stimuli to determine feeding and avoidance responses. In the predatory marine snail Pleurobranchaea californica, food chemostimuli induced proboscis extension and biting at concentration thresholds that varied directly with satiation state. However, food stimuli also tended to elicit avoidance behavior (withdrawal and avoidance turns) at concentration thresholds that were relatively low and fixed. When the feeding threshold for active feeding (proboscis extension with biting) was exceeded, ongoing avoidance and locomotion were interrupted and suppressed. Noxious chemostimuli usually stimulated avoidance, but, in animals with lower feeding thresholds for food stimuli, they often elicited feeding behavior. Thus, sensory pathways mediating appetitive and noxious stimuli may have dual access to neural networks of feeding and avoidance behavior, but their final effects are regulated by satiation state. These observations suggest that a simple cost-benefit computation regulates behavioral switching in the animal's foraging behavior, where food stimuli above or below the incentive level for feeding tend to induce feeding or avoidance, respectively. This decision mechanism can weigh the animal's need for nutrients against the potential risk from other predators and the cost of relative energy outlay in an attack on prey. Stimulation of orienting and attack by low-level noxious stimuli in the hungriest animals may reflect risk-taking that can enhance prey capture success. A simple, hedonically structured neural network model captures this computation.
To optimize foraging behavior, animals often must make decisions based on the likely costs and benefits of a feeding attempt. One way in which they may do so is by integrating the percepts of a potential food source with their own internal state. That is, the predicted gains and losses of a feeding attempt, in terms of nutrient gain, energy expenditure, and risks from noxious prey defense and predation while foraging, are weighed against the organism's nutrient need as represented in terms of hunger. How animals do this must be basic to their ecosystem interactions and a major organizing factor in strategies of optimal foraging. However, the computational mechanisms animals use to decide between expression of feeding and avoidance behaviors are not well understood.
In the carnivorous opisthobranch snail Pleurobranchaea californica, feeding and avoidance behaviors are largely exclusive of each other, as is the case for most animals. For instance, induction of active feeding behavior (rhythmic biting) suppresses avoidance withdrawal to a mechanical stimulus (1). Escape swimming, a stereotypic predator avoidance behavior, takes precedence over most other behaviors, including feeding (2, 3). Some data indicate that the transitions between feeding and avoidance behaviors can be modulated by experience, such that the feeding response to a food stimulus is replaced by withdrawal and avoidance turns after associative conditioning of food and shock (4).
We have further examined the influences of hunger state on the alternative expression of feeding and avoidance behavior. We have found that feeding stimuli tend to activate avoidance behavior at relatively low thresholds, such that partly satiated animals often actively avoid weak food stimuli; conversely, hungry animals with low feeding thresholds suppress avoidance to feed. Moreover, in hungry animals, even noxious stimuli come to stimulate feeding, which is replaced by avoidance as animals are satiated. These relationships seem to more fully outline the organization of this predator's foraging behavior in the contexts of sensation and internal state, and they suggest how the nervous system could be structured to effect this organization.
Methods
P. californica were supplied by Rimmon Fay (Pacific Biomarine, Santa Monica, CA) and Michael Morris (SeaLife Supply, Sand City, CA). Before experiments, animals were maintained for one to several weeks without feeding in aquaria in artificial sea water at 15°C. Feeding thresholds of satiated animals largely recover during a week's deprivation (5). In some experiments, animals were fed to partial or full satiation (cessation of feeding) with pieces of previously frozen shrimp purchased locally.
We define and use the term “hunger” here in terms of “readiness to feed,” which we recognize to be multiply influenced by satiation state, learning, hormonal state, and general health. We manipulated satiation state in these studies and measured readiness to feed as chemosensory thresholds for proboscis extension and biting.
Solutions.
Betaine (trimethylglycine) was chosen as the main feeding stimulant in this study from comparison in 10 animals of 17 substances, which ranked descendingly in effectiveness as betaine, glycine = cysteine, glutamine, taurine, proline, methionine, alanine, lysine, asparagine, phenylalanine, aspartate, arginine, glutamate, trimethylamine oxide, 5′-AMP, and protein (BSA, 1 mg/ml). Betaine is a common feeding stimulant for marine carnivores (6) and was nearly 10-fold more potent than glycine and cysteine. Betaine is an osmolyte found in high concentrations (tens of millimolar) in most marine invertebrates and stimulates feeding in many marine carnivores (7–9). Comparison of betaine as a feeding stimulant with a homogenate of squid, formerly used routinely as a feeding stimulant for Pleurobranchaea (1), showed no significant difference in feeding thresholds for dilutions of standard squid homogenate and molar concentrations of betaine (P > 0.3 for six animals; Wilcoxon signed rank test). Advantages of betaine as a feeding stimulant are its simple preparation and precise nature. Taurine, also present in marine invertebrates at >10 mM, gave mixed results as described later.
Behavioral Observations.
Behavioral measures were performed as described (1). Responses were observed for solutions applied in 1.5-ml volumes with a hand-held Pasteur pipette to the oral veil over 10 s in a series of ascending concentrations from 10−6 to 10−1 M. Feeding thresholds measured were those concentrations at which animals showed proboscis extension and biting (Fig. 1A). When specimens failed to respond to the highest concentration (10−1 M), the next highest value, 100, was assigned. Tests began with a control sea-water application that was assigned a value of 10−7. This convention assigns conservative finite values to essentially infinitely high or low thresholds. Data are presented as means and standard errors (SEMs) of the averaged negative logarithms of the dilutions; thus, 10−1 is 1.0, and so on (1). The resulting data were analyzed with nonparametric tests that best accommodate the threshold conventions described above.
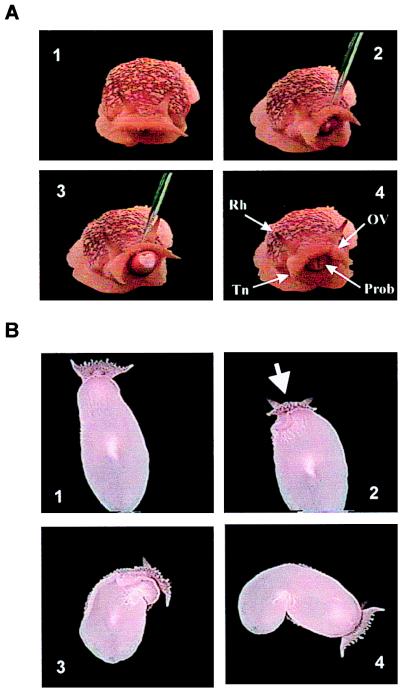
Figure 1.
Feeding and avoidance behaviors. (A) Feeding induced by betaine application (pipette is visible) showing a slight orienting turn, proboscis extension, and biting. Chemosensory structures indicated by arrows are rhinophore (Rh), oral veil (OV), and tentacle (Tn); the proboscis (Prob) also is shown. (B) In a ventral view of an animal on the aquarium glass, an avoidance turn is stimulated by application of taurine.
Avoidance behaviors defined and observed here were: local withdrawal of affected body parts, head withdrawal involving more active contractures of the anterior head region and oral veil, and active avoidance consisting of an avoidance turn followed by crawling substrate locomotion. The avoidance turn is a stereotypic behavior observed in at least five notaspid opisthobranch species (Fig. 1B; see ref. 10). During the avoidance turn, locomotion is suppressed while the anterior part of the foot is lifted slightly off the substrate. The animal twists right or left 45–180° away from the stimulus, pivoting on its broadened tail/posterior foot region, which remains attached to the substrate. Completing the turn, the anterior foot region reattaches and forward locomotion commences. At this time, the tail narrows to a sharper tip and frequently is detached from the substrate. Thresholds for expression of avoidance behaviors were measured as for the feeding behaviors, with dilutions of betaine and taurine.
Results
Subthreshold Feeding Stimuli Induce Avoidance Behavior.
In the course of testing the effectiveness of the various feeding stimuli (see Methods), we were struck that avoidance behavior seemed to be a frequent response to subthreshold dilutions. This observation stimulated the following experiments in which we found that avoidance behavior could be stimulated by feeding stimuli, but generally was replaced by active feeding at higher stimulus strengths. Head withdrawal, avoidance turning, and locomotion were interrupted by biting, but not by simple proboscis extension.
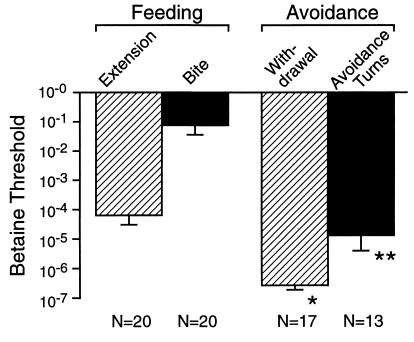
Betaine thresholds for the appearance of avoidance and feeding behaviors were tested in a population of 20 animals. The results (Fig. 2) were that 17 of the 20 animals displayed avoidance behavior, withdrawal, and avoidance turns at weaker stimuli than those at which they showed feeding behavior; the 20th animal showed an initial active avoidance response followed by proboscis extension at the same stimulus strength. Thirteen of the 20 animals showed avoidance turns; these turns were initiated in every case at betaine thresholds significantly lower than for biting, but not for proboscis extension. Thresholds for withdrawal were lower still, by almost 2 orders of magnitude; in this population, withdrawal behavior occurred in many cases solely in response to the light mechanical stimulation of control sea-water applications. Avoidance turns often followed withdrawal to a betaine stimulus in a smooth sequence. Proboscis extension not uncommonly accompanied the expression of avoidance behavior and was compatible with locomotion, withdrawal, and avoidance turns. However, it was notable that, in every case where biting occurred, avoidance turns and locomotion were completely halted for at least the entire duration of the biting sequence.
Figure 2.
Feeding and avoidance behaviors induced by betaine. *, Thresholds for head withdrawal were significantly lower than for proboscis extension in 17 subjects that expressed both behaviors (P < 0.0001; two-tailed Mann–Whitney U test), of a population of 20. **, Thresholds for avoidance turns differed from thresholds for biting (P < 0.0001) but were not significantly different from proboscis extension (P > 0.15) in the same 13 animals expressing both behaviors.
The mixed effects of betaine are general properties of food stimulation. The observations that betaine can stimulate either feeding or avoidance at different stimulus strengths resemble numerous informal and unpublished observations on effects of squid homogenate made in the course of previous studies, but whose significance was not then pursued.
Suppression of Active Avoidance and Locomotion by Active Feeding (Biting).
We further observed the interruption of locomotion and avoidance turns by induced biting in 29 animals during repeated threshold measurements. Animals were actively locomoting either at the beginning of the threshold measures or were induced to do so at lower stimulus concentrations. Avoidance turns were stimulated in some animals at stimulus concentrations that were the same as or lower than biting thresholds. For these animals, when the onset of biting occurred during avoidance turns (21 observations in 14 animals) or in simple forward locomotion (64 observations in 22 animals), in every instance, turning and locomotion were suppressed for the duration of the biting sequence. In contrast, simple proboscis extension was coincident with, but did not interrupt, locomotion or avoidance turns in 118 observations on the same 29 animals. These observations suggest that consummatory behavior actively suppresses avoidance turning and locomotion.
Satiation Raises Feeding Thresholds and Releases Avoidance.
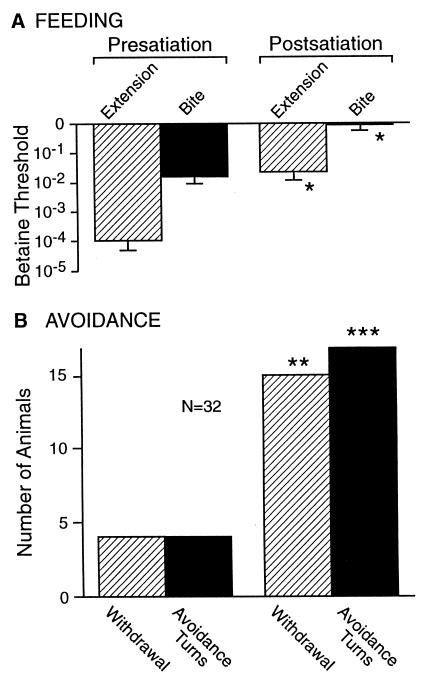
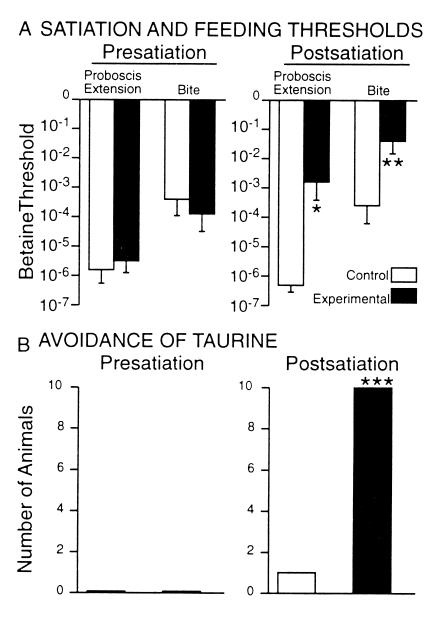
The observation that food stimuli induced avoidance behavior (withdrawal and avoidance turns) at subfeeding threshold concentrations suggested that the decision between avoidance or feeding behavior depended on both hunger state and stimulus strength. We tested this by measuring the effects of partial satiation on the appearance of avoidance behavior during measures of betaine feeding threshold in 32 animals. Partial satiation that raised feeding thresholds on average 204- and 64-fold (for proboscis extension and biting, respectively) caused the appearance of avoidance behavior in a significant fraction of the population (Fig. 3).
Figure 3.
Satiation-induced avoidance responses to betaine. (A) Partial satiation raised feeding thresholds significantly over presatiation measures. (B) Partial satiation increased the frequency of avoidance responses (withdrawal and turns) to the feeding stimulus during threshold measures. *, P < 0.0001, two-tailed paired Wilcoxon signed rank test. **, P < 0.006; ***, P < 0.002; two-sided values, Fisher's exact tests.
Avoidance and Feeding Responses to a Noxious Stimulus Are Related to Feeding Threshold.
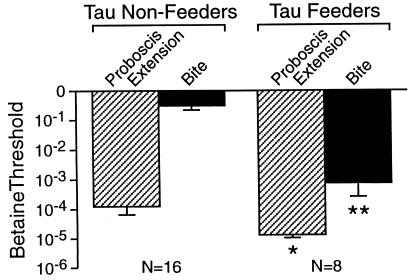
The amino acid derivative taurine is a potent noxious stimulus for Pleurobranchaea, causing avoidance at concentrations from 10−5 to 10−1 M (10). Thus, it was a matter of curiosity to find in the course of testing that taurine caused feeding behavior in some animals, and that whether animals attacked or avoided a taurine stimulus was related to their feeding thresholds. We investigated this effect in a population of 24 animals in which we measured betaine feeding thresholds and characterized taurine effects on inducing feeding and avoidance behaviors. This Pleurobranchaea population sorted into two groups (Fig. 4). One group of animals, with relatively high feeding thresholds, showed only avoidance responses to taurine (n = 16). The second group had significantly lower feeding thresholds and showed both feeding and avoidance responses (n = 8).
Figure 4.
Animals showing feeding responses to taurine had significantly lower betaine feeding thresholds than taurine nonfeeders. *, P < 0.025; **, P < 0.0001 (Mann–Whitney U tests).
The two groups differed only in feeding thresholds; there were no significant differences in avoidance thresholds to taurine between the two groups. In both groups, avoidance responses increased in frequency with increasing taurine concentration. The feeding and avoidance responses of the low feeding threshold animals usually occurred in a specific sequence. The initial response was local withdrawal of the oral veil, often along with a more involved withdrawal of the head region. This was succeeded within seconds by oriented proboscis extension that endured variably from a few to tens of seconds in trials where taurine was repeatedly pipetted. In this particular group, the strongest feeding response recorded was proboscis extension; however, repeated cycles of biting have since been elicited in numerous other animals by taurine stimulation (see below); in these animals, avoidance responses did not always occur before feeding. With exceptions of those animals that bit continuously during the stimulus, the feeding response was followed by an active avoidance turn away from the stimulus and locomotion.
The above results suggested that feeding responses to both betaine or taurine depended on hunger state, whereas avoidance responses to taurine were not (or less so). If so, satiating the animals might selectively suppress the feeding responses to taurine, while leaving avoidance responses intact. Such proved to be the case. In a controlled experiment, selected animals that bit vigorously to 10−2 M taurine ceased to bite after satiation and in every case responded to taurine with avoidance turns. The control group of unsatiated animals retained low feeding thresholds to betaine and continued to bite vigorously to the taurine stimulus, except in a single case (Fig. 5). Thus, although betaine is the more effective appetant and taurine the more potent aversive stimulus, both betaine and taurine may stimulate either feeding or avoidance behavior, depending on an animal's hunger state.
Figure 5.
Satiation suppresses feeding responses to taurine and releases avoidance. Twenty animals were identified that exhibited biting responses to 10−2 M taurine and assigned to experimental (satiated) and control groups. (A) Satiation raised average betaine thresholds for feeding responses tested 1 h later by 30- to 50-fold. (B) Presatiation, no animals showed avoidance behavior to taurine, whereas postsatiation all fed animals responded with avoidance turns. A single control animal showed avoidance to taurine, associated with a spontaneously elevated feeding threshold. Significant postsatiation changes: *, P < 0.015, P < 0.01 (two-tailed paired Wilcoxon signed rank tests); ***, P < 0.001 (Fisher's exact test).
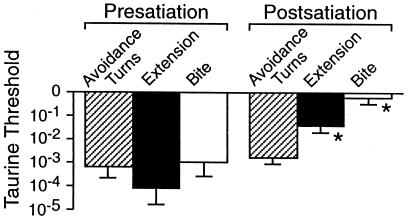
The effects of satiation on taurine thresholds for avoidance behavior were tested in another experiment by using 10 more animals that showed strong feeding responses to 10−2 M taurine. Thresholds were measured for taurine-induced avoidance and feeding responses and for betaine-induced feeding responses. They were then fed and chemosensory thresholds were measured again after 1 h (Fig. 6). Partial satiation that markedly increased animals' feeding thresholds to both taurine and betaine did not significantly alter the averaged thresholds for taurine-induced avoidance behavior.
Figure 6.
Satiation significantly raised taurine thresholds for feeding but not for avoidance turns. *, P = 0.002, two-tailed paired Wilcoxon signed rank tests. For taurine avoidance, P = 0.58.
Taurine is noxious to Pleurobranchaea for its ability to stimulate skin acid secretion (10), which is normally a defensive response in which the skin pH plummets from 7.0–8.0 to 1.5–2.0 and is noxious to predators (11). The secretion also potentiates the snail's own avoidance behavior, inducing local and head withdrawal, avoidance turns, and occasionally escape swimming (10). To test whether taurine-induced feeding behavior could be mediated by the nociceptive pathway stimulated by induced acid secretion, we identified six animals that showed strong feeding responses to 10−2 M taurine and tested their responses to acidified seawater (pH 1.7). In each case, these animals responded to acid with vigorous proboscis extension and multiple cycles of biting, similar to taurine responses. Feeding responses were specific to oral veil stimulation; taurine or acid stimulation of other body regions caused local withdrawal. Taurine applied anywhere on the animal's surface caused local acid secretion, measured with pH electrode (10), contrasting with the specific chemosensitivity to betaine restricted to oral veil, rhinophores, and mouth area. In another set of six animals that fed to both taurine and acidified seawater, satiation changed responses to both stimuli to avoidance (P < 0.005; two-sided values, Fisher's exact test). Thus, acid secretion is a likely common mechanism through which taurine stimulates both avoidance behavior and feeding, and these results confirm that a directly noxious stimulus by itself can induce appetitive behavior in hungry animals.
Discussion
Appetant Stimulation of Both Avoidance and Feeding Behavior.
The finding that avoidance behavior is stimulated by feeding stimuli subthreshold to actual feeding complement and explain prior observations that food-avoidance training can lead to active avoidance replacing feeding responses to food stimuli. Food-avoidance conditioning, where a food stimulus is paired with electric shock, raises feeding thresholds on average between 100- and 1,000-fold (4, 12). The present observations have shown that (i) in the absence of active feeding, food stimuli normally induce avoidance, and (ii) satiation, which like food-avoidance training raises feeding thresholds, also replaces feeding with avoidance behavior. It follows that the effects of both food-avoidance training and satiation are interpretable in terms of the release of avoidance behaviors when feeding thresholds are elevated.
At the neural network level, these observations suggest that food chemosensory inputs excite both avoidance and feeding circuitry simultaneously, and that when active feeding occurs, as noted in these experiments, avoidance and locomotion are suppressed. Because the thresholds for feeding behavior vary as a function of hunger, and those for food-induced avoidance behaviors are relatively fixed at low levels, the animal avoids or ignores low-level feeding stimuli when not hungry and suppresses avoidance when threshold for active feeding is exceeded.
Noxious Agent Stimulation of Both Feeding and Avoidance.
Nociceptive input, like food chemosensation, also can stimulate either avoidance or feeding, depending on an animal's internal state. Taurine is a generally noxious stimulus to Pleurobranchaea, acting by stimulation of acid secretion from the skin; acidic stimuli are aversive and induce avoidance behavior as shown here and elsewhere (10). However, in hungrier animals, taurine tended to induce elements of feeding behavior, and, in the most hungry, it actually stimulated vigorous, repetitive biting. The lack of the biting response to noxious stimuli in satiated animals argues against it being merely a well-differentiated defensive behavior. Satiation of these animals raised feeding thresholds for both taurine and betaine and left avoidance thresholds for taurine at low levels. That is, satiation suppressed feeding responses to the noxious agent and effectively replaced them with strong avoidance behavior.
Two observations suggest that the sensory pathways stimulated by taurine are at least partly distinct from those that mediate effects of betaine and other food stimuli: (i) chemosensitivity to taurine is broadly distributed on the animal's surface, whereas betaine sensitivity is specifically localized to food sensory areas, and (ii) acidified seawater, a noxious stimulus to most invertebrates including Pleurobranchaea, mimicked all effects of taurine. Thus, we hypothesize the existence of a basically nociceptive pathway that excites avoidance behavior, but that also provides excitation to the feeding motor network. In very hungry animals, this excitation is sufficient to induce patterned consummatory output.
Like taurine, noxious acidic solutions applied to the oral veil stimulated avoidance in less hungry animals, but induced feeding behavior in the hungriest. The observations that noxious stimuli can provoke feeding behavior has an interesting and close parallel in another predatory mollusk, Octopus vulgaris. Hungry octopuses vigorously attack a noxious electric shock, but after feeding reduce attack duration or avoid shock entirely; at this time, the well satiated octopus may actively avoid food chemostimuli§ as do Pleurobranchaea.
A Network Model.
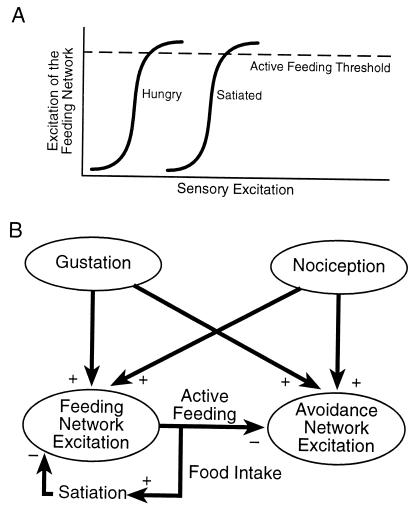
The experimental results can be summarized in a simple, testable model. Patterned feeding motor output by the feeding network is assumed to be a function of both sensory input and satiation state, such that motor pattern excitability is low in satiated animals and requires stronger sensory excitation to release active feeding behavior (Fig. 7A). Effectively, the excitability of the feeding motor network manifests the satiated state. Fig. 7B shows the model in terms of potential neural network interactions. In this scheme, a food stimulus is assumed to simultaneously excite networks for both feeding and avoidance. When the feeding network is active, it inhibits avoidance. A possible reciprocal inhibitory connection is not shown here. Food stimulus excitation of the motor networks is assumed to be mediated by a dedicated chemosensory afferent pathway, and the excitatory path to the motor networks activated by pain may be the nociceptive afferent pathway already well defined in gastropods (13, 14). Nociceptive afferents may directly excite feeding network elements, based on observations of effects of mechanical stimuli on identified cells (15, 16).
Figure 7.
A model of satiation-influenced decision for appetitive vs. avoidance behavior. (A) Satiation state is postulated to be reflected in the excitability of the feeding motor network, whose activity is depicted arbitrarily as a sigmoid function (see text). (B) The possible organization of sensory and motor pathways of feeding and avoidance behavior. The model embodies the observations that appetant stimuli may stimulate feeding in hungry animals and avoidance in satiated and food-avoidance trained animals, by postulating that the gustatory and nociceptive pathways each have parallel outputs to both the pattern generator networks of feeding and avoidance behaviors. Activity in the feeding network inhibits the avoidance network. The effects of satiation are represented by a negative feedback loop from active feeding output to the feeding network by means of food intake.
Although other models are possible, experiments in progress suggest that elements of the feeding network may indeed change their excitability because of changes in levels of endogenous neuromodulator chemicals (unpublished work). This and other simple models that can account for the present results are testable, because a variety of neurons critical to feeding and avoidance behaviors are identified, and multiple interactions between feeding and avoidance motor networks are already documented at the level of single neurons (3, 15–24).
Significance of Hunger/Satiation Regulation of Affect in a Predatory Snail.
A growing body of observations indicates that animals can choose between cautious or riskier tactics in situations where perceived threat and the relative benefits of foraging both influence behavioral decisions (25, 26). Our results suggest an underlying mechanism in the dynamic organization of foraging behavior, where an animal can predict the cost-benefit values of a feeding attempt from the appetant and noxious characters of a stimulus and its own state of satiation. In hungry animals with low feeding thresholds, expression of active feeding inhibits avoidance. In partly or fully satiated animals, where the benefit of a feeding attempt would be relatively low, avoidance may be expressed in response to weak feeding stimuli; such avoidance is suppressed when stimulus incentive reaches feeding threshold. Additional hedonic dimension is added by nociceptive regulation of both avoidance and feeding behaviors, such that noxious stimulation of food chemosensory areas stimulates avoidance in less hungry animals, but induces elements of feeding behavior in hungrier animals and releases active feeding in the hungriest. The immediate significance of these relationships to foraging may be that (i) orienting and attack to food stimuli at varying thresholds must certainly reflect nutrient need, (ii) the avoidance of food stimuli by satiated animals protects them from other predators drawn to the stimuli, and prevents both expenditure of energy and accidental damage in unnecessary feeding attempts, and (iii) potentiation of feeding behavior by noxious stimuli in hungry animals may link more extreme nutrient need to greater effort to overcome the defenses of prey unwilling to be eaten.
Acknowledgments
We thank Dr. Arthur Ghent for statistical advice and Dr. Kurt Potgieter and Ms. Jessica Wenzel for technical assistance. This work was supported by National Science Foundation Grant IBN-9808400 and National Institutes of Health Grant MH59339 (to R.G.). Significant parts of this work were conceived from observations and/or carried out at the Laboratoire Arago, Banyuls-sur-Mer, France, and the University of Washington Friday Harbor Marine Laboratory.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Gillette, R., Cephalopod International Advisory Council Meeting, June 5–11, 1994, Vico Equense, Italy, p. 12 (abstr.).
References
- 1.Davis W J, Mpitsos G J, Pinneo J M. J Comp Physiol. 1974;90:207–224. [Google Scholar]
- 2.Davis W J, Mpitsos G J. Z Vergl Physiol. 1971;75:205–232. [Google Scholar]
- 3.Jing J, Gillette R. J Neurophysiol. 1995;74:1900–1910. doi: 10.1152/jn.1995.74.5.1900. [DOI] [PubMed] [Google Scholar]
- 4.Mpitsos G J, Collins S. Science. 1975;188:954–957. doi: 10.1126/science.1138366. [DOI] [PubMed] [Google Scholar]
- 5.Davis W J, Mpitsos G J, Pinneo J M, Ram J L. J Comp Physiol. 1977;117:99–125. [Google Scholar]
- 6.Mackie A M, Grant P T. In: Chemoreception in Marine Organisms. Grant P T, Mackie A M, editors. New York: Academic; 1974. pp. 143–175. [Google Scholar]
- 7.Hara T J. In: Perception of Behavioral Chemicals. Norris D M, editor. Amsterdam: Elsevier/North–Holland; 1981. pp. 29–57. [Google Scholar]
- 8.McClintock J B, Klinger T S, Lawrence J M. Mar Biol (Berlin) 1984;84:47–52. [Google Scholar]
- 9.Carr W E S, Netherton J C, Gleeson R A, Derby C D. Biol Bull (Woods Hole, MA) 1996;190:149–160. doi: 10.2307/1542535. [DOI] [PubMed] [Google Scholar]
- 10.Gillette R, Saeki M, Huang R-C. J Exp Biol. 1991;156:335–347. [Google Scholar]
- 11.Thompson T E. J Mar Biol Assoc UK. 1960;39:115–122. [Google Scholar]
- 12.Davis W J, Gillette R. Science. 1978;199:801–804. doi: 10.1126/science.622572. [DOI] [PubMed] [Google Scholar]
- 13.Walters E T. Biol Bull (Woods Hole, MA) 1991;180:241–251. doi: 10.2307/1542394. [DOI] [PubMed] [Google Scholar]
- 14.Getting P A. J Comp Physiol. 1977;110:271–286. [Google Scholar]
- 15.Gillette R, Davis W J. J Comp Physiol. 1977;116:129–159. [Google Scholar]
- 16.London J A, Gillette R. J Exp Biol. 1984;113:423–446. doi: 10.1242/jeb.113.1.423. [DOI] [PubMed] [Google Scholar]
- 17.Gillette R, Kovac M P, Davis W J. Science. 1978;199:798–801. doi: 10.1126/science.622571. [DOI] [PubMed] [Google Scholar]
- 18.Gillette R, Gillette M U, Davis W J. J Neurophysiol. 1980;43:669–685. doi: 10.1152/jn.1980.43.3.669. [DOI] [PubMed] [Google Scholar]
- 19.Gillette R, Kovac M P, Davis W J. J Neurophysiol. 1982;47:885–908. doi: 10.1152/jn.1982.47.5.885. [DOI] [PubMed] [Google Scholar]
- 20.Kovac M P, Davis W J, Matera E M, Croll R P. J Neurophysiol. 1983;49:1517–1538. doi: 10.1152/jn.1983.49.6.1517. [DOI] [PubMed] [Google Scholar]
- 21.Kovac M P, Davis W J, Matera E M, Croll R P. J Neurophysiol. 1983;49:1539–1556. doi: 10.1152/jn.1983.49.6.1539. [DOI] [PubMed] [Google Scholar]
- 22.London J A, Gillette R. Proc Natl Acad Sci USA. 1986;83:4058–4062. doi: 10.1073/pnas.83.11.4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovac M P, Davis W J. J Neurophysiol. 1980;43:469–487. doi: 10.1152/jn.1980.43.2.469. [DOI] [PubMed] [Google Scholar]
- 24.Jing J, Gillette R. J Neurophysiol. 2000;83:1346–1355. doi: 10.1152/jn.2000.83.3.1346. [DOI] [PubMed] [Google Scholar]
- 25.Lima S L, Dill L M. Can J Zool. 1990;68:619–640. [Google Scholar]
- 26.Ydenberg R C. In: Cognitive Ecology. Dukas R, editor. Chicago: Univ. of Chicago Press; 1998. pp. 343–378. [Google Scholar]