Abstract
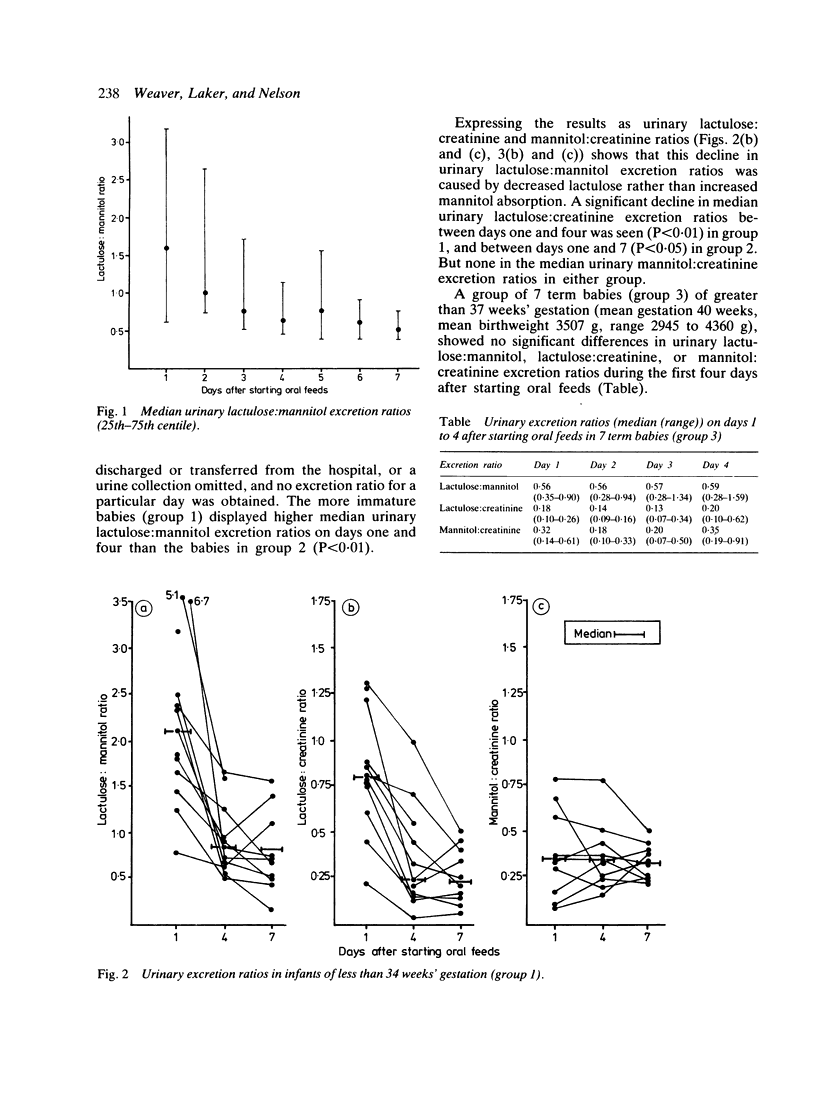
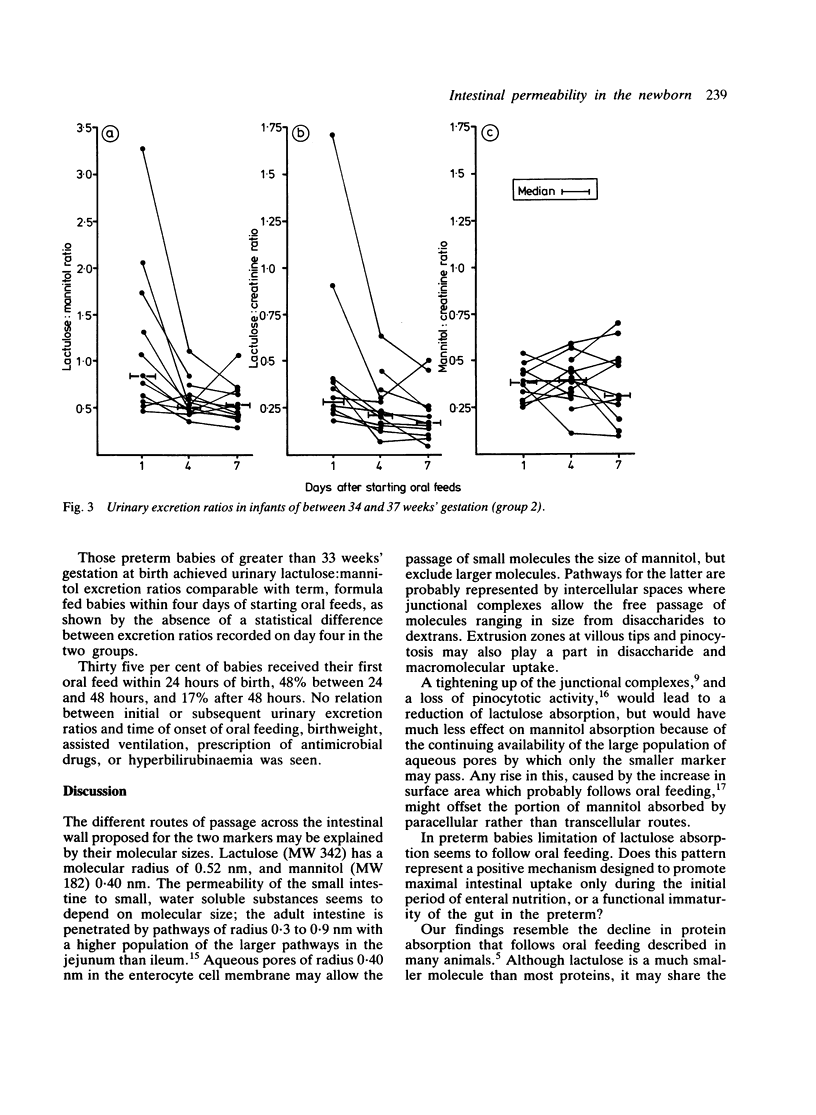
Passive intestinal permeability in 33 newborn babies was studied using feeds containing lactulose and mannitol. Each marker is thought to pass across the gut wall by a different route; lactulose by a paracellular and mannitol by a transcellular pathway. Neither is metabolised and both are wholly and solely excreted by the kidney; urinary recovery is a measure of the intestinal uptake. Babies born before 34 weeks' gestation exhibited a higher intestinal permeability to lactulose than more mature babies, and all preterm babies showed an appreciable decline in lactulose absorption during the first week of oral feeds. Babies of 34 to 37 weeks' gestation achieved a 'mature' intestinal permeability to lactulose within four days of starting oral feeds. These findings may reflect the immaturity of the gut of the preterm baby rather than a process essential to adaptation to enteral nutrition.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonowicz I., Lebenthal E. Developmental pattern of small intestinal enterokinase and disaccharidase activities in the human fetus. Gastroenterology. 1977 Jun;72(6):1299–1303. [PubMed] [Google Scholar]
- Beach R. C., Menzies I. S., Clayden G. S., Scopes J. W. Gastrointestinal permeability changes in the preterm neonate. Arch Dis Child. 1982 Feb;57(2):141–145. doi: 10.1136/adc.57.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach R. C., Menzies I. S. Lactulose and other non-absorbable sugars in infant milk feeds. Lancet. 1983 Feb 19;1(8321):425–426. doi: 10.1016/s0140-6736(83)91548-9. [DOI] [PubMed] [Google Scholar]
- Eastham E. J., Lichauco T., Grady M. I., Walker W. A. Antigenicity of infant formulas: role of immature intestine on protein permeability. J Pediatr. 1978 Oct;93(4):561–564. doi: 10.1016/s0022-3476(78)80888-9. [DOI] [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Jr, Ewton M. F., Soter N., Kinney J. Permeability characteristics of the human small intestine. J Clin Invest. 1965 Dec;44(12):1935–1944. doi: 10.1172/JCI105299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRYBOSKI J. D. THE SWALLOWING MECHANISM OF THE NEONATE. I. ESOPHAGEAL AND GASTRIC MOTILITY. Pediatrics. 1965 Mar;35:445–452. [PubMed] [Google Scholar]
- Grand R. J., Watkins J. B., Torti F. M. Development of the human gastrointestinal tract. A review. Gastroenterology. 1976 May;70(5 PT1):790–810. [PubMed] [Google Scholar]
- Laker M. F., Bull H. J., Menzies I. S. Evaluation of mannitol for use as a probe marker of gastrointestinal permeability in man. Eur J Clin Invest. 1982 Dec;12(6):485–491. doi: 10.1111/j.1365-2362.1982.tb02230.x. [DOI] [PubMed] [Google Scholar]
- Laker M. F. Estimation of disaccharides in plasma and urine by gas-liquid chromatography. J Chromatogr. 1979 May 1;163(1):9–18. doi: 10.1016/s0378-4347(00)81163-9. [DOI] [PubMed] [Google Scholar]
- Laker M. F., Menzies I. S. Increase in human intestinal permeability following ingestion of hypertonic solutions. J Physiol. 1977 Mar;265(3):881–894. doi: 10.1113/jphysiol.1977.sp011750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecce J. G., Broughton C. W. Cessation of uptake of macromolecules by neonatal guinea pig, hamster and rabbit intestinal epithelium (closure) and transport into blood. J Nutr. 1973 May;103(5):744–750. doi: 10.1093/jn/103.5.744. [DOI] [PubMed] [Google Scholar]
- Lucas A., Bloom S. R., Aynsley-Green A. Metabolic and endocrine events at the time of the first feed of human milk in preterm and term infants. Arch Dis Child. 1978 Sep;53(9):731–736. doi: 10.1136/adc.53.9.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogra S. S., Weintraub D., Ogra P. L. Immunologic aspects of human colostrum and milk. III. Fate and absorption of cellular and soluble components in the gastrointestinal tract of the newborn. J Immunol. 1977 Jul;119(1):245–248. [PubMed] [Google Scholar]
- Parkin J. M., Hey E. N., Clowes J. S. Rapid assessment of gestational age at birth. Arch Dis Child. 1976 Apr;51(4):259–263. doi: 10.1136/adc.51.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger C. H., Rothberg R. M. Development of the capacity to produce specific antibody to an ingested food antigen in the premature infant. J Pediatr. 1975 Oct;87(4):515–518. doi: 10.1016/s0022-3476(75)80811-0. [DOI] [PubMed] [Google Scholar]
- Roberton D. M., Paganelli R., Dinwiddie R., Levinsky R. J. Milk antigen absorption in the preterm and term neonate. Arch Dis Child. 1982 May;57(5):369–372. doi: 10.1136/adc.57.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg R. M. Immunoglobulin and specific antibody synthesis during the first weeks of life of premature infants. J Pediatr. 1969 Sep;75(3):391–399. doi: 10.1016/s0022-3476(69)80264-7. [DOI] [PubMed] [Google Scholar]
- Saunders D. R., Wiggins H. S. Conservation of mannitol, lactulose, and raffinose by the human colon. Am J Physiol. 1981 Nov;241(5):G397–G402. doi: 10.1152/ajpgi.1981.241.5.G397. [DOI] [PubMed] [Google Scholar]



