Abstract
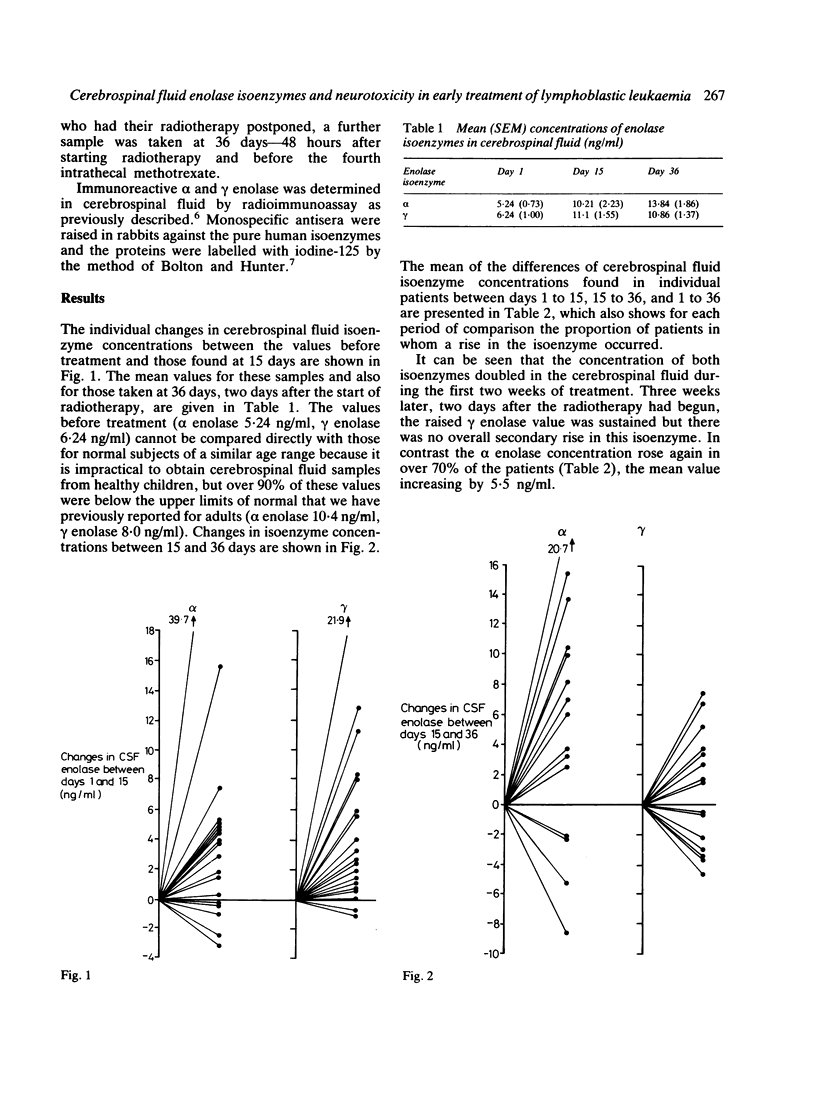
Serial measurements of enolase in the cerebrospinal fluid were made in 19 children with lymphoblastic leukaemia undergoing their first 6 weeks of antineoplastic treatment. The neurone-specific gamma enolase value rose appreciably in nearly all patients during the first two weeks of treatment, which comprised chemotherapy only, but the mean values for this isoenzyme failed to show any further rise during the subsequent cranial irradiation. In contrast the alpha enolase value, which is derived predominantly from glial tissue, rose progressively to attain its highest value during radiotherapy. A consideration of the likely rate of clearance of gamma enolase from the cerebrospinal fluid and the time sequence of administration of the several chemotherapeutic agents in UKALL VIII suggests that asparaginase may be the main causative agent in the rise of this marker of neuronal damage.
Full text
PDF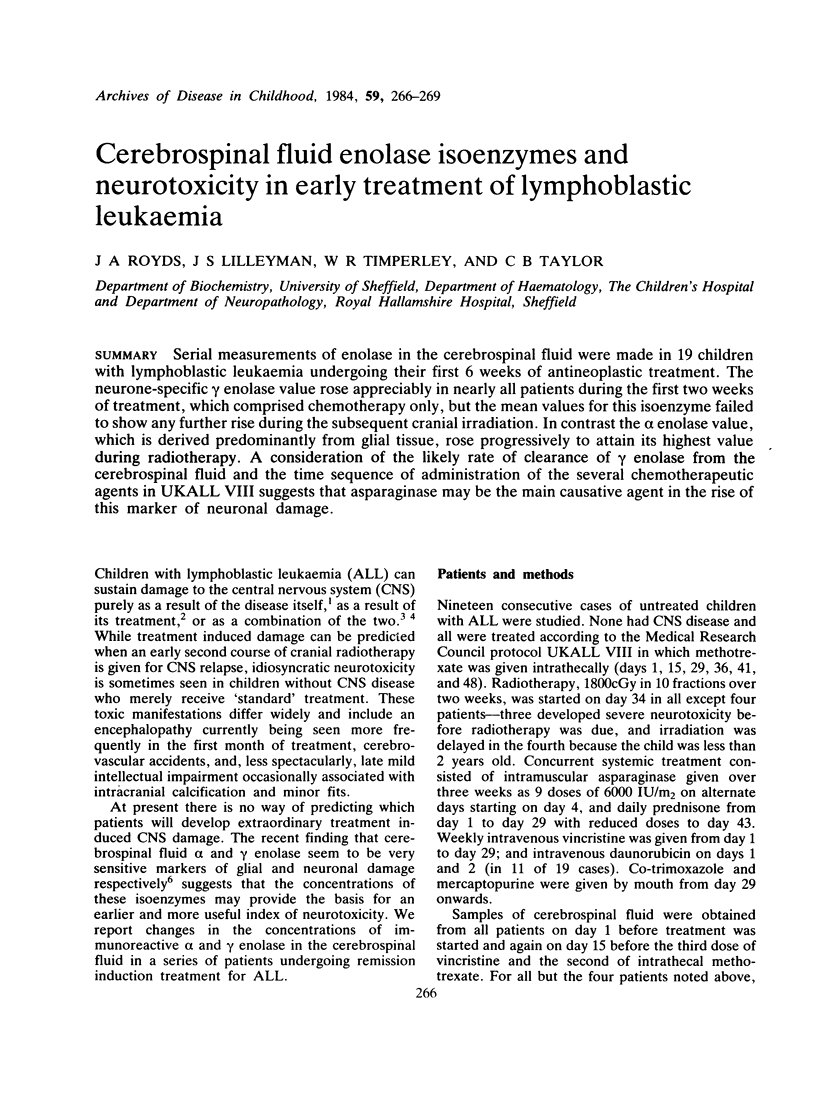


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bode U., Magrath I. T., Bleyer W. A., Poplack D. G., Glaubiger D. L. Active transport of methotrexate from cerebrospinal fluid in humans. Cancer Res. 1980 Jul;40(7):2184–2187. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell C. M. L-Asparaginase: human toxicology and single agent activity in nonleukemic neoplasms. Cancer Treat Rep. 1981;65 (Suppl 4):57–59. [PubMed] [Google Scholar]
- Holland J., Fasanello S., Onuma T. Psychiatric symptoms associated with L-asparaginase administration. J Psychiatr Res. 1974 May;10(2):105–113. doi: 10.1016/0022-3956(74)90030-2. [DOI] [PubMed] [Google Scholar]
- Kay H. E., Knapton P. J., O'Sullivan J. P., Wells D. G., Harris R. F., Innes E. M., Stuart J., Schwartz F. C., Thompson E. N. Encephalopathy in acute leukaemia associated with methotrexate therapy. Arch Dis Child. 1972 Jun;47(253):344–354. doi: 10.1136/adc.47.253.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land V. J., Sutow W. W., Fernbach D. J., Lane D. M., Williams T. E. Toxicity of L-asparginase in children with advanced leukemia. Cancer. 1972 Aug;30(2):339–347. doi: 10.1002/1097-0142(197208)30:2<339::aid-cncr2820300206>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Moure J. M., Whitecar J. P., Jr, Bodey G. P. Electroencephalogram changes secondary to asparaginase. Arch Neurol. 1970 Oct;23(4):365–368. doi: 10.1001/archneur.1970.00480280079009. [DOI] [PubMed] [Google Scholar]
- Oliff A., Bleyer W. A., Poplack D. G. Acute encephalopathy after initiation of cranial irradiation for meningeal leukaemia. Lancet. 1978 Jul 1;2(8079):13–15. doi: 10.1016/s0140-6736(78)91323-5. [DOI] [PubMed] [Google Scholar]
- Price R. A., Jamieson P. A. The central nervous system in childhood leukemia. II. Subacute leukoencephalopathy. Cancer. 1975 Feb;35(2):306–318. doi: 10.1002/1097-0142(197502)35:2<306::aid-cncr2820350203>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Royds J. A., Davies-Jones G. A., Lewtas N. A., Timperley W. R., Taylor C. B. Enolase isoenzymes in the cerebrospinal fluid of patients with diseases of the nervous system. J Neurol Neurosurg Psychiatry. 1983 Nov;46(11):1031–1036. doi: 10.1136/jnnp.46.11.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H. D., Walker M. D., Wiernik P. H. Neurotoxicity of commonly used antineoplastic agents (first of two parts). N Engl J Med. 1974 Jun 11;291(2):75–81. doi: 10.1056/NEJM197407112910205. [DOI] [PubMed] [Google Scholar]


