Abstract
Estrogen replacement therapy in women is associated with improvement of cognitive deficits and reduced incidence of Alzheimer's disease. The present study indicates that estrogen is neuroprotective against N-methyl-d-aspartate (NMDA)- and kainate-mediated neurotoxicity, an effect mediated by tyrosine kinase/mitogen-activated protein kinase (MAPK) pathways. Estrogen also stimulates tyrosine phosphorylation of NMDA receptors via an src tyrosine kinase/MAPK pathway. Finally, estrogen-mediated enhancement of long-term potentiation in hippocampal slices is mediated by activation of an src tyrosine kinase pathway. Thus, estrogen, by activating an src tyrosine kinase and the extracellular signal-related protein kinase/MAPK signaling pathway, both enhances NMDA receptor function and long-term potentiation and retains neuroprotective properties against excitotoxicity. These findings warrant further evaluation of the usefulness of estrogenic compounds for the treatment of Alzheimer's disease and other neurodegenerative diseases.
Estrogen replacement therapy in postmenopausal women decreases the probability of developing Alzheimer's disease and slows the progress of the disease (1). In addition, estrogen replacement therapy improves cognitive performance in women with Alzheimer's disease (2–4). These effects of estrogen suggest that estrogen acts both as a cognitive enhancer and as a neuroprotective agent. Although the mechanisms underlying these effects remain unknown, estrogen has been shown to increase basal synaptic responses and the magnitude of long-term potentiation (LTP) in acute hippocampal slices (5–7). In addition, estrogen increased both α-amino-3-hydroxy-5-methylisoxazole propionic acid (AMPA) and N-methyl- d-aspartate (NMDA) receptor-mediated responses in hippocampal neurons (7–10). Such effects could account for the estrogen-mediated cognitive improvement in humans, because LTP is widely considered to represent a cellular model of learning and memory (11). Recent studies have also shown that estrogen is neuroprotective against excitotoxicity in primary neuronal cultures (12, 13). Estrogen-mediated neuroprotection involved the tyrosine kinase/mitogen-activated protein kinase (MAPK) signal transduction cascade, because estrogen rapidly activated tyrosine kinase and MAPK activity (14, 15) and because the neuroprotective effect of estrogen against glutamate toxicity was blocked by inhibitors of tyrosine kinases and MAPK (16). The MAPK pathway is thought to play an important role in the action of neurotrophins and in synaptic plasticity (17, 18), and its activation could lead to increased expression of antiapoptotic genes. A vast literature indicates that tyrosine kinase directly phosphorylates some NMDA receptor subunits, thereby enhancing NMDA receptor function (19–23). In addition, fyn knockout mice have LTP impairment, suggesting that the tyrosine kinase pathway could be involved in LTP (24). In the present study, we used acute and cultured hippocampal slices to study the role of tyrosine kinase/MAP kinase pathways in estrogen-mediated neuroprotection against excitotoxicity, phosphorylation of NMDA receptors, and LTP enhancement.
Materials and Methods
Preparation of Hippocampal Slice Cultures.
Transverse hippocampal slice cultures were prepared from 7- to 10-day-old rat pups according to standard techniques (25). Briefly, hippocampi were dissected and sectioned transversely at 400 μm by using a McIlwain tissue chopper (Westbury, NY) in a cutting medium consisting of MEM (with Earle's salts; GIBCO), 25 mM Hepes, 10 mM Tris, 10 mM glucose, and 3 mM MgCl2 (pH 7.2). Hippocampal sections were then placed onto membrane inserts (Millipore) in 6-well culture trays with 1 ml of growth medium [MEM with Hanks' salts (GIBCO)/20% (vol/vol) horse serum/3 mM glutamine/25 mM Hepes/5 mM NaHCO3/25 mM glucose/0.5 mM ascorbate/2 mM CaCl2/2.5 mM MgCl2/0.5 mg/liter insulin/1 μg/ml penicillin, pH 7.2].
After 7–14 days in culture, hippocampal sections were incubated in serum-free medium with or without 1 nM 17-β-estradiol (E2) for 24 h. They were then treated with 50 μM NMDA or 50 μM kainic acid (KA) for 3 h. The MAPK inhibitor PD98059 and the tyrosine kinase inhibitor PP2 were added at a concentration of 50 μM and 10 μM, respectively, together with E2. Cultured slices were then incubated in serum-free medium for another 24 h. Neuronal damage was assessed by semiquantitative analysis of propidium iodide (PI) uptake and lactate dehydrogenase (LDH) assay (26).
Immunoblotting.
For Western blots, cultured hippocampal slices were collected and sonicated in 10 mM Tris⋅HCl buffer (pH 7.4) containing 0.32 M sucrose, 2 mM EDTA, 2 mM EGTA, and 0.1 mM leupeptin. Aliquots of homogenates (30–40 μg protein per lane) were diluted with equal volumes of 2× sample buffer [2% (vol/vol) SDS/50 mM Tris⋅HCl, pH 6.8/10% (vol/vol) 2-mercaptoethanol/10% (vol/vol) glycerol/0.1% bromophenol blue]. After heating samples at 90–100°C for 5 min, proteins were subjected to SDS/PAGE (27) and transferred onto nitrocellulose membranes (28). GluR1 proteins were probed with a C-terminal domain antibody (C-Ab, 1:500; Chemicon). Anti-NR2 and anti-phosphotyrosine antibodies were obtained from Chemicon and Upstate Biotechnology (Lake Placid, NY), respectively. Immunoblots were scanned, and the digitized images were analyzed quantitatively by densitometry with the imagequant program providing peak areas and apparent molecular masses.
Immunoprecipitation.
Hippocampal slice cultures were removed from the membrane insert and placed in a microfuge tube with 200 μl of ice-cold homogenization buffer [20% (vol/vol) sucrose in PBS with 3.0 mM EGTA] and sonicated. Samples were centrifuged at 14,000 × g for 15–20 min. The supernatant was discarded; the pellet was resuspended in 100 μM EGTA in distilled water and centrifuged again as above. The pellet was resuspended in Tris-acetate (100 mM; pH 7.4) containing 100 μM EGTA and centrifuged again. The supernatant was discarded, and this last step was repeated at least once. The final pellet was resuspended in the Tris-acetate buffer and frozen at −70°C until used. Membranes fractions (≈300 μg of protein) were lysed in PBS containing protease inhibitors (0.1 mM PMSF/1 μg/ml pepstatin A/1 μg/ml leupeptin) and 2 mM EDTA. Membranes were then sonicated and centrifuged at 4°C for 10 min at 100,000 × g. Membranes fractions were resuspended in lysis buffer containing 1% (vol/vol) SDS and heated at 95°C for 5 min, followed by dilution in four volumes of cold lysis buffer containing 2% (vol/vol) Triton X-100. Insoluble materials were removed by centrifugation at 100,000 × g for 10 min. Protein A-agarose (Upstate Biotechnology)/anti-NR2A/B antibody mixtures (3 μg each) were added to the supernatant and incubated with gentle agitation overnight at 4°C. Immunoreactive complexes were recovered by centrifugation at 14,000 × g for 5 s. The pellets were washed first with lysis buffer [containing 2% (vol/vol) Triton X-100] and then with PBS. Proteins were eluted finally in 2× sample buffer [1× sample buffer consists of 2% (vol/vol) SDS, 50 mM Tris⋅HCl (pH 6.8), 10% (vol/vol) 2-mercaptoethanol, 10% (vol/vol) glycerol, and 0.1% bromophenol blue] and incubated at 95°C for 5 min. Immunoprecipitated proteins were subjected to SDS/PAGE with 8% polyacrylamide gels, and to immunoblotting as described above.
Electrophysiology in Acute Hippocampal Slices.
Transverse hippocampal slices (400-μm-thick) were prepared from adult Sprague–Dawley rats with a McIlwain tissue chopper. Slices were immediately placed in ice-cold cutting buffer containing (in mM) NaCl (124), KCl (3), KH2PO4 (1.25), CaCl2 (1), MgCl2 (3), NaHCO3 (26), glucose (10), and l-ascorbate (2). Slices were then transferred to an interface chamber constantly oxygenated with a 95% O2: 5% CO2 mixture and perfused with an artificial cerebrospinal fluid containing (in mM) NaCl (124), KCI (3), KH2PO2, (1.25), CaCl2 (3), MgCl2 (1), NaHCO3 (26), glucose (10), and l-ascorbate (2). Perfusion was stopped when recordings began, such that all experiments were done under static bath conditions, allowing for the application of small volumes of drugs.
Recording of extracellular field potentials evoked in CA1 after electrical stimulation of the Schaffer collateral pathway was performed with a bipolar stimulating electrode and a glass recording electrode positioned in stratum radiatum. Stimulus intensity was set to approximately one-third of the intensity required to evoke a population spike, and responses were evoked every 30 s (0.033 Hz; pulse duration of 0.1 ms). LTP was induced by a high frequency burst (100 Hz for 1 s). All drugs were applied directly into the recording chamber.
Results
Estrogen-Mediated Neuroprotection Against Excitotoxicity in Hippocampal Slice Cultures.
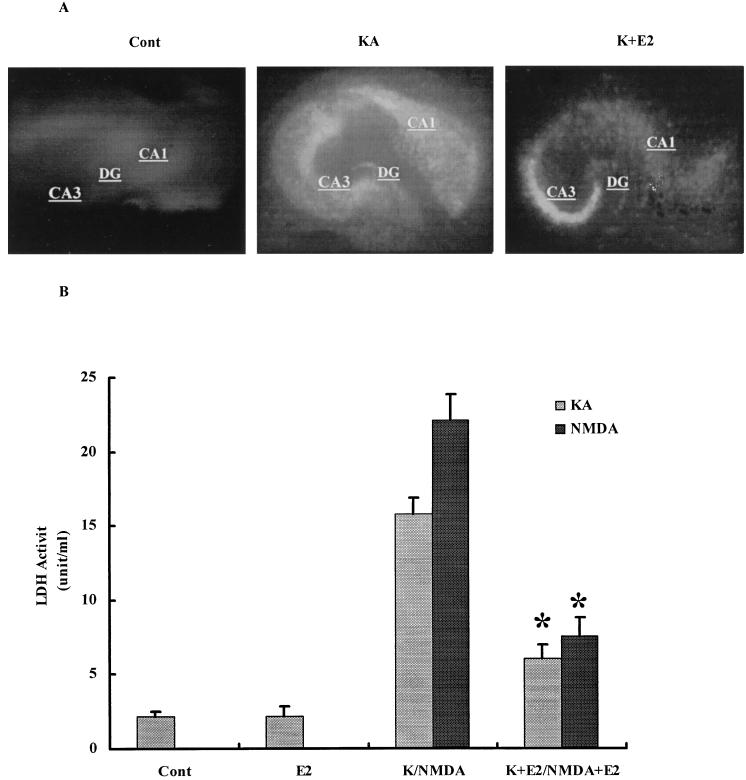
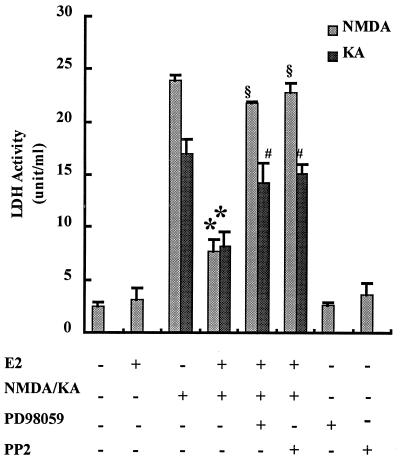
Treatment of hippocampal slice cultures with 50 μM KA or NMDA for 3 h resulted in neuronal degeneration assessed by both the extent of PI uptake and LDH release in the culture medium (Fig. 1). Control cultures showed no obvious PI uptake, whereas KA- or NMDA-treated cultures had intense and sustained PI uptake, particularly in CA1 and CA3 areas and dentate gyrus. Pretreatment of cultures with 1 nM E2 for 24 h significantly decreased KA- (Fig. 1A) or NMDA-induced PI uptake (not shown). LDH activity in the medium was markedly increased 24 h after KA or NMDA (Fig. 1B) treatment. Pretreatment with 1 nM E2 significantly reduced LDH release in the medium with both excitotoxins (Fig. 1B). The effect of E2 was similar whether cultures were pretreated for 24 h or for 10 min, suggesting that the neuroprotection effect did not require transcriptional activation. Because estrogen had been shown to protect neuronal cultures against glutamate toxicity via the activation of the tyrosine kinase/MAPK pathway (13, 16), we tested the effects of an inhibitor of src tyrosine kinase, PP2, and an inhibitor of the MAPK kinase, PD98059, on control and E2-treated cultured hippocampal slices (Fig. 2). Treatment with the inhibitors alone did not modify PI uptake or LDH release in the medium. Both inhibitors completely blocked E2-mediated neuroprotection assessed with PI uptake (not shown) or LDH release (Fig. 2).
Figure 1.
Effects of E2 on KA- and NMDA-elicited neurotoxicity. Hippocampal slices were prepared as described in Materials and Methods and were maintained in cultures for 10–14 days. They were treated with NMDA or KA (50 μM) for 3 h and returned to normal medium for 24 h. When present, E2 was added at 1 nM 24 h before NMDA or KA and was present for the duration of the experiment. (A) PI uptake in control, 24 h after a 3-h treatment with KA (50 μM) in the absence or presence of E2. DG, dentate gyrus. (B) LDH activity in medium measured in control (Cont), 48 h after treatment with E2 (E2), 24 h after KA or NMDA treatment in the absence (K/NMDA) or presence of E2 (K+E2/NMDA+E2). Results are means ± SEM of six to eight experiments. *, P < 0.001 compared with KA or NMDA (Student's t test).
Figure 2.
Effects of tyrosine kinase inhibitors on E2-mediated neuroprotection against KA and NMDA neurotoxicity. Hippocampal slices were treated as described for Fig. 1. PP2 (10 μM) or PD98059 (50 μM) was added at the same time and for the same duration as E2. Toxicity was assessed by measuring LDH release in the medium 24 h after KA or NMDA treatment. Results are means ± SEM of six to eight experiments. *, P < 0.001 compared with KA or NMDA alone; §, not significantly different from NMDA alone; #, not significantly different from KA alone (Student's t test).
Estrogen-Mediated Modifications of AMPA and NMDA Receptor Subunits.
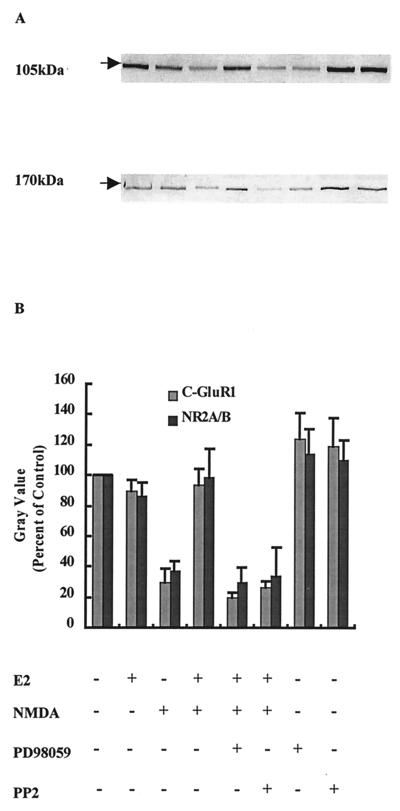
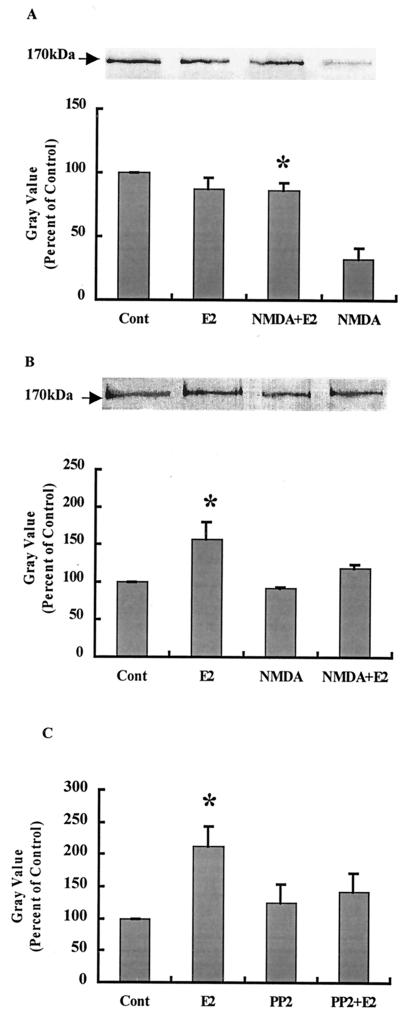
Treatment of hippocampal slice cultures with NMDA (50 μM) for 3 h produced a significant decrease in GluR1 and NR2 immunoreactivity in Western blots labeled with antibodies recognizing their C-terminal domains (Fig. 3). We discussed elsewhere the evidence indicating that the decrease in immunoreactivity is caused by calpain-mediated truncation of the C-terminal domains of several AMPA and NMDA receptor subunits (29, 30). Pretreatment with E2 did not modify the amount of GluR1 or NR2 subunits but completely prevented NMDA-induced decrease in both subunits. Combined treatment of cultures with E2 and PD98059 or PP2 completely blocked the effects of E2 on GluR1 and NR2 subunits (Fig. 3). Similar effects were obtained with KA treatment (not shown). To evaluate E2-induced changes in NMDA receptor subunit properties further, membrane preparations from hippocampal slice cultures incubated under various conditions were solubilized, and NMDA receptors were immunoprecipitated with antibodies against the C-terminal domain of NR2 subunits. Western blots were then stained with either anti-NR2 antibodies or anti-phosphotyrosine antibodies (Fig. 4 A and B). As shown previously, anti-phosphotyrosine antibodies labeled a band with an apparent molecular mass of 170 kDa that corresponds to NR2 subunits (ref. 31 and R.B. and M.B., unpublished work). NMDA treatment resulted in a large decrease in NR2 subunits stained with anti-NR2 antibodies, whereas it had no effect on the 170-kDa band stained with anti-phosphotyrosine antibodies. Pretreatment of hippocampal slice cultures with E2 did not modify the amount of NR2 subunits labeled with anti-NR2 antibodies but increased that labeled with anti-phosphotyrosine antibodies. However, pretreatment with E2 did prevent NMDA-induced decrease in the amount of NR2 subunits labeled with the anti-NR2 antibodies. NMDA treatment did not decrease the amount of NR2 labeled with anti-phosphotyrosine antibodies. Finally, E2 treatment of hippocampal slice cultures for 30 min significantly increased tyrosine phosphorylation of NR2 subunits as evidenced by the increase in immunolabeling of the 170-kDa band in membrane fractions (Fig. 4C). This effect was blocked completely by PP2, whereas PP2 alone did not modify the extent of immunolabeling.
Figure 3.
Effects of tyrosine kinase inhibitors on E2-mediated protection of the C-terminal domain of GluR1 or NR2 subunits against NMDA-elicited truncation. Hippocampal slices were treated with NMDA (50 μM) for 3 h. E2 and PP2 or PD98059 were added as described for Fig. 2. Immunoblots were performed as described in Materials and Methods and were labeled with antibodies against the C-terminal domains of GluR1 (105 kDa) or NR2 (170 kDa) subunits. (A) Representative blot, with the order of treatment as indicated in B. (B) Quantification of blots similar to the ones shown in A. Results are expressed as the percentage of values found in control conditions and are means ± SEM of six to eight experiments.
Figure 4.
Effects of E2 on NR2 subunits of NMDA receptors. Hippocampal slices were treated as described for Fig. 3. At the end of the experiments, membrane fractions were prepared, and proteins were immunoprecipitated with anti-NR2A/B antibodies. Immunoblots were then stained with antibodies against anti-NR2A/B (A) or anti-phosphotyrosine (B). Alternatively, slices were incubated for 30 min with E2 in the presence or absence of PP2 (10 μM). Immunoblots of samples from membrane fractions were stained with anti-phosphotyrosine antibodies and quantified as described (C). (A Upper) Representative Western blot. (A Lower) Quantitative analysis of blots similar to the blot shown in A Upper. Results are expressed as percentage of values found in control conditions (Cont) and are means ± SEM of four experiments. *, P < 0.001 as compared with control (Student's t test). (B Upper) Representative Western blot. (B Lower) Quantitative analysis of blots similar to the blot shown in B Upper. Results are expressed as percentage of values found in control conditions (Cont) and are means ± SEM of four experiments. *, P < 0.001 compared with control (Student's t test). (C) Quantitative analysis of immunoblots of membrane fractions stained with anti-phosphotyrosine antibodies. Results are expressed as percentage of values found in control conditions (Cont) and are means ± SEM of four experiments. *, P < 0.001 compared with control (Student's t test).
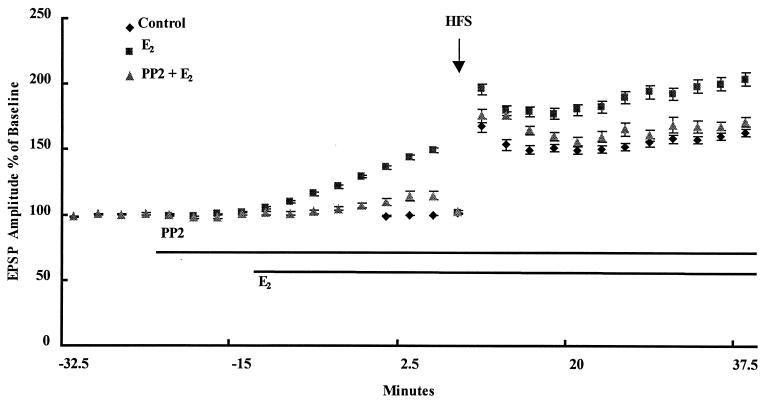
Effects of E2 on LTP in Acute Hippocampal Slices.
As previously reported (5, 7), incubation of acute hippocampal slices with 1 nM E2 resulted in a rapid increase in excitatory postsynaptic potential (EPSP) amplitude evoked in CA1 by electrical stimulation of the Schaffer collateral pathway (Fig. 5). To evaluate the effects of E2 on the magnitude of LTP, the intensity of stimulation was decreased to reset baseline values to the predrug level immediately before delivering the high frequency stimulation train. Under these conditions, E2 also resulted in an increase in the amplitude of LTP (Fig. 5; Table 1). When similar experiments were performed in the presence of the src inhibitor PP2, E2 no longer increased basal EPSP amplitude, nor did it increase LTP magnitude (Fig. 5; Table 1). Note that, under the same conditions, PP2 alone did not significantly influence LTP magnitude (Table 1).
Figure 5.
Effects of an src inhibitor on E2-mediated enhancement of EPSP amplitude and degree of LTP in acute hippocampal slices. Acute hippocampal slices were prepared as described in Materials and Methods. A stimulating electrode was located in CA3, and a recording electrode was located in stratum radiatum of CA1. Extracellular EPSPs were evoked by stimulation every 30 s, and the amplitude of the EPSP was measured. After a stable baseline was recorded, PP2 (10 μM) was added at the indicated time. Likewise, E2 (1 nM) was added at the indicated time. After resetting the stimulation intensity to obtain an EPSP of the same amplitude as before treatment with PP2 or E2, high frequency stimulation was delivered, and low frequency stimulation was resumed. Results are expressed as percentage of predrug values and are means ± SEM of 6–10 experiments. HFS, high-frequency stimulation.
Table 1.
Effects of E2 and PP2 on baseline responses and LTP amplitude measured 30 min after tetanus
| Treatment (no.) | Baseline response, % | LTP amplitude, % |
|---|---|---|
| Control (10) | 101 ± 1 | 163 ± 3 |
| E2 (10) | 150 ± 2* | 204 ± 5* |
| PP2 (6) | 107 ± 2 | 184 ± 7 |
| PP2 + E2 (7) | 115 ± 3† | 172 ± 4† |
See legend to Fig. 5. Results for the baseline response represent the amplitude of EPSPs before high frequency stimulation and are expressed as percentage of the predrug levels. LTP amplitudes represent the amplitudes of EPSPs measured 30 min after high frequency stimulation and are expressed as percentage of the average values measured immediately before high frequency stimulation. Data are means ± SEM of the indicated number of experiments. *, P < 0.05 compared with the respective control values. †, P < 0.05 when compared to E2 alone but not significant from control (Student's t test).
Discussion
The present results indicate that E2 exerts a multiplicity of effects on the properties of glutamate ionotropic receptors, LTP, and excitotoxicity through the activation of the tyrosine kinase/MAPK kinase pathway. First, we showed that E2 treatment of hippocampal slice cultures produced a significant degree of protection against both KA and NMDA neurotoxicity. This effect was observed whether the steroid was added 24 h or 10 min before the excitotoxins, suggesting that the neuroprotective effect was probably not caused by the activation of a nuclear receptor and the activation of genes with estrogen regulatory elements. E2 neuroprotective effect was blocked completely by an inhibitor of src tyrosine kinase or of the MAPK kinase, suggesting that the effect is caused by the activation of a pathway that is shared with a variety of neurotrophic factors. Our results are in good agreement with a recent report indicating that estrogen neuroprotection against glutamate toxicity in cortical neurons in cultures is mediated by the activation of the same pathways (16). Likewise, our results are in good agreement with those of Singh et al. (18), who showed that estrogen rapidly activates src tyrosine kinase and the MAPK kinase pathway in cortical organotypical cultures.
We previously reported that NMDA or KA treatment of hippocampal slice cultures is accompanied by calpain activation and the resulting truncation of the C-terminal domains of several subunits of AMPA and NMDA receptors (29, 30). Although the functional significance of the truncation is not clear at the moment, we also found that phosphorylation of the subunits protects them from calpain-mediated truncation (32). Moreover, we demonstrated that src-mediated phosphorylation of NR2 subunits of NMDA receptors completely protects the subunits from such truncation (R.B., Y. Rong, and M.B., unpublished work). The results we observed in this study indicate clearly that estrogen treatment of hippocampal slice cultures results in activation of src tyrosine kinase and tyrosine phosphorylation of NR2A subunits and protects them from calpain-mediated truncation of their C-terminal domains that takes place after excitotoxin treatment. Numerous reports have shown that NR2 subunits of NMDA receptors are a major substrate for src tyrosine kinases (19–23). Our results imply that MAPK kinase could also phosphorylate NR2 subunits, because PD98059 is an inhibitor more specific for MAPK than for src tyrosine kinases that also prevents estrogen-mediated blockade of calpain truncation of NR2 subunits. Interestingly, estrogen also protected the C-terminal domain of GluR1 receptors from KA- or NMDA-induced truncation, an effect also blocked by inhibitors of src tyrosine kinase or MAPK kinase. Because GluR1 is not known to be a substrate of tyrosine kinases, it is possible that tyrosine kinases activate other protein kinases capable of phosphorylating AMPA receptor subunits and thereby of protecting them from calpain-mediated truncation of their C-terminal domains.
Estrogen had been shown to enhance both AMPA and NMDA receptor-mediated responses as well as to enhance the degree of LTP elicited in CA1 pyramidal neurons by high frequency stimulation of the Schaffer collateral pathway (5–7). Our results confirmed both the estrogen-induced increase in the amplitude of AMPA receptor-mediated EPSP and in the degree of LTP in hippocampal slices. Both effects of estrogen were blocked completely by a src inhibitor. As mentioned above, NMDA receptors are a major substrate for src tyrosine kinase, and src-mediated tyrosine phosphorylation of NR2 subunits has been shown to increase NMDA receptor-mediated responses (19–23, 33, 34). Thus, the estrogen effect on LTP is likely caused by the activation of src and the tyrosine phosphorylation of NR2 subunits. The effect of estrogen on basal EPSP has been proposed to be caused by the activation of adenylate cyclase (10). Our results would imply that the tyrosine kinase pathway might be coupled to the adenylate cyclase pathway. Further studies should be directed at this issue.
Our results then indicate that estrogen, by activating the tyrosine kinase/MAPK pathways, exerts both a “cognitive” effect and a neuroprotective effect. The estrogen-mediated enhancement of the degree of LTP as a result of the activation of this pathway fits well with several data indicating that this pathway is important for LTP (35–37). Thus, fyn knockout mice have LTP and learning impairment, whereas MAPK inhibitors impair spatial learning (38). However, it is important to note that, under our conditions, the inhibitor of src tyrosine kinase PP2 did not modify the degree of LTP and that src knockout mice have normal LTP (24). Thus, different tyrosine kinases might play different roles in regulating NMDA receptors and LTP characteristics. Although the estrogen-mediated increase in NMDA receptor function resulting from tyrosine phosphorylation should have been expected to enhance NMDA neurotoxicity, the activation of the MAPK pathway provided neuroprotection presumably by activating neuroprotective cascades downstream from NMDA receptor activation. This idea also fits well with the notion that estrogen and neurotrophins might share similar signaling pathways (18). Our results therefore warrant further studies to evaluate the usefulness of estrogenic compounds for the treatment of Alzheimer's disease and other neurodegenerative diseases.
Acknowledgments
This research was supported by National Institute on Aging Grant AG-14751 (Principal investigator: Dr. C. Finch, University of Southern California).
Abbreviations
- NMDA
N-methyl-d-aspartate
- KA
kainic acid
- AMPA
α-amino-3-hydroxy-5-methylisoxazole propionic acid
- MAPK
mitogen-activated protein kinase
- E2
17-β-estradiol
- LDH
lactate dehydrogenase
- PI
propidium iodide
- LTP
long-term potentiation
- EPSP
excitatory postsynaptic potential
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.060034497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.060034497
References
- 1.Henderson V W. Neurology. 1997;48, Suppl. 7:S27–S35. doi: 10.1212/wnl.48.5_suppl_7.27s. [DOI] [PubMed] [Google Scholar]
- 2.Schneider L S, Farlow M R, Henderson V W, Pogoda J M. Neurology. 1996;46:1580–1584. doi: 10.1212/wnl.46.6.1580. [DOI] [PubMed] [Google Scholar]
- 3.Doraiswamy P M, Bieber F, Kaiser L, Krishnan K R, Reuning-Scherer J, Gulanski B. Neurology. 1997;48:1511–1517. doi: 10.1212/wnl.48.6.1511. [DOI] [PubMed] [Google Scholar]
- 4.Simpkins J W, Green P S, Gridley K E, Singh M, de Fiebre N C, Rajakumar G. Am J Med. 1997;103:19S–25S. doi: 10.1016/s0002-9343(97)00260-x. [DOI] [PubMed] [Google Scholar]
- 5.Teyler T J, Vardaris R M, Lewis D, Rawitch A B. Science. 1980;209:1017–1019. doi: 10.1126/science.7190730. [DOI] [PubMed] [Google Scholar]
- 6.Ito K, Skinkle K L, Hicks T P. J Physiol. 1999;515:209–220. doi: 10.1111/j.1469-7793.1999.209ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foy M R, Xu J, Xie X, Brinton R D, Thompson R F, Berger T W. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 8.Moss R L, Gu Q. Steroids. 1999;64:14–21. doi: 10.1016/s0039-128x(98)00092-0. [DOI] [PubMed] [Google Scholar]
- 9.Wong M, Moss R L. J Neurosci. 1992;12:3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu Q, Moss R L. J Physiol. 1998;506:745–754. doi: 10.1111/j.1469-7793.1998.745bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baudry M, Davis J L, editors. Long-Term Potentiation. Cambridge, MA: MIT Press; 1997. [Google Scholar]
- 12.Singer C A, Rogers K L, Strickland T M, Dorsa D M. Neurosci Lett. 1996;212:13–16. doi: 10.1016/0304-3940(96)12760-9. [DOI] [PubMed] [Google Scholar]
- 13.Goodman Y, Bruce A J, Cheng B, Mattson M P. J Neurochem. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 14.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 15.Watters J J, Campbell J S, Cunningham M J, Krebs E G, Dorsa D M. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- 16.Singer C A, Figueroa-Masot X A, Batchelor R H, Dorsa D M. J Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Impey S, Obrietan K, Storm D R. Neuron. 1999;23:11–14. doi: 10.1016/s0896-6273(00)80747-3. [DOI] [PubMed] [Google Scholar]
- 18.Singh M, Setalo G, Jr, Guan X P, Warren M, Toran-Allerand C D. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X M, Askalan R, Keil G J, Salter M W. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- 20.Zheng F, Gingrich M B, Traynelis S F, Conn P J. Nat Neurosci. 1998;1:185–191. doi: 10.1038/634. [DOI] [PubMed] [Google Scholar]
- 21.Lancaster B, Rogers M V. Eur J Neurosci. 1998;10:2302–2308. doi: 10.1046/j.1460-9568.1998.00241.x. [DOI] [PubMed] [Google Scholar]
- 22.Lu W Y, Xiong Z G, Lei S, Orser B A, Dudek E, Browning M D, MacDonald J F. Nat Neurosci. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- 23.Salter M W. Biochem Pharmacol. 1998;56:789–798. doi: 10.1016/s0006-2952(98)00124-5. [DOI] [PubMed] [Google Scholar]
- 24.Grant S G, O'Dell T J, Karl K A, Stein P L, Soriano P, Kandel E R. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 25.Stoppini L, Buchs P A, Muller D. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 26.Bruce A J, Sakhi S, Schreiber S S, Baudry M. Exp Neurol. 1995;132:209–219. doi: 10.1016/0014-4886(95)90026-8. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bi X, Rong Y, Chen J, Dang S, Wang Z, Baudry M. Brain Res. 1998;790:245–253. doi: 10.1016/s0006-8993(98)00067-5. [DOI] [PubMed] [Google Scholar]
- 30.Bi X, Chen J, Baudry M. Brain Res. 1998;781:355–357. doi: 10.1016/s0006-8993(97)01365-6. [DOI] [PubMed] [Google Scholar]
- 31.Moon I S, Apperson M L, Kennedy M B. Proc Natl Acad Sci USA. 1994;91:3954–3958. doi: 10.1073/pnas.91.9.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bi R, Bi X, Baudry M. Brain Res. 1998;797:154–158. doi: 10.1016/s0006-8993(98)00433-8. [DOI] [PubMed] [Google Scholar]
- 33.Thomas S M, Brugge J F. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T, Okumura N J. Biochem Biophys Res Commun. 1995;216:582–588. doi: 10.1006/bbrc.1995.2662. [DOI] [PubMed] [Google Scholar]
- 35.Atkins C M, Selcher J C, Petraitis J J, Trzaskos J M, Sweatt J D. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 36.Orban P C, Chapman P F, Brambilla R. Trends Neurosci. 1999;22:38–44. doi: 10.1016/s0166-2236(98)01306-x. [DOI] [PubMed] [Google Scholar]
- 37.Lisman J E, Fallon J R. Science. 1999;283:339–340. doi: 10.1126/science.283.5400.339. [DOI] [PubMed] [Google Scholar]
- 38.Blum S, Moore A N, Adams F, Dash P K. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]