Abstract
Regulation of the Drosophila pigment-dispersing factor (pdf) gene products was analyzed in wild-type and clock mutants. Mutations in the transcription factors CLOCK and CYCLE severely diminish pdf RNA and neuropeptide (PDF) levels in a single cluster of clock-gene-expressing brain cells, called small ventrolateral neurons (s-LNvs). This clock-gene regulation of specific cells does not operate through an E-box found within pdf regulatory sequences. PDF immunoreactivity exhibits daily cycling, but only within terminals of axons projecting from the s-LNvs. This posttranslational rhythm is eliminated by period or timeless null mutations, which do not affect PDF staining in cell bodies or pdf mRNA levels. Therefore, within these chronobiologically important neurons, separate elements of the central pacemaking machinery regulate pdf or its product in novel and different ways. Coupled with contemporary results showing a pdf-null mutant to be severely defective in its behavioral rhythmicity, the present results reveal PDF as an important circadian mediator whose expression and function are downstream of the clockworks.
Daily rhythms of physiology and behavior are generated by endogenous circadian oscillators. In Drosophila, this time-keeping is controlled by several clock genes (reviewed in ref. 1). The current model posits that the Clock- and cycle-encoded products—basic helix–loop–helix proteins that dimerize by means of their PAS domains—activate period (per) and timeless (tim) transcription by interactions with E-boxes located in the 5′-flanking region of these clock genes and a circadian enhancer upstream of (at least) per's transcription unit (2, 3). After PER and TIM proteins accumulate in the cytoplasm and form heterodimers, they translocate to the nucleus and negatively regulate their own genes by interfering with CLK:CYC function (3, 4).
A similar negative-feedback loop operates in the circadian clocks of other animals. Mammalian homologs of Drosophila clock genes have been identified or originally discovered in mice and humans (1). Circadian oscillations of mPer mRNAs occur in the suprachiasmatic nucleus of mouse, a circadian-pacemaker structure in the brain. The amplitude of these mRNA rhythms is reduced in the murine Clock mutant, consistent with the fly paradigm (5). In addition to the central time-keeping functions of clock transcription factors, they may also regulate downstream rhythm-relevant genes, which are thought to control physiological and behavioral rhythms. In this respect, CLK:BMAL1 heterodimers can activate transcription of a clock-regulated vasopressin gene in mice (5). In Drosophila, cyclically expressed genes whose mRNA oscillations are affected by clock mutations have been described (6, 7). Additional factors putatively functioning as clock outputs were originally identified by mutations (8, 9). However, the manner by which clock genes act—presumably upon these and other output factors—to effect the pacemaker's control of an overt rhythm is unknown.
It has been suggested that a neuropeptide, pigment-dispersing factor (PDF), is involved in clock output in insects (reviewed in ref. 10). PDF is also known as pigment-dispersing hormone, which was named after its function in crustaceans; it does not appear to play such a role in insects (11). PDH-immunoreactive neurons in Drosophila melanogaster co-express the per gene (12). These brain cells—called Lateral Neurons—are believed to play an important role in the control of circadian behavioral rhythms (reviewed in ref. 13).
To permit examination of a possible role for PDF as a circadian effector, we previously isolated the pdf gene in Drosophila (14). Subsequently we presented evidence that PDF is necessary for normal locomotor-activity rhythms (15). Yet, this behavioral genetic study did not address the question of whether pdf or its encoded product are influenced by the circadian pacemaker. In this report, we show that this gene and PDF are regulated by clock genes in remarkably diverse and unexpected ways. That a pdf mutant is rhythm-defective (15) could mean that the neuropeptide is a generic maintenance factor for behavioral rhythmicity, but the current study reveals that PDF exhibits daily cycling in a subcellular compartment of certain brain neurons.
Materials and Methods
Fly Strains.
Most of the per, tim, Clk, and cyc mutant strains used were as described previously (16, 17). A new cyc0 mutant was found in a screen (of ca. 6,000 lines descended from chemically mutagenized flies) independent of that which resulted in cyc01. cyc02 is a recessive locomotor-arrhythmic, as is cyc01; the mutations are noncomplementing both for behavior and for a severe reduction in levels of PER protein (data not shown; cf. ref. 17). By sequence analysis like that performed on cyc01—showing it to be a nonsense mutation in the PAS-encoding region (17)—cyc02 was found to be similarly mutated (Q113/CAA → UAA) within a part of the gene that specifies amino acids between the relatively N-terminal basic helix–loop–helix domain and the PAS domain. To obtain ClkJrk hemizygotes, flies carrying a 3rd-chromosome deletion missing the Clock locus [Df(3L)pbl-X/In(3LR)TM6B, Tb Hu (16)] were crossed to mutant homozygotes.
RNA Assessments.
In situ hybridizations were performed as in ref. 18 on dissected central nervous systems (CNSs). The reagents and procedures were held constant among specimens to permit quantitative analysis of the signals. Numbers of brain cells stained after application of the pdf probe (full-length cDNA, digoxigenin-labeled) were counted blindly with respect to genotype. For temporal analysis and mutant vs. normal comparisons of in situ-hybridized brains, staining levels were scored blindly (on a scale of 0 to 4, in increments of 1) with respect to time of sacrifice and genotype, In the temporally controlled experiment, wild-type male flies were put through five 12-h:12-h light–dark (12:12 LD) cycles, then killed at four time-points for in situ hybridization. Staining scores for the per01 and tim01 mutants were statistically analyzed by the Kruskal–Wallis nonparametric ANOVA test, followed by Dunn's multiple comparisons test. Northern blotting was performed and quantified as in ref. 14.
Immunohistochemistry.
The Drosophila PDF 18-mer was synthesized on the basis of its inferred amino acid sequence (14). The product was amidated at its C terminus, as is the case for naturally occurring PDH (11), then conjugated separately to three different carrier proteins (ovalbumin, BSA, keyhole limpet hemocyanin). These materials were injected sequentially into two rats; subsequent bleedings led to antisera, which from each rat produced identical immunohistochemical results. Whole-mounted CNSs were dissected 2–5 h after lights-on (ZT2–5) and subjected to staining procedures as in ref. 19, except for varying incubation times: overnight for the primary antibody (dilution 1:500 for anti-fly-PDF, 1:2000 for anti-crab-PDH); and 2- to 5-h incubations for the fluorescein isothiocyanate (FITC)-, tetramethylrhodamine (TRITC)-, or peroxidase-conjugated secondary antibody (dilution 1:200). Both peroxidase- and FITC-mediated signals led to the cell counts in the legend to Fig. 2, which were obtained blindly with respect to genotype.
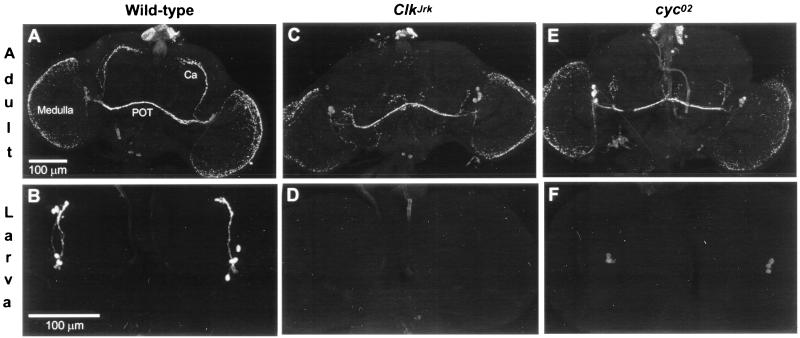
Figure 2.
Effects of Clock and cycle mutations on PDF immunostaining in brain neurons. (A) Wild-type adults (n = 14); numbers of signal-containing cells per brain hemisphere: s-LNvs, 3.0 ± 0.2; l-LNvs, 3.6 ± 0.1 (n = 27 scorable hemispheres). Ca, calyx of the dorsal-brain mushroom body, in the vicinity of which are termini of neurites projecting from s-LNv cells; POT, posterior optic tract, one type of projection from l-LNv cells. (B) Wild-type larvae (n = 6); four PDF-containing LNs were invariably detected in the 12 brain hemispheres examined. (C) ClkJrk adults (n = 10); no s-LNvs were detected in the 20 brain hemispheres examined for mutant homozygotes, nor were there any such signals in 7 brains of flies heterozygous for ClkJrk and a deletion (Df) of the locus; counts of stained l-LNvs in ClkJrk/ClkJrk: 3.8 ± 0.2 (n = 17 scorable hemispheres). Arrow, abnormal projections. (D) ClkJrk larvae (n = 14); no LNs were detected in any of the 28 hemispheres examined, nor were any observed in the brains of 8 larvae heterozygous for ClkJrk and the Df. (E) cyc0 adults; this cyc02 specimen exemplifies the subnormalities and abnormalities of s-LNv cells exhibited by the 10 adults examined that were homozygous this allele—e.g., no PDF neurites extending toward the top of the brain. Arrowhead, abnormal projections. For the companion cyc01 mutant (n = 9 adult CNSs dissected and stained) cell counts: s-LNvs, 1.0 ± 0.2; l-LNvs, 3.4 ± 0.2 (n = 15 scorable hemispheres). (F) cyc0 larvae. Consistent with the in situ hybridizations (Fig. 1), the staining intensities in PDF cells were much weaker than in wild-type and variable within a given specimen; regardless of the number of LNs detected in either cyc01 or cyc02 larvae (n = 16 and 10, respectively), no stained axonal processes were detectable. Cell counts: cyc01, 3.1 ± 0.3 (n = 32 hemispheres); cyc02, 2.9 ± 0.3 (n = 20).
Temporally based immunostaining of sectioned material was performed by sacrificing flies at 3-h intervals in 12:12 LD cycles or constant darkness; heads were fixed, dehydrated, embedded in paraffin, sectioned at 7 μm, and stained with anti-PDH as in ref. 19. Signal intensities were scored as in ref. 20, blindly with respect to time-points and per genotypes. Period values were determined by applying curve-fitting software. To compare the signal intensities at two times within an LD cycle, dissected whole-mounted adult brains were obtained from 10-day-old flies (that had been entrained to 12:12 LD) at ZT1 and ZT13; anti-PDH immunohistochemistry (involving TRITC) was performed as in ref. 21. Pictures of the stained specimens were taken with a digital camera mounted on a fluorescence microscope; the images were converted to “gray scale” by a corel photo paint program (Corel, Dublin, Ireland). The gray values of the stained structures and of the background were scored by the doku program (Olympus) with a scale from 0 (black) to 255 (white). Gray values of the background were subtracted from corresponding ones of the stained nerve terminals to obtain the intensity indices on the ordinate of Fig. 4D. These values were statistically analyzed as indicated for the quantified in situ hybridizations.
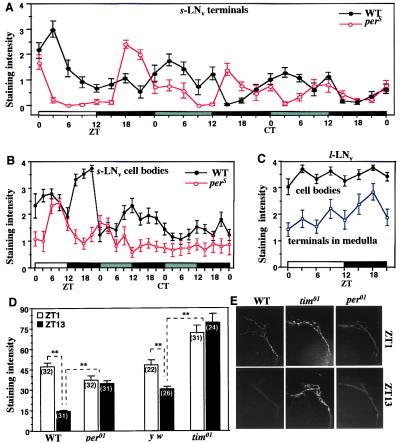
Figure 4.
Temporal analysis of PDF-like immunoreactivity in brain cells and their projections. (A) Immunohistochemical time-course of wild-type head sections and those of the perS and per01 mutants, in both LD-cycling and constant-dark (DD) conditions (n = 10–13 per time-point for each genotype). ZTs, as in Fig. 1; CT, circadian times during DD (gray bar, subjective day, corresponding to actual daytime in the preceding LD condition; black bar, subjective night). The apparent oscillations were formally analyzed for the DD portions of the records, resulting in 24.2-h and 19.9-h free-running period estimates for wild-type and perS, respectively. In per01 specimens, examined at eight equally spaced times in LD, the nerve-terminal signals were chronically low: at five time-points, the staining levels were 0; at the other three, the average scores ranged from 0.1 to 0.2. (B) s-LNv cell-body staining scores for the same specimens as in A. (C) l-LNv-cell staining for cell bodies and centrifugally projecting fibers; the specimens scored are a subset of those that led to the scoring for A and B (here, only the flies that were in the LD cycle). (D) Immunohistochemical comparison of wild type (WT) to two clockless mutants. The nerve-terminal signals were examined in wild type, per01, and tim01 at the peak and trough times (cf. A) in an LD cycle; these brains were whole-mounted, and the relevant dorsally located anti-PDH-mediated signals were quantified as described in the text; y w is a tim+ control for the arrhythmic loss-of-function timeless mutant (which was in a y w genetic background); based on the numerical read-outs from analysis of the nerve-terminal staining intensities, certain of the average signals were determined to be significantly different (P < 0.05), as indicated by ∗∗; the numbers of brain hemispheres examined are shown in parentheses; error bars, SEM. (E) Representative dorsal-brain images of s-LNv nerve terminals (cf. Fig. 2A), stained after sacrificing flies of three genotypes in the early morning (ZT1) or the early night (ZT13).
pdf Transgenics.
A genomic fragment from the locus (14), inserted into pBluescript, was digested with EcoNI, ligated with EcoRI linker, cut with BamHI/EcoRI, then subcloned into pBluescript (P2.4-pBS). This DNA fragment, called P2.4 (promoter-region, 2.4 kb), was sequenced; its 3′ end extends 16 bp downstream of the transcription-start site and terminates 58 bp upstream of the translation-start (see ref. 14). An XbaI/EcoRI fragment from P2.4-pBS was subcloned into the pPTGAL vector (which includes the mini-w+ marker gene), then injected into y w embryos with pUChpsΔ2–3 helper plasmid. For the “promoter-bashed” constructs, P2.4-pBS was digested with BsaAI/EcoRI (for P1.4), AccI/EcoRI (for P1.0), or NsiI/EcoRI (for P0.8); then each of these fragments was subcloned into pBluescript, from which XbaI/EcoRI fragments were isolated and subcloned to pPTGAL vector. To produce the most severely bashed P0.5 construct, P2.4-pBS was NheI/SpeI-digested; then the vector was isolated and religated intramolecularly, from which XbaI/EcoRI fragments were isolated and subcloned into pPTGAL. After generating transgenic strains from the five construct types, such flies were crossed to ones from a UAS-lacZ reporter strain, which produces cytoplasmic β-galactosidase (β-GAL) in the presence of GAL4 (22). 5-Bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) histochemistry was performed on dissected CNSs of the progeny as in ref. 20. Five or more independent pdf-GAL4 lines for each construct were obtained; three or more lines for each construct were crossed to UAS-lacZ, and at least four larval and four adult CNSs from among the progeny of each cross were examined for β-GAL expression.
Results
Expression of a Neuropeptide-Encoding Gene in the CNS.
To assess the effects of clock mutations on pdf expression, we first determined the normal cellular distribution of the Drosophila gene's native products. By in situ hybridization, we showed that the expression pattern of pdf RNA is very similar to that determined previously with anti-crab-PDH (19, 23). There are four positive cells in each larval brain hemisphere (Fig. 1B); these persist into adulthood (Fig. 1 A, C, and E) and become the small ventrolateral neurons (s-LNvs), whose neurites project into a dorsal region of the adult brain. Four large ventrolateral neurons (l-LNvs) also express pdf (Fig. 1A); these emerge during metamorphosis and send projections into the optic lobe and across the brain midline. Larvae and adults also contain pdf mRNA in the posterior extremity of the CNS (Fig. 1).
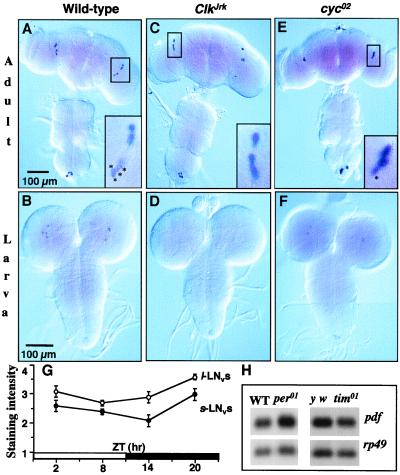
Figure 1.
Spatial and temporal expression of pdf RNA. Whole-mounted nervous systems were processed for pdf in situ hybridization. (A) Wild-type (Canton-S) adults (n = 5 dissected CNSs). Almost invariably, there were four signal-containing s-LNvs and four l-LNvs in each of the 10 brain hemispheres. The boxed area is a 4× magnification of the lateral-brain region; asterisked cells are the s-LNvs. (B) Wild-type larvae (n = 6); four signal-containing brain cells were invariably stained in each of the 12 brain hemispheres. (C) ClkJrk mutant adults (n = 7); there were no discernible s-LNvs in any of the 14 hemispheres. (D) ClkJrk larvae (n = 6); no pdf-RNA-containing cells were detected in the 12 hemispheres. (E) cyc0 mutant adults. The results depicted here for cyc02 (n = 7) were very similar those obtained from cyc01 (n = 8); in both allelic mutant types, there were subnormal numbers of s-LNvs labeled by the pdf probe; this is exemplified by the one asterisked cell in the 4×-magnified box for the lateral-brain region of this cyc02 adult (compare with the box in A); the reductions in s-LNv cell numbers in cyc0 adults were best appreciated after immunohistochemistry (see Fig. 2). (F) cyc0 larvae; for cyc02 (as exemplified in this image), 2.6 ± 0.3 lateral neurons (LNs) were labeled per hemisphere (n = 14), and these RNA signals were weak compared with wild-type; corresponding values for cyc01: 3.4 ± 0.2 (n = 8). Near the bottom of A–F is the abdominal ganglion, within which there is a small cluster of pdf cells. (G) Timecourse of pdf mRNA in separate clusters of peptide-containing neurons; in situ-hybridized specimens were blind-scored for staining levels, using 7–16 adult brains per time-point (the scoring scale ranged from 0 to 4, but only 3% of the individual values ranged as low as 1); ZTs are Zeitgeber times in which light comes on at noon during a 12-h light (open bar)–12-h dark (black bar) cycle. (H) pdf RNA levels in period and timeless mutants; the Northern-blot results were obtained from head extracts after sacrificing adults at ZT2–4; normalizing the per+ and tim+ levels (set = 1.00) to the rp49 loading control, the abundance in per01 was 1.07 and that for tim01 was 0.95. In situ hybridizations on y per01 w, y w;tim01, and y w control brains (dissected from adults at ZT2–5) led to the following staining scores (as on the ordinate of G): s-LNvs, in the genotypic order just given: 2.1 ± 0.2 (n = 16 brain hemispheres), 1.8 ± 0.3 (n = 10), 2.4 ± 0.2 (n = 8); l-LNvs: 3.0 ± 0.2, 2.7 ± 0.3, 3.6 ± 0.2; by ANOVA, the differences among s-LNv scores were not significant (P = 0.1); those among l-LNv scores were significant (P = 0.03), but only one pairwise comparison was marginally so (tim01 vs. control, P ≈ 0.05).
Northern blots revealed no daily rhythm of pdf mRNA abundance (14), but they could have failed to detect pdf mRNA cycling in a subset of the cells. Thus we performed temporal in situ hybridizations; neither category of pdf-expressing neurons exhibited systematic fluctuations in signal intensities (Fig. 1G). Therefore, there is no pdf mRNA rhythm for clock mutations to affect.
Our newly produced anti-Drosophila PDF antibodies led to cell labeling identical to that obtained by in situ hybridization (Fig. 2 A and B). Neither method led to marking of cells in the dorsal brain of adults that are stained by anti-crab-PDH (23). This and other evidence (Fig. 3, below; and ref. 15) indicates that the dorsally located antigen is cross-reacting material and does not have to be considered in terms of effects of clock mutations on pdf expression.
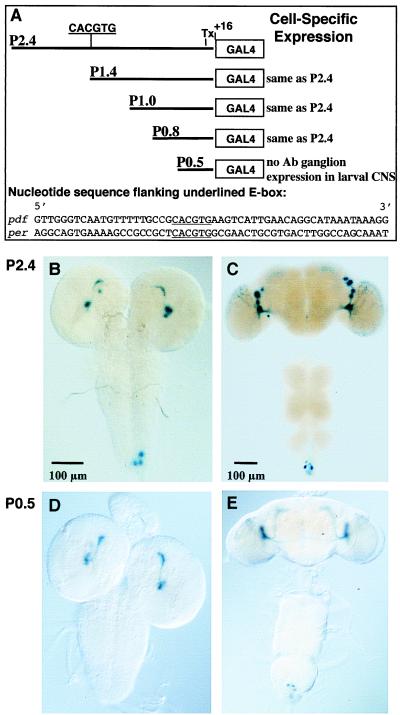
Figure 3.
Analysis of the pdf promoter region. (A) E-box sequence ca. 1.4 kb upstream of the transcription start site (Tx) in the P2.4 construct. Half of the E-box (GTG) is present in P1.4 but eliminated in the other three constructs; note that there is no homology in the nucleotide sequences flanking per's and pdf's E-boxes (per upstream sequence: ref. 2). (B) 5-Bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) histochemistry for a P2.4 + UAS-lacZ larval CNS. (C) Adult CNS of the same transgenic type as in B. Note that in the imaginal specimen there are only LN signals, no dorsal signals (cf. Figs. 1 and 2). (D) X-Gal-mediated staining in a P0.5 + UAS-lacZ larval CNS. There is no reporter expression in the abdominal ganglion. (E) Adult CNS of the same transgenic type as in D.
Cell-Specific Regulation of pdf mRNA and PDF by Clock and cycle.
Expression of pdf in the arrhythmic ClkJrk mutant was found to be strikingly abnormal. In ClkJrk brains, neither pdf mRNA nor PDF was detectable in larval LN cells and in the s-LNvs of adults (Figs. 1 and 2). The same defects were observed in mutant animals heterozygous for ClkJrk and a deletion of the locus (legend to Fig. 2). These results suggest that CLK is required for pdf transcription, although only in certain cells: the larval LNs and the s-LNvs into which they develop. Dorsally projecting axonal processes arising from the s-LNv cells terminate near the calyx of the dorsal-brain mushroom body. In accord with the absence of perikaryal s-LNv immunoreactivity, these projections are absent from ClkJrk brains (Fig. 2C). In contrast, expression in the l-LNvs and abdominal-ganglionic cells of adults was apparently unaffected by ClkJrk (Figs. 1 C and D and 2 C and D); this included normal staining of centrifugal and interhemispheric projections within the fly's head (Fig. 2A). However, certain features of projections from l-LNv cells are aberrant in ClkJrk. Approximately 50% of the mutant brains showed abnormal projections (as exemplified in Fig. 2C); in others, one or two axons from this region projected farther and irregularly toward a dorsal or median region of the brain. None of these projections was similar to the more dorsal-reaching projections in the brains of wild-type adults (Fig. 2A).
Because the CYC protein cooperates with CLK in their transcriptional-activation roles (1), we also analyzed pdf expression in cycle mutants (Figs. 1 and 2). The effects were similar to but less severe than those caused by ClkJrk. Most of the larval LNs homozygous for either of two cyc0 mutations showed much weaker expression of both mRNA and peptide compared with wild type, but the mutant expression levels were variable even within a single brain hemisphere: some cells contained signal, whereas others were extremely difficult to detect. While this report was in preparation, we learned that similar ClkJrk and cyc01 effects on pdf expression in larvae were obtained by others (9). The numbers of antibody-stained s-LNvs in cyc0 adults were well above zero (details in the legend to Fig. 2), compared with the elimination of such signals in ClkJrk flies. Numbers of l-LNv cells in the brains of cyc-mutated adults were normal (Fig. 2), similar to the results obtained in the ClkJrk background. About 25% of the adult cyc0 brains exhibited an abnormal dorsal projection similar to that shown in Fig. 2E. In approximately 30% of these mutant specimens, the projections were asymmetric within a single individual: one hemisphere would contain a bundle of dorsally projected axons; but in the contralateral hemisphere, only one or two axons projected into the dorsal brain. In other cyc0 adults, axons stemming from the region indicated by an arrow in Fig. 2E projected irregularly into a median brain region.
The major conclusions from examining pdf expression in the Clock and cycle mutants are that (i) both genes appear to be positive regulators of pdf RNA levels but only in the s-LNvs and their larval precursors; (ii) the effects of ClkJrk are stronger than those of the cyc0 mutations; and (iii) there are developmental defects, as PDF-containing processes in the adult CNS are aberrant in both types of mutants.
A pdf E-Box Is Not Necessary for Normal Spatial Expression.
Do CLK and CYC activate pdf transcription directly? If that were the case, there could be an E-box in this gene's regulatory region (cf. refs. 2 and 3). Indeed, within a 2.4-kb segment 5′ to the pdf ORF we found a CACGTG sequence ≈1.4 kb upstream of the transcription-start site (Fig. 3A). The 2.4-kb DNA fragment was fused to the (yeast) GAL4 gene; transgenic strains were generated and crossed to flies carrying UAS-lacZ. The doubly transgenic progeny showed faithful β-galactosidase-reported expression of pdf (Fig. 3). To determine whether the E-box is important for the pdf's transcriptional activation, further transgenics were generated (Fig. 3A). Deletions missing the half or all of the E-box were sufficient to drive brain expression indistinguishable from that observed in wild type (Fig. 3). Interestingly, the smallest 5′-flanking region examined mediated the normal brain pattern but did not lead to abdominal-ganglionic expression in the larval CNS (Fig. 3D). That the influences on pdf expression of Clock and cycle do not operate through a circadian E-box, and thus seem to be indirect, is consistent with the lack of pdf mRNA cycling and Clock/cycle-independent expression in the l-LNv cells.
Posttranscriptional Regulation of PDF by period and timeless.
No effect of a period-null mutation on pdf mRNA levels was detectable in previous Northern blottings (14). We confirmed and extended this result by showing that neither per01 nor a timeless-null mutation affects the RNA's abundance, by Northern blottings and by in situ hybridizations (Fig. 1H and accompanying legend).
To search further for regulation by per or tim, we stained adult brains with anti-PDH at different times of day and night. Strikingly, nerve terminals in a dorsal region of the central brain exhibited rhythms of anti-PDH-mediated staining. The neurites that terminate in this region project from the s-LNv cells (13). In an LD cycle, the peak and trough times for the nerve-terminal cycling were 1 h after lights-on and lights-off, respectively (Fig. 4A). Staining levels in the perikarya of s-LNvs exhibited some fluctuations but no regular pattern (Fig. 4B). The adult-specific, larger PDF neurons also exhibited no appreciable cycling of anti-PDH-mediated staining, either in l-LNv cell bodies or in the termini of their neurites that ramify over the surface of the medulla optic lobe (Fig. 4C).
The dorsal-brain, nerve-terminal cycling persisted in constant darkness with an ≈24-h period in wild type. In that condition the cycle duration was shortened to ≈20 h by the perS mutation (Fig. 4A), which causes behavioral periodicities to be about 5 h shorter than normal (e.g., ref. 24). In the dorsal brains of the per01 null mutant, nerve-terminal cycling was abolished, and the signal strengths were very low (legend to Fig. 4A). However, the immunohistochemical procedure performed on these brain sections is not very sensitive. Therefore, we used a quantitative fluorescence method, better to judge PDF staining intensities in whole-mounted brains (Fig. 4D). At the peak and trough time-points, nerve-terminal signals in wild type were again higher in the early morning compared with the early night. This temporal difference was not observed in the dorsal brains of the arrhythmic per01 and tim01 mutants. In per01, the staining intensities at both times were nearly identical and at levels intermediate between the per+ peaks and troughs (Fig. 4 D and E). In tim01, the PDF terminal signals were also the same at the two time-points but significantly higher than in tim+ (Fig. 4 D and E). The mutational effects of these clock genes on daily fluctuations of PDF abundance at certain nerve terminals indicate that an aspect of this peptide's regulation is on the one hand clock controlled, and on the other posttranslationally regulated.
Discussion
This paper focuses on the remarkable cell-type specificity manifest by Drosophila clock mutations. They affect a particular subset of adult neuropeptide-containing cells, small neurons in a ventrolateral region of the brain (Figs. 1 and 2). The larval precursors of such cells are similarly affected (Fig. 1), an observation also made by others (9) for two of the mutants, ClkJrk and cyc01. Although the larval assay makes a connection between these clock mutants and pdf expression, it does not detect the exquisite cell-type selectivity of the mutations. This is because the affected precursor cells are the only PDF-expressing ones in larvae. In adults, a second class of PDF cells has arisen. These, the l-LNvs, are largely insensitive to effects of Clk and cyc0 (Figs. 1 and 2). Analogous cell-specific effects were uncovered for per and tim mutations (Fig. 4), whose effects on the PDF oscillation are manifest only in the small cells despite the presence of cycling PER and TIM in both the l-LNvs and the s-LNvs (25).
These results provide further evidence pointing to the s-LNv cells as the most important circadian-pacemaker neurons in the fly's circadian system, at least insofar as behavioral rhythmicity is concerned (cf. ref. 15). Supporting this inference are the facts that (i) s-LNv cells are the only clock-gene-expressing LNs projecting to a central brain region—as opposed to out into the visual system, which is dispensable for behavioral rhythmicity (13); (ii) a brain-damaged mutant, in which LNs are mostly eliminated, exhibits behavioral rhythms very rarely, and this is almost always correlated with the presence of centripetal (PDF-stained) projections from the s-LNvs (21); (iii) a cryptochrome (cryb) mutation that eliminates per and tim gene-product cyclings throughout most of the fly permits behavioral rhythmicity, which is correlated with PER and TIM cycling in the s-LNvs (25); (iv) the precursors of s-LNv cells are present throughout postembryonic development (19, 20), and adult locomotor-activity rhythms can be entrained as early as the first-instar larval stage (24, 26).
The cell-type specificity of these mutants is unprecedented, as the central pacemaker mechanism—the CLK:CYC/PER:TIM feedback loop—is believed to operate similarly in a large number of clock cells all over the fly. So why is PDF expression uniquely sensitive to ClkJrk and cyc0 in the s-LNvs? There must be additional clock-relevant elements or developmental events that distinguish between the small and large cells. An extreme view of the second possibility is that CLK:CYC or some related circadian clock transcription complex is necessary for s-LNv specification; thus, these cells would be absent from the ClkJrk and cyc0 mutants. A developmental role for these genes is consistent with the aberrant projections of certain PDF-containing neurites in the mutant animals (Fig. 2). However, the key PDF cells are not eliminated by these mutations, in that the s-LNvs are retained in the ClkJrk and cyc0 mutants. This retention was revealed by weak per and tim expression within these neurons (27), even though the levels of the latter two gene products are grossly subnormal in ClkJrk and cyc0 flies (16, 17). Moreover, the cry gene, which is co-expressed with pdf in larval LNs (28), marks those cells as present in the ClkJrk and cyc01 mutants (P. Emery and M.R., unpublished observations).
Why does ClkJrk have more severe effects on pdf expression than do the cyc0 mutations? This question is especially intriguing, because the former is not a null mutant; the semidominance of this mutation is a reflection of the residual activity of CLKJrk (16). In contrast, both cyc0 mutants are likely to be complete loss-of-function mutants (Materials and Methods; ref. 17). The milder effects of cyc0s on pdf (Figs. 1 and 2) suggest the existence of another PAS-containing transcription factor, which in cyc0 mutants would partner with CLK and provide a chronobiologically relevant function. The stronger effects of ClkJrk on pdf expression could be due to this mutant protein's ability to sequester in inactive complexes not only CYC but also the other putative PAS factor.
In the Clock mutant mouse, the aberrant product is similar to that encoded by ClkJrk in Drosophila; both proteins are missing substantial portions of their activation domains (1). By analogy to the cell specificity of ClkJrk effects on pdf, Clock eliminates a vasopressin mRNA rhythm in the mouse suprachiasmatic nucleus (SCN), whereas the (normally) constitutive level of that transcript in another hypothalamic nucleus is unaffected (5). Regulation of the vasopressin RNA rhythm in the SCN probably results from a canonical interaction of the mCLK:BMAL1 heterodimer with an E-box present in the 5′-flanking region of the vasopressin gene (5). As implied by Fig. 3, clock regulation of pdf expression within Drosophila lateral neurons is unlikely to be based on similar principles.
Against a background of the transcriptional feedback-loop paradigm that dominates the current circadian landscape (1), it is remarkable that the robust temporal oscillation of PDF involves the peptide itself and is restricted to the axon terminals projecting from certain cells. Moreover, this cycling and its alteration by per and tim mutations (Fig. 4) do not stem from changes in pdf mRNA abundance (Fig. 1). Although the implied posttranslational regulation could take place at any level (such as peptide processing or axonal transport), we prefer the idea that the clock regulates release of PDF from the centripetally projecting s-LNv terminals. Under this hypothesis, there are abundant PDF-containing axonal termini early in the morning; clock-mediated release would occur during the day, such that there are relatively few PDF-positive termini in the early night (Fig. 4). Inappropriately high nighttime levels of PDF in per01 and tim01 (especially the latter: Fig. 4D) suggest that release is inhibited in these mutants. Consistent with this supposition is that PDF axonal signals normally rise in the morning (Fig. 4) as PER and TIM levels are falling (1). This is not to say that “high PER and TIM” directly potentiate nighttime release of PDF. These clock proteins could contribute to temporal gating of release by hindering the production of an inhibitor of that process.
In summary, our results indicate remarkable cell-type specificity of several circadian clock genes as well as an important role for posttranslational regulation of the PDF neuropeptide. Taken together with the affects of a pdf mutation (15), these findings present insights into how a behaviorally meaningful humoral output factor is regulated by central pacemaker cells and clock genes functioning within them. Given the similarities between circadian-clock mechanisms in Drosophila and mouse, it will be interesting to see whether these features of the insect system also apply to mammals.
Acknowledgments
We thank Jörg Wulf, Robina Reinecke, Maki Kaneko, and Edward Dougherty for assistance with histology; Neal E. White for isolating and characterizing cyc02; Joan E. Rutila for sequencing; and Justin Blau for comments on the manuscript. This work was supported by National Institutes of Health Grant GM-33205 (J.C.H. and M.R.), National Research Service Award MH-1196 (J.H.P.), National Institutes of Health Training Grant NS-07292 (G.L.), and an award from the Deutsche Forschungsgemeinschaft (C.H.-F.).
Abbreviations
pigment-dispersing factor
- s-LNvs and l-LNvs
small and large ventrolateral neurons
- LNs
Lateral Neurons
- CNS
central nervous system: LD, light–dark cycle
- ZT
Zeitgeber time in LD cycle
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070036197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070036197
References
- 1.Dunlap J C. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 2.Hao H, Allen D, Hardin P E. Mol Cell Biol. 1997;17:3687–3693. doi: 10.1128/mcb.17.7.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darlington T K, Wager-Smith K, Ceriani M F, Staknis D, Gekakis N, Steeves T D L, Weitz C J, Takahashi J S, Kay S A. Science. 1998;280:1599–1603. doi: 10.1126/science.280.5369.1599. [DOI] [PubMed] [Google Scholar]
- 4.Lee C, Bae K, Edery I. Mol Cell Biol. 1999;19:5316–5325. doi: 10.1128/mcb.19.8.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin X, Shearman L P, Weaver D R, Zylka M J, de Vries G J, Reppert S M. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- 6.Van Gelder R N, Krasnow M A. EMBO J. 1996;15:1625–1631. [PMC free article] [PubMed] [Google Scholar]
- 7.Rouyer F, Rachidi M, Pikielny C, Rosbash M. EMBO J. 1997;16:3944–3954. doi: 10.1093/emboj/16.13.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNeil G P, Zhang X, Genova G, Jackson F R. Neuron. 1998;20:297–303. doi: 10.1016/s0896-6273(00)80457-2. [DOI] [PubMed] [Google Scholar]
- 9.Blau J, Young M W. Cell. 1999;99:961–971. doi: 10.1016/s0092-8674(00)81554-8. [DOI] [PubMed] [Google Scholar]
- 10.Helfrich-Förster C, Stengl M, Homberg U. Chronobiol Int. 1998;15:567–594. doi: 10.3109/07420529808993195. [DOI] [PubMed] [Google Scholar]
- 11.Rao K R, Riehm J P. Biol Bull. 1989;177:225–229. [Google Scholar]
- 12.Helfrich-Förster C. Proc Natl Acad Sci USA. 1995;92:612–616. doi: 10.1073/pnas.92.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko M. Curr Opin Neurobiol. 1998;8:652–658. doi: 10.1016/s0959-4388(98)80095-0. [DOI] [PubMed] [Google Scholar]
- 14.Park J H, Hall J C. J Biol Rhythms. 1998;13:219–228. doi: 10.1177/074873098129000066. [DOI] [PubMed] [Google Scholar]
- 15.Renn S C P, Park J H, Rosbash M, Hall J C, Taghert P. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 16.Allada R, White N E, So W V, Hall J C, Rosbash M. Cell. 1998;93:791–804. doi: 10.1016/s0092-8674(00)81440-3. [DOI] [PubMed] [Google Scholar]
- 17.Rutila J E, Suri V, Le M, So W V, Rosbash M, Hall J C. Cell. 1998;93:805–814. doi: 10.1016/s0092-8674(00)81441-5. [DOI] [PubMed] [Google Scholar]
- 18.Tautz D, Pfeifle C. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- 19.Helfrich-Förster C. J Comp Neurol. 1997;380:335–354. doi: 10.1002/(sici)1096-9861(19970414)380:3<335::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko M, Helfrich-Förster C, Hall J C. J Neurosci. 1997;17:6745–6760. doi: 10.1523/JNEUROSCI.17-17-06745.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helfrich-Förster C. J Comp Physiol A. 1998;182:435–453. doi: 10.1007/s003590050192. [DOI] [PubMed] [Google Scholar]
- 22.Brand A H, Manoukian A S, Perrimon N. Methods Cell Biol. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- 23.Helfrich-Förster C, Homberg U. J Comp Neurol. 1993;337:177–190. doi: 10.1002/cne.903370202. [DOI] [PubMed] [Google Scholar]
- 24.Kaneko M, Hamblen M J, Hall J C. J Biol Rhythms. 2000;15:13–30. doi: 10.1177/074873040001500103. [DOI] [PubMed] [Google Scholar]
- 25.Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay S A, Rosbash M, Hall J C. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 26.Sehgal A, Price J, Young M W. Proc Natl Acad Sci USA. 1992;89:1423–1427. doi: 10.1073/pnas.89.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneko, M. & Hall, J. C. (2000) J. Comp. Neurol., in press. [DOI] [PubMed]
- 28.Emery, P., Stanewsky, R., Helfrich-Förster, C., Emery-Le, M., Hall, J. C. & Rosbash, M. (2000) Neuron, in press. [DOI] [PubMed]