Abstract
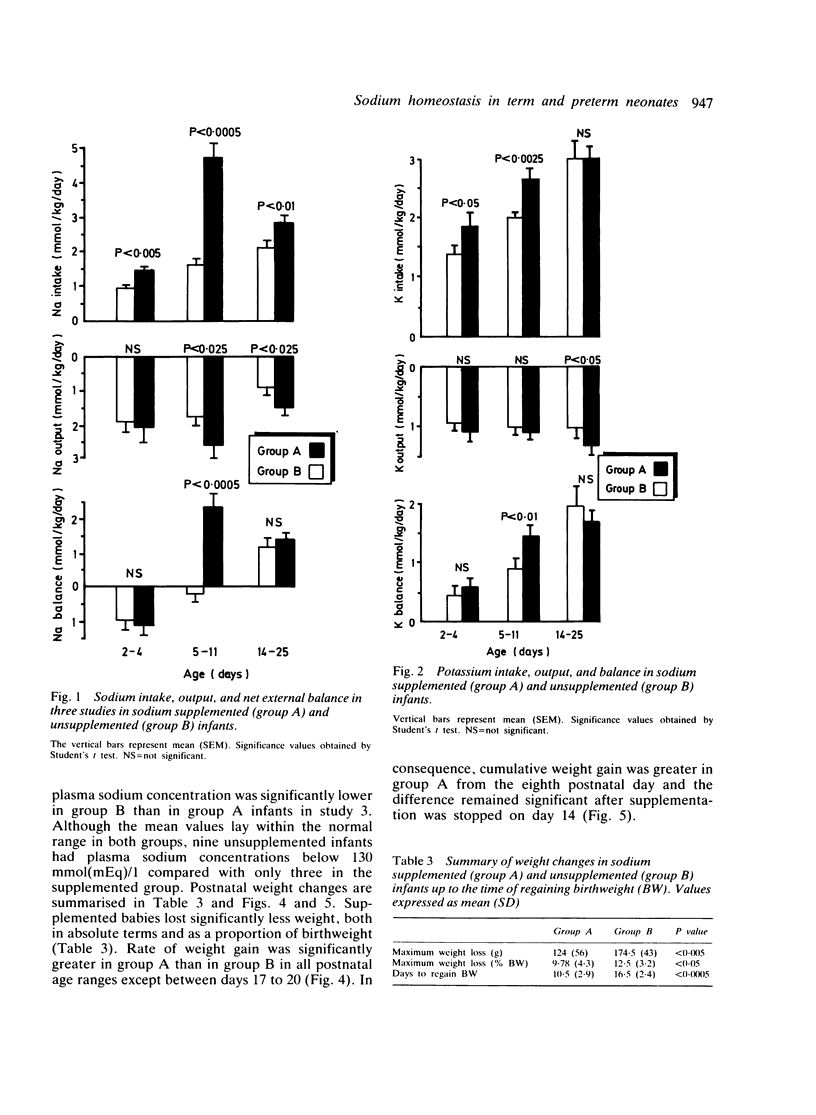
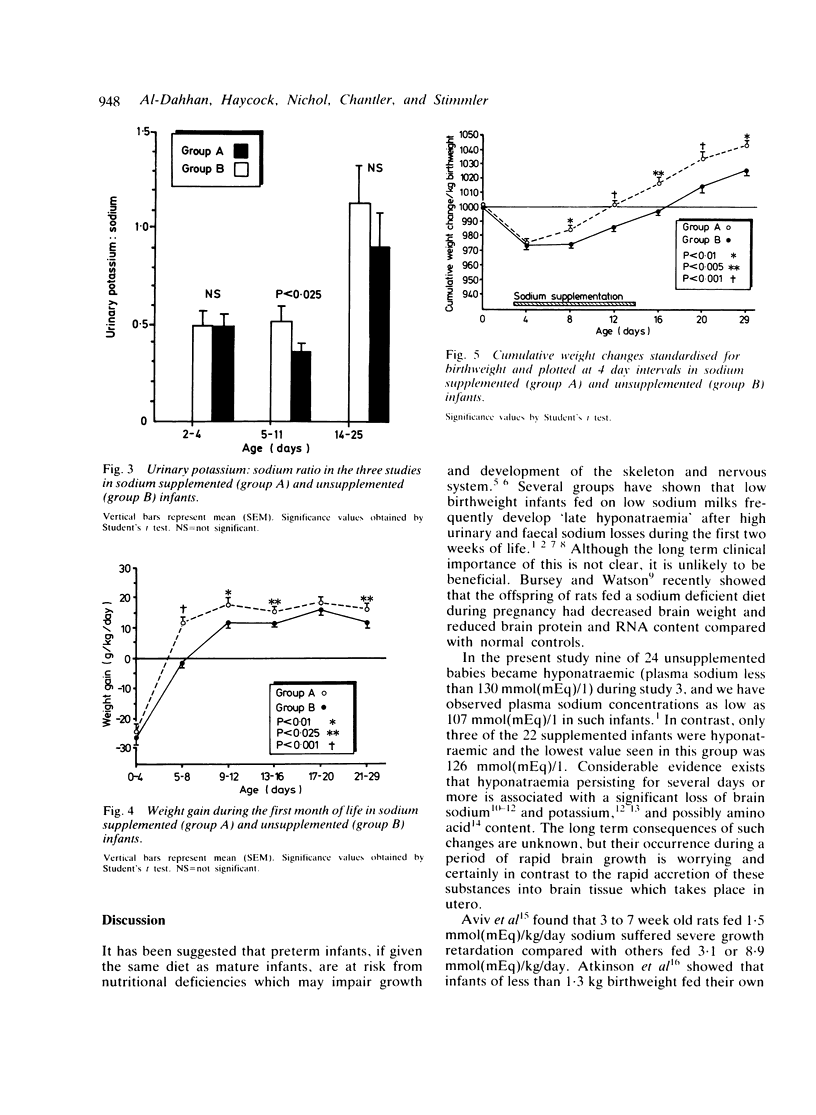
Clinical and biochemical effects of supplementing dietary sodium intake to 4 to 5 mmol(mEq)/kg/day from days 4 to 14 of life were studied in 22 infants of gestational age 27 to 34 weeks. These infants were compared with a group of 24 unsupplemented babies. Supplemented infants lost less weight postnatally and regained birthweight more quickly: their improved weight gain continued after supplementation was stopped. Sodium balance was positive at age 5 to 11 days in supplemented babies but slightly negative in controls. Potassium balance was more strongly positive in the supplemented group. Plasma sodium concentration was higher in supplemented infants during weeks 3 and 4. Hyponatraemia was significantly more common in unsupplemented (37.5%) than supplemented (13.6%) infants. No infant became oedematous, hypernatraemic, or showed evidence of circulatory overload. The incidence of patent ductus arteriosus and necrotising enterocolitis was not increased; no intracranial haemorrhages occurred. Urinary potassium:sodium ratio was lower in supplemented babies than controls suggesting responsiveness of the distal tubule to mineralocorticoids. Providing 4 to 5 mmol(mEq)/kg/day of sodium to infants born before 34 weeks' gestation for the first two postnatal weeks improves growth and biochemical status and causes no undesirable side effects.
Full text
PDF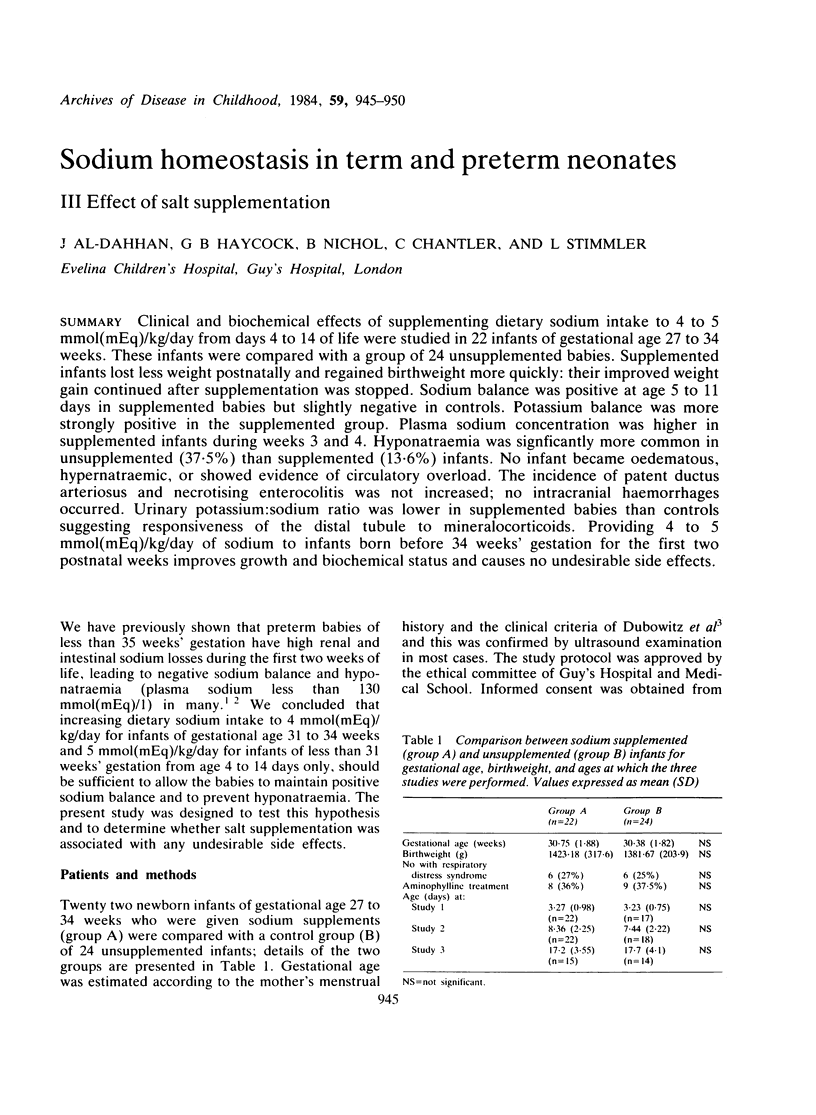



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Dahhan J., Haycock G. B., Chantler C., Stimmler L. Sodium homeostasis in term and preterm neonates. I. Renal aspects. Arch Dis Child. 1983 May;58(5):335–342. doi: 10.1136/adc.58.5.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dahhan J., Haycock G. B., Chantler C., Stimmler L. Sodium homeostasis in term and preterm neonates. II. Gastrointestinal aspects. Arch Dis Child. 1983 May;58(5):343–345. doi: 10.1136/adc.58.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aperia A., Broberger O., Herin P., Zetterström R. Salt content in human breast milk during the three first weeks after delivery. Acta Paediatr Scand. 1979 May;68(3):441–442. doi: 10.1111/j.1651-2227.1979.tb05034.x. [DOI] [PubMed] [Google Scholar]
- Arieff A. I., Llach F., Massry S. G. Neurological manifestations and morbidity of hyponatremia: correlation with brain water and electrolytes. Medicine (Baltimore) 1976 Mar;55(2):121–129. doi: 10.1097/00005792-197603000-00002. [DOI] [PubMed] [Google Scholar]
- Atkinson S. A., Radde I. C., Anderson G. H. Macromineral balances in premature infants fed their own mothers' milk or formula. J Pediatr. 1983 Jan;102(1):99–106. doi: 10.1016/s0022-3476(83)80302-3. [DOI] [PubMed] [Google Scholar]
- Bursey R. G., Watson M. L. The effect of sodium restriction during gestation of offspring brain development in rats. Am J Clin Nutr. 1983 Jan;37(1):43–51. doi: 10.1093/ajcn/37.1.43. [DOI] [PubMed] [Google Scholar]
- Chance G. W., Radde I. C., Willis D. M., Roy R. N., Park E., Ackerman I. Postnatal growth of infants of less than 1.3 kg birth weight: effects of metabolic acidosis, of caloric intake, and of calcium, sodium, and phosphate supplementation. J Pediatr. 1977 Nov;91(5):787–793. doi: 10.1016/s0022-3476(77)81043-3. [DOI] [PubMed] [Google Scholar]
- Chessex P., Reichman B., Verellen G., Putet G., Smith J. M., Heim T., Swyer P. R. Quality of growth in premature infants fed their own mothers' milk. J Pediatr. 1983 Jan;102(1):107–112. doi: 10.1016/s0022-3476(83)80303-5. [DOI] [PubMed] [Google Scholar]
- Cook J. G. Creatinine assay in the presence of protein. Clin Chim Acta. 1971 May;32(3):485–486. doi: 10.1016/0009-8981(71)90452-9. [DOI] [PubMed] [Google Scholar]
- Davies D. P. Adequacy of expressed breast milk for early growth of preterm infants. Arch Dis Child. 1977 Apr;52(4):296–301. doi: 10.1136/adc.52.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day G. M., Radde I. C., Balfe J. W., Chance G. W. Electrolyte abnormalities in very low birthweight infants. Pediatr Res. 1976 May;10(5):522–526. doi: 10.1203/00006450-197605000-00003. [DOI] [PubMed] [Google Scholar]
- Dila C. J., Pappius H. M. Cerebral water and electrolytes. An experimental model of inappropriate secretion of antidiuretic hormone. Arch Neurol. 1972 Jan;26(1):85–90. doi: 10.1001/archneur.1972.00490070103013. [DOI] [PubMed] [Google Scholar]
- Dubowitz L. M., Dubowitz V., Goldberg C. Clinical assessment of gestational age in the newborn infant. J Pediatr. 1970 Jul;77(1):1–10. doi: 10.1016/s0022-3476(70)80038-5. [DOI] [PubMed] [Google Scholar]
- Esmann V., Hobolth N., Jorgensen J. I. Heredity of leukocyte phosphorylase and amylo-1, 6-glucosidase deficiency. J Pediatr. 1969 Jan;74(1):90–94. doi: 10.1016/s0022-3476(69)80012-0. [DOI] [PubMed] [Google Scholar]
- Fomon S. J., Ziegler E. E., Vázquez H. D. Human milk and the small premature infant. Am J Dis Child. 1977 Apr;131(4):463–467. doi: 10.1001/archpedi.1977.02120170089018. [DOI] [PubMed] [Google Scholar]
- Gall D. G., Chung M. Effect of body weight on postnatal development of the proximal small intestine of the rabbit. Biol Neonate. 1982;42(3-4):159–165. doi: 10.1159/000241590. [DOI] [PubMed] [Google Scholar]
- Gross S. J. Growth and biochemical response of preterm infants fed human milk or modified infant formula. N Engl J Med. 1983 Feb 3;308(5):237–241. doi: 10.1056/NEJM198302033080501. [DOI] [PubMed] [Google Scholar]
- Harkavy K. L., Scanlon J. W., Jose P. The effects of theophylline on renal function in the premature newborn. Biol Neonate. 1979;35(3-4):126–130. doi: 10.1159/000241163. [DOI] [PubMed] [Google Scholar]
- Holliday M. A., Kalayci M. N., Harrah J. Factors that limit brain volume changes in response to acute and sustained hyper- and hyponatremia. J Clin Invest. 1968 Aug;47(8):1916–1928. doi: 10.1172/JCI105882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R. N., Chance G. W., Radde I. C., Hill D. E., Willis D. M., Sheepers J. Late hyponatremia in very low birthweight infants. (less than 1.3 kilograms). Pediatr Res. 1976 May;10(5):526–531. doi: 10.1203/00006450-197605000-00004. [DOI] [PubMed] [Google Scholar]
- Rymer M. M., Fishman R. A. Protective adaptation of brain to water intoxication. Arch Neurol. 1973 Jan;28(1):49–54. doi: 10.1001/archneur.1973.00490190067009. [DOI] [PubMed] [Google Scholar]
- Schanler R. J., Oh W. Composition of breast milk obtained from mothers of premature infants as compared to breast milk obtained from donors. J Pediatr. 1980 Apr;96(4):679–681. doi: 10.1016/s0022-3476(80)80738-4. [DOI] [PubMed] [Google Scholar]
- Shaw J. C. Malnutrition in very low birth-weight, pre-term infants. Proc Nutr Soc. 1974 Sep;33(2):103–111. doi: 10.1079/pns19740021. [DOI] [PubMed] [Google Scholar]
- Sulyok E., Németh M., Tényi I., Csaba I. F., Varga L., Varga F. Relationship between the postnatal development of the renin-angiotensin-aldosterone system and the electrolyte and acid-base status in the sodium chloride supplemented premature infant. Acta Paediatr Acad Sci Hung. 1981;22(1-2):109–121. [PubMed] [Google Scholar]
- Thurston J. H., Hauhart R. E., Jones E. M., Ater J. L. Effects of salt and water loading on carbohydrate and energy metabolism and levels of selected amino acids in the brains of young mice. J Neurochem. 1975 May;24(5):953–957. doi: 10.1111/j.1471-4159.1975.tb03661.x. [DOI] [PubMed] [Google Scholar]


