Abstract
Gonadal steroid feedback to oxytocin neurons during pregnancy is in part mediated via the neurosteroid allopregnanolone (3α-OH-DHP), acting as allosteric modulator of postsynaptic γ-aminobutyric acid type A (GABAA) receptors. We describe here a form of nongenomic progesterone signaling by showing that 3α-OH-DHP not only potentiates GABAA receptor-channel activity but also prevents its modulation by protein kinase C (PKC). Application of oxytocin or stimulation of PKC suppressed the postsynaptic GABA responses of oxytocin neurons in the absence, but not in the presence of 3α-OH-DHP. This finding was true at the juvenile stage and during late pregnancy, when the GABAA receptor is sensitive to 3α-OH-DHP. In contrast, after parturition, when the GABAA receptors expressed by oxytocin neurons are less sensitive to 3α-OH-DHP, this neurosteroid no longer counteracts PKC. The change in GABAA-receptor responsiveness to 3α-OH-DHP helps to explain the onset of firing activity and thus the induction of oxytocin release at parturition.
Oxytocin plays a key role in the initiation of parturition and lactation in female rats. Synchronous firing of magnocellular neurons in the supraoptic nucleus (SON) and the paraventricular nucleus triggers oxytocin release. The spiking frequency of these neurons is under control of a GABAergic input (1–3). The postsynaptic γ-aminobutyric acid type A (GABAA) receptors that mediate this input are susceptible to allosteric interaction with the neurosteroid allopregnanolone 3α-OH-DHP during some stages of the female reproductive cycle, in particularly during pregnancy (4, 5). In addition, somatodendritic release of oxytocin within the SON acting on autoreceptors (6) decreases the fast synaptic inhibition of the magnocellular cells, via suppression of postsynaptic GABAA receptor activity in a Ca2+-dependent manner (7, 8). Because 3α-OH-DHP and oxytocin both affect the activity of the postsynaptic GABAA receptor, we investigated whether allosteric interaction of 3α-OH-DHP with this receptor may influence its modulation by metabotropic pathways. If so, this finding would imply a novel pathway of nongenomic steroid hormone signaling in the central nervous system.
Methods
Dissection and Recordings.
Juvenile male (21–24 days postnatal) or adult female Wistar rats, either after 20 days of pregnancy (P20) or on the first day after parturition (PPD1) were decapitated, and 400-μm-thick coronal hypothalamus slices incorporating the middle portion of the SON were prepared as described (4, 5, 7) by using a Leica (Nussloch, Germany) vibratome slicer. Spontaneous GABAergic synaptic currents were recorded at room temperature (20°C) at a holding potential of −70 mV with an Axopatch 200A amplifier (Axon Instruments, Foster City, CA) in the whole-cell voltage clamp mode (see refs. 4, 5, and 7 for recording criteria). Electrodes had a tip resistance of around 2 MΩ and uncompensated series resistance < 12 MΩ (which usually was compensated for 70%). The external solution contained 125 mM NaCl, 25 mM NaHCO3, 3 mM KCl, 1.2 mM NaH2PO4⋅H2O, 2.4 mM CaCl2⋅2H2O, 1.3 mM MgSO4⋅7H2O, 10 mM d(+)-glucose (304 mosmol, carboxygenated in 5% CO2/95% O2, pH 7.4). 6,7-Dinitroquinoxaline-2,3-dione and (±)-2-amino-5-phosphoropentanoic acid (Research Biochemicals, both at 20 μM) were continuously present in the external solution. Bicuculline (Research Biochemicals, 20 μM) blocked all remaining synaptic activity (data not shown, see ref. 7). The frequency of spontaneous inhibitory postsynaptic currents (sIPSCs), obtained in the absence of bicuculline, was not sensitive to tetrodotoxin or nominal zero extracellular Ca2+ (see ref. 5). The pipette was filled with freshly prepared medium containing 142 mM CsCl, 10 mM Hepes, 2 mM MgATP, and 0.1 mM GTP (acid free) and was adjusted to pH 7.2 by using CsOH (296 mosmol). With this solution, the equilibrium potential for Cl− ions (ECl) was 0 mV. Neuropeptide and other applications were performed via a homemade so-called Y-tube microperfusion system (see ref. 7). Oxytocin (Bachem) effects were effectively blocked by a specific oxytocin antagonist called d(CH2)5-OVT (Bachem; data not shown). Allopregnanolone (5α-pregnan-3α-ol-20-one), abbreviated here as 3α-OH-DHP, was obtained from Research Biochemicals and dissolved in DMSO at 10 mM and further diluted in external solution before application. The lipophylicity of this substance prevents accurate estimates of the final concentration of 3α-OH-DHP at the site of action, as has been extensively discussed previously (4, 5). Staurosporine, TPA (phorbol 12-myristate 13-acetate, PMA), and the inactive form of PMA, 4-α-PMA, were all from Research Biochemicals and dissolved as stocks in DMSO at 10 mM.
Photolysis.
1,2-Dioctanoyl-3-(2-nitrobenzyl)-sn-glycerol (NB-caged DOG, Molecular Probes) was dissolved in DMSO as a stock of 2.1 mM (i.e., the final intracellular DMSO concentration was 1% when using NB-caged DOG at 20 μM). The UV flashes (1 ms, 360 nm) for photolysis of NB-caged DOG in the postsynaptic cell were delivered via the 40× objective of the Zeiss Axioscope microscope, using a 35 S Mercury arch Flashtube and a Stobex model 238 power pack (Chadwick–Helmuth, El Monte, CA), delivering > 12.5 J per flash at the plane of focus.
Quantal Analysis of Synaptic Currents.
Digital detection of sIPSCs and quantal analysis, curve fitting procedures to quantify the decay rate of individual sIPSCs, and statistical analysis of the lognormally distributed sIPSC amplitude and decay values all have been described (4, 5, 7). Within individual experiments after lognormal transformation, Wilcoxon rank sum testing was used to detect putative changes in either the sIPSC amplitude or decay time constant (averaged per 20 events as shown in Fig. 1a). Per experiment and per condition an unimodal lognormal function was fitted to histograms of binned data (either amplitude or decay time constants) obtained from a minimum of 250 IPSCs per histogram. Average values thus obtained were compared by using the paired t test. Alternatively the ratio of cells in which an effect was observed or not, was tested by using the χ2 test.
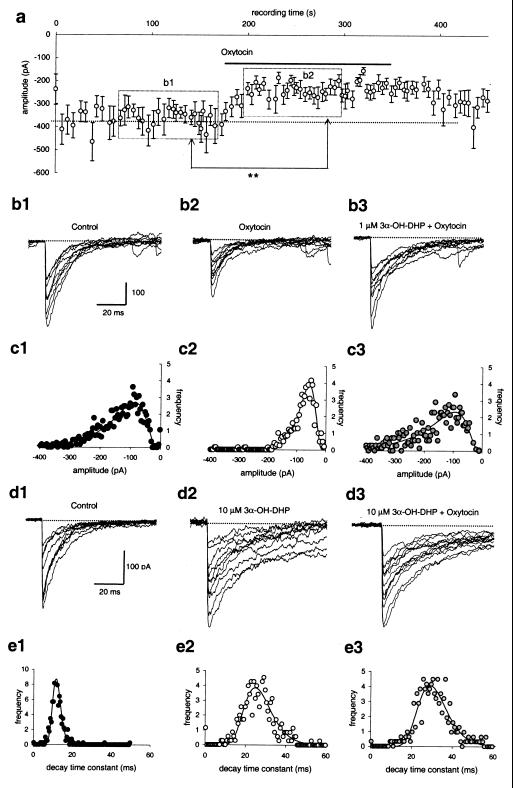
Figure 1.
3α-OH-DHP prevents oxytocin-induced suppression of sIPSCs. (a) Application of oxytocin (5 μM) during recording of sIPSCs of SON neurons in a slice from a juvenile male animal induces a significant (**, P < 0.01) suppression of averaged sIPSC amplitudes (plotted here per 20 events). (b1 and b2) Superimposed traces showing individual sIPSCs before and after application of oxytocin, respectively, and (b3) after 2 min of pretreatment with 3α-OH-DHP (1 μM). (c1–c3) Amplitude histograms of the experiment shown in b1–b3, with in each case a single lognormal function fitted to the binned data. Whereas in the absence of 3α-OH-DHP, oxytocin reduced the average sIPSC amplitude from 175 ± 113 pA to 88 ± 42 pA, pretreatment with 3α-OH-DHP blocked this suppressive action action of oxytocin (amplitude in c3: 219 ± 159 pA). (d1–d3) Application of 3α-OH-DHP at 10 μM significantly attenuated the decay rate of individual sIPSCs both in the absence (d2) and the presence (d3) of oxytocin (traces shown were taken after 2 min of application to allow for equilibration). (e1–e3) Decay time constant histograms of the experiment shown in d1–d3, with in each case a single lognormal function fitted to the binned data. Both in the absence and the presence of oxytocin, 3α-OH-DHP attenuated the synaptic current decay (e1: 14 ± 2 ms; e2: 29 ± 8 ms; e3: 32 ± 8 ms).
Results
3α-OH-DHP Prevents Oxytocin-Induced Suppression of sIPSCs.
In situ whole-cell voltage clamp recordings of GABAA receptor-mediated sIPSCs in acutely prepared rat brain slices were performed in the presence of 6,7-dinitroquinoxaline-2,3-dione and (±)-2-amino-5-phosphoropentanoic acid (both at 20 μM). Under these conditions the sIPSCs are resistant to tetrodotoxin and nominal zero extracellular calcium (not shown, refs. 5 and 7), which implies that they are monosynaptic. The ultimate goal of this study was to show whether allosteric interaction of 3α-OH-DHP with the GABAA receptor during pregnancy might influence its modulation by oxytocin. For ethical reasons, however, in initial experiments juvenile animals were used (Figs. 1–3). However, during the juvenile stage the GABAA receptors of the SON show biophysical and pharmacological properties similar to those observed during pregnancy (4, 5). We distinguished oxytocinergic neurons from vasopressinergic neurons by their sensitivity to oxytocin. To this end, we compared sIPSCs during > 100 s of control recording to those obtained during > 100 s in the presence of oxytocin, starting 20 s after application of the neuropeptide (Fig. 1 a, b1, b2, c1, and c2). Cells in which the sIPSC amplitude before and after neuropeptide application differed with P ≤ 0.01 (Wilcoxon rank sum test) were considered to be oxytocin-sensitive. In 13 of 19 recordings we observed such a significant reduction of the sIPSC amplitude in response to 5 μM oxytocin. Repeated oxytocin applications gave reproducible responses (8). We previously have shown that this effect is mediated via a postsynaptic mechanism and occurs in a Ca2+-dependent manner (7).
Figure 3.
Activation of PKC in the postsynaptic cell mimics the effect of oxytocin and TPA on sIPSC amplitude. During recording of SON neurons at the juvenile stage, NB-caged DOG (20 μM) was perfused into the interior of the recorded cell; flash photolysis of this agent (arrows) gave a significant (***, P < 0.001) suppression of the sIPSC amplitudes. Flash photolysis in the absence of this agent did not affect the amplitude nor the current decay rate; prolonged dialysis with this agent without applying UV flashes also failed to reduce the sIPSC amplitudes (not shown).
At the juvenile stage, 3α-OH-DHP (10 μM) caused an increase of the synaptic current decay time constant of 185 ± 53% (n = 10, compare Fig. 1 d1 and d2, P < 0.02, Wilcoxon rank sum test). At a concentration of 1 μM, this increase is much less (20 ± 11%, n = 8); however, a similar dose dependence previously was observed for the pregnant stage (4). Although 3α-OH-DHP affected the decay rate of the sIPSCs, it had no significant effect on the amplitude (Fig. 1 d1 and d2, Wilcoxon rank sum test). We hypothesized that interference between oxytocin modulation and 3α-OH-DHP potentiation of GABAA receptors might occur when both mechanisms are simultaneously activated. In six of six cells that responded to oxytocin before 3α-OH-DHP treatment (Fig. 1 b2 and c2), no response to oxytocin could be obtained in the presence of > 1 μM of 3α-OH-DHP (Fig. 1 b3 and d3). Thus, the sIPSC amplitude in oxytocin alone was significantly reduced compared with the control level (compare Fig. 1 c1 and c2; paired t test for pooled data, P < 0.004), whereas in the presence of 3α-OH-DHP and oxytocin it was not significantly altered (compare Fig. 1 c1 and c3). In four additional cells that were not tested for oxytocin sensitivity before 3α-OH-DHP application, only one showed a small (24%) reduction of the amplitude in response to oxytocin after neurosteroid addition (Wilcoxon rank sum test, P < 0.02). Thus, in a 9:1 ratio, application of oxytocin could not evoke a suppression of sIPSCs when 3α-OH-DHP was present, which contrasts to the 6:13 ratio found under control conditions (see above; χ2 test, P < 0.01).
Pretreatment with 3α-OH-DHP appears to interfere with the ability of oxytocin to reduce the sIPSC amplitude in oxytocin neurons. One might wonder, whether vice versa, oxytocin also interferes with the ability of 3α-OH-DHP to prolong the sIPSC decay in these cells (Fig. 1 b3 and d3). To test this we quantified the decay time constants of all individual sIPSCs in these experiments under the different conditions (Fig. 1 e1–e3). Analysis showed that the sIPSC decay time constant was 52.8 ± 9.7 ms in the presence of 10 μM 3α-OH-DHP (versus 18.5 ± 1.1 ms for controls; n > 10), whereas upon additional application of 5 μM of oxytocin, this value was 63 ± 11 ms (not significantly altered compared to 3α-OH-DHP alone, paired t test, n = 10). Therefore it is unlikely that oxytocin interferes with the usual allosteric interaction of 3α-OH-DHP with the GABAA receptor.
Oxytocin-Induced Suppression of sIPSCs Depends on Protein Kinase C (PKC).
In the SON oxytocin binds to a G-protein coupled receptor (7, 8), giving rise to inositol trisphosphate (IP3) and diacylglycerol (DAG) (9). Although IP3 is known to release Ca2+ from intracellular stores, a rise in intracellular [Ca2+] combined with DAG also might activate PKC. To test whether the oxytocin-mediated response is caused by activation of PKC, we tested the effect of the phorbol ester TPA (25 nM). This agent induced a suppression of the monoquantal sIPSC amplitude by 39 ± 8% (significant, P < 0.01, paired t test) in all cells tested (n = 11, Fig. 2a). This effect was interpreted as a postsynaptic effect (see below). An additional presynaptic effect of TPA, not observed with oxytocin, may occur, because in five of 11 cells a 61 ± 38% reduction of sIPSC interval was observed (P < 0.01, t test). TPA had no effect on the sIPSC decay time constants in any of these experiments (Fig. 2b). Application of 4-α-TPA, an inactive phorbol ester, had no effect on the sIPSC amplitude, decay, or interval (n = 4, data not shown). We also tested the effect of oxytocin on the sIPSC amplitude in the presence of staurosporin to block PKC activity. In seven of eight cells we failed to find any response to oxytocin in the presence of staurosporine (Fig. 2c). Only in one of these cells, did we observe a 19% reduction in sIPSC amplitude because of oxytocin application in the presence of staurosporine (P < 0.04, Wilcoxon rank sum test, not shown). This 1:7 ratio is significantly different compared with the 13:6 cell ratio found in the initial control experiments (Fig. 2d, χ2 test, P < 0.01). These data suggest that in the SON oxytocin mediates the suppression of postsynaptic GABAA receptors via a PKC-dependent mechanism.
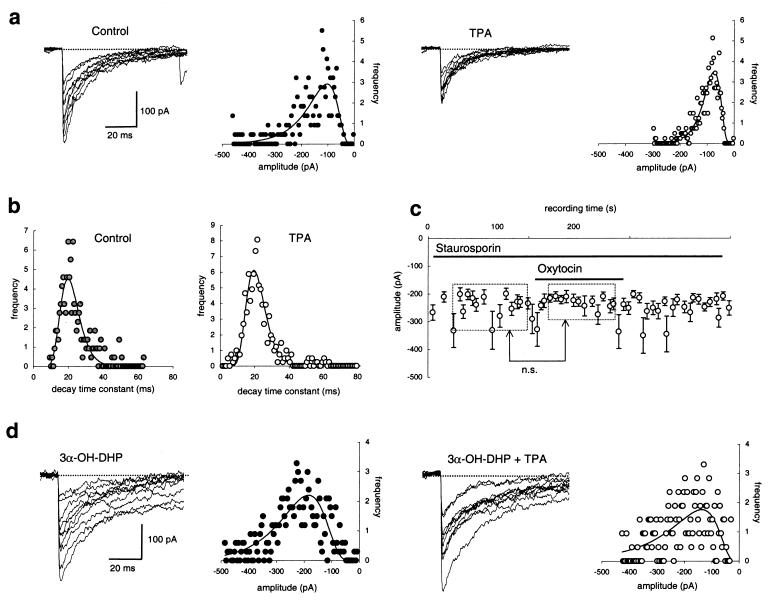
Figure 2.
Oxytocin effect depends on PKC but 3α-OH-DHP prevents PKC-induced suppression of sIPSCs. (a) Superimposed sIPSCs before and after a 2-min application of TPA (25 nM) with the respective sIPSC amplitude distributions. TPA induced a significant reduction of the average sIPSC amplitude as is shown by the sIPSC amplitude distributions to which unimodal lognormal curves have been fitted. (b) TPA does not affect the monoexponential decay time constant of sIPSC in these experiments. Again unimodal lognormal functions were fitted to the distributions to obtain the average per condition in each experiment. (c) Oxytocin (5 μM) gave no effect (n.s. = not significant) after 2 min of pretreatment with 2 μM of staurosporine, although this cell was observed to be oxytocin-sensitive before application of staurosporine (not shown here). (d) 3α-OH-DHP (1–10 μM) prevents the suppression of the sIPSC amplitude normally observed after 2-min application of TPA (compare with a). Juvenile animals as in Fig. 1.
To substantiate the postsynaptic nature of the PKC-dependent action on the sIPSC amplitude, we tested whether the effect of oxytocin could be mimicked by means of photo activation of a chemically caged diacylglycerol-metabolite, NB-caged DOG, exclusively applied to the interior of the postsynaptic neuron. Upon photolysis of this agent a significant 30 ± 5% suppression of the sIPSC amplitude occurred when two or more UV flashes were delivered (Fig. 3; n = 4 cells, P < 0.001 for each cell tested with Wilcoxon rank sum test; P < 0.01 for pooled data, paired t test). In none of these experiments did we observe an effect on the interval between sIPSCs (non shown).
3α-OH-DHP Prevents PKC-Induced Suppression of sIPSCs.
Because it is well known that 3α-OH-DHP affects postsynaptic activity via a direct allosteric interaction with the GABAA receptor (10), we hypothesized that the 3α-OH-DHP-induced block of the metabotropic modulation of sIPSCs occurred downstream of PKC. This implied that 3α-OH-DHP also would suppress the effect of TPA. To test this hypothesis, TPA was applied to eight cells after preincubation with 3α-OH-DHP. In none of these cells did TPA cause a significant reduction of the sIPSC amplitude (Fig. 2d, relative sIPSC amplitude in the presence of 3α-OH-DHP and TPA was 95 ± 6% of that with 3α-OH-DHP alone, paired t test, not significant).
PKC-Dependent Action of Oxytocin Is Prevented by 3α-OH-DHP During Late Pregnancy.
The above results indicate that in juvenile rats, GABAA receptors, potentiated by 3α-OH-DHP, are no longer susceptible to oxytocin receptor signaling. Therefore in P20 rats a similar approach was chosen as in juvenile rats. Cells were tested for oxytocin sensitivity both in the absence and the presence of 3α-OH-DHP. Under control conditions (but after extensive rinsing of the slice preparation to wash out endogenous 3α-OH-DHP, see refs. 4 and 5) in seven of 14 SON cells we observed a significant response to oxytocin (P < 0.01 for each of these seven cells, Wilcoxon rank sum test, Fig. 4a). In contrast, none of eight cells recorded in the presence of 3α-OH-DHP was sensitive to oxytocin (P > 0.06 for each of these cells, Wilcoxon rank sum test, Fig. 4b, χ2 test for contrast in pooled data: P < 0.01). At this reproductive stage, the sIPSC decay time constant in controls was 15.8 ± 0.8 ms (n = 14), whereas in the presence of 3α-OH-DHP it was significantly increased (with 89 ± 21%, P < 0.01, paired t test). The latter observation implies that under this condition allosteric interaction of 3α-OH-DHP with the GABAA receptor takes place, in line with previous findings (4, 5).
Figure 4.
3α-OH-DHP blocks the action of oxytocin during late pregnancy. (a) Application of oxytocin (5 μM) during recording of SON neurons at the P20 stage gave a significant (**, P < 0.01) suppression of the sIPSCs amplitudes in line with effect observed in juveniles. (b) Pretreatment (2–4 min) with 3α-OH-DHP prevented the effect of oxytocin in all recordings tested (n.s. = not significant).
To show that during late pregnancy the 3α-OH-DHP action also is mediated at a level downstream of PKC activation, rather than at the level of the oxytocin receptor, we tested whether TPA was capable of suppressing the sIPSC amplitude in the presence of exogenous 3α-OH-DHP. TPA applied in the absence of 3α-OH-DHP induced a 49% reduction of the sIPSC amplitude (Fig. 5 a Top and b, n = 4, P < 0.01, t test), without any effect on the sIPSC decay (analysis not shown). In contrast, while significantly potentiating the decay of sIPSCs (Fig. 5a Middle), 3α-OH-DHP prevented the action of TPA in four of four cells tested (Fig. 5 a Bottom and b, not significant, paired t test for pooled data).
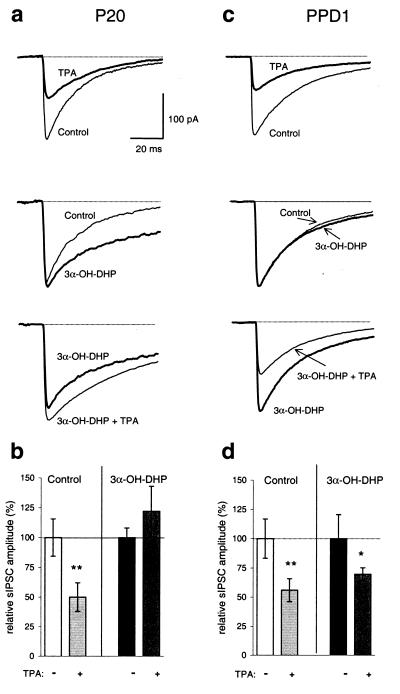
Figure 5.
PKC-dependent modulation of sIPSCs is prevented by 3α-OH-DHP during late pregnancy, but not after parturition. (a and b) TPA (25 nM) induced a significant (**, P < 0.01) suppression in the absence of 3α-OH-DHP, but not after pretreatment with 3α-OH-DHP (1–10 μM) in P20 stage animals. (c and d) At PPD1, the effect of TPA was observed regardless of the presence of 3α-OH-DHP (** and *, P < 0.01 and 0.02, respectively). Note that 3α-OH-DHP also fails to affect the decay of the IPSCs at this stage. Traces in a and c are averages of a minimum of 50 individual sIPSCs per condition. All tests summarized in b and d are from n = 4 recordings from n = 4 animals.
PKC Action on GABAA Receptors Is Not Prevented by 3α-OH-DHP After Parturition.
The potentiating effect of allosteric modulators on the ion channel activity of GABAA receptors may depend on the subunit composition of the postsynaptic receptor subtype being expressed at a certain stage of development. To investigate whether this holds true for the PKC blocking action of 3α-OH-DHP, the interaction between the effect of TPA and 3α-OH-DHP was tested on SON neurons on PPD1 and compared with the P20 stage. At PPD1 the ratio of α1/α2 subunit expression is changed such that α2-dominated receptors prevail, whereas at P20 α1-dominated receptors are found (4, 5). This subunit switch was shown previously to correlate well with a down-regulation of the 3α-OH-DHP sensitivity of these receptors at PPD1 stage(s) (4, 5). If allosteric interaction of 3α-OH-DHP with the GABAA receptors is required for block of PKC-dependent modulation, one would predict that at PPD1, for lack of significant effects of 3α-OH-DHP on the decay of sIPSCs (Fig. 5c Middle), activation of PKC in the presence of 3α-OH-DHP would still suppress the sIPSC amplitude. Both in the absence and the presence of 3α-OH-DHP, TPA was capable of inducing a significant suppression of the sIPSC amplitude (Fig. 5 c Top versus Bottom and d, n = 4 for both conditions, P < 0.01 and 0.02 for control and 3α-OH-DHP, respectively, paired t tests).
Discussion
The concept that we present here is that nongenomic, allosteric interaction of 3α-OH-DHP with the neurosteroid-sensitive GABAA receptor subtype in oxytocin neurons of the SON prevents PKC from phosphorylating the GABAA receptor itself or one of its interacting proteins. The conformational change that prolongs the ion channel open time of the receptor (11) would either make one or more PKC phosphorylation site(s) of the GABAA receptor inaccessible or, alternatively, it would alter hitherto unknown receptor-protein interactions that may indirectly depend on PKC activity. Our findings do not necessarily demonstrate that direct phosphorylation of GABAA receptors by PKC is prevented; however, several phosphorylation sites are present at the γ2 and the β2 subunit (12, 13), both of which are expressed in the SON (see ref. 14). Furthermore activation of PKC has variable effects on GABAA receptor activity (15–18), which are likely to be caused by cell-specific differentiation in GABAA receptor subunit composition. In addition, differences in PKC-dependent effects may be brought about by heterogeneity in the expression of other proteins that interact or associate with the GABAA receptor from the intracellular side (19–21).
Progesterone(-metabolites) might prevent binding of oxytocin to its receptor (22). However, in such a scenario, activation of PKC, thereby bypassing the activation of postsynaptic oxytocin receptors, would still induce the suppression of the GABAA receptor, even in the presence of 3α-OH-DHP. Because this was not the case either in juvenile rats or during late pregnancy, two developmental stages known to give rise to 3α-OH-DHP-sensitive GABAA receptors, the 3α-OH-DHP block of metabotropic signaling must be downstream of PKC. In contrast, after parturition, when the GABAA receptors are less sensitive to 3α-OH-DHP (2), 3α-OH-DHP no longer counteracted PKC. Thus, allosteric interaction of the neurosteroid with the GABAA receptor is required to give rise to a block of the PKC modulation.
These observations have implications for our view of the induction of oxytocin release at the onset of parturition. The physiological relevance of 3α-OH-DHP signaling is largest during pregnancy, when the combination of prolongation of the sIPSC decay and the block of PKC-dependent suppression of the GABAA receptors in the oxytocin neurons might provide an efficient mechanism to prevent premature oxytocin release. The effect of 3α-OH-DHP on the sIPSC decay leads to a > 2-fold increase in the synaptic efficacy of the GABA input (5). Thus during pregnancy, the overall impact of GABAergic transmission in the SON is “locked” in a potentiated mode, a condition that is not under control of oxytocin autoregulation, because of the continuous presence of 3α-OH-DHP. As previously shown, this is sufficient to silence the firing activity of oxytocin neurons (4). Then, at parturition, 3α-OH-DHP no longer controls the firing activity (see ref. 4) and also fails to prevent the autoregulatory action of oxytocin within the SON (this paper). As a result, disinhibition of oxytocin neurons occurs via a reduction in tonic GABAergic synaptic input (7), giving way to other excitatory synaptic input (see ref. 23). It is noteworthy that a previous microdialysis study failed to observe any alteration in the amount of GABA being released around parturition (ref. 23; A.E. Herbison, personal communication).
Abbreviations
- PKC
protein kinase C
- GABAA
γ-aminobutyric acid type A
- 3α-OH-DHP
allopregnanolone
- SON
supraoptic nucleus
- P20
20 days of pregnancy
- PPD1
first day after parturition
- sIPSC
spontaneous inhibitory postsynaptic current
- TPA
phorbol 12-myristate 13-acetate
- NB-caged DOG
1,2-dioctanoyl-3-(2-nitrobenzyl)-sn-glycerol
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050424697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050424697
References
- 1.Van de Pol A N. J Neurosci. 1985;5:2940–2945. doi: 10.1523/JNEUROSCI.05-11-02940.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wuarin J P, Dudek F E. J Neurosci. 1993;13:2323–2331. doi: 10.1523/JNEUROSCI.13-06-02323.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voisin D L, Herbison A E, Poulain D A. J Physiol (London) 1995;483:211–224. doi: 10.1113/jphysiol.1995.sp020579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brussaard A B, Kits K S, Baker R E, Willems W P A, Leyting-Vermeulen J W, Voorn P, Smit A B, Bicknell R J, Herbison A E. Neuron. 1997;19:139–150. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- 5.Brussaard A B, Piroska D, Leyting-Vermeulen J, Kits K S. J Physiol (London) 1999;516:513–524. doi: 10.1111/j.1469-7793.1999.0513v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwig M. J Neuroendocr. 1998;10:881–895. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 7.Brussaard A B, Kits K S, De Vlieger T A. J Physiol (London) 1996;497:495–507. doi: 10.1113/jphysiol.1996.sp021783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brussaard A B. Adv Exp Med Biol. 1995;395:105–115. [PubMed] [Google Scholar]
- 9.Lambert R C, Dayanithi G, Moos F C, Richard P H. J Physiol (London) 1994;485:485–492. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert J J, Belelli D, Hill-Venning C, Peters J A. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 11.Twyman R E, McDonald R L. J Physiol (London) 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kellenberger S, Malherbe P, Sigel E. J Biol Chem. 1992;267:25660–25663. [PubMed] [Google Scholar]
- 13.Moss S J, Doherty C A, Huganir R L. J Biol Chem. 1992;267:14470–14476. [PubMed] [Google Scholar]
- 14.Fenelon V S, Herbison A E. J Neurosci. 1996;16:4872–4880. doi: 10.1523/JNEUROSCI.16-16-04872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigel E, Baur R. Proc Natl Sci USA. 1998;85:6192–6196. doi: 10.1073/pnas.85.16.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leidenheimer N J, McQuilkin S J, Hahner L D, Whiting P, Harris A. Mol Pharmacol. 1992;41:1116–1123. [PubMed] [Google Scholar]
- 17.Leidenheimer N J, Chapell R. Mol Brain Res. 1997;52:173–181. doi: 10.1016/s0169-328x(97)00255-6. [DOI] [PubMed] [Google Scholar]
- 18.Poisbeau P, Cheney M C, Browning M D, Mody I. J Neurosci. 1999;19:674–683. doi: 10.1523/JNEUROSCI.19-02-00674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Bedford F K, Brandon N J, Moss S J, Olsen R W. Nature (London) 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- 20.Kneussel M, Brandstatter J H, Laube B, Stahl S, Muller U, Betz H. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandon N J, Uren J M, Kittler J T, Wang H, Olsen R, Parker P J, Moss S J. J Neurosci. 1999;19:9228–9234. doi: 10.1523/JNEUROSCI.19-21-09228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grazzini E, Guillon G, Mouillac B, Zingg H H. Nature (London) 1998;392:509–512. doi: 10.1038/33176. [DOI] [PubMed] [Google Scholar]
- 23.Herbison A E, Voisin D V, Douglas A J, Chapman C. Endocrinology. 1997;138:33–40. doi: 10.1210/endo.138.1.4859. [DOI] [PubMed] [Google Scholar]