Abstract
Senescence may contribute to the pathogenesis of atherosclerosis. Although the bioavailability of nitric oxide (NO) is limited in senescence, the effect of NO on senescence and its relationship to cardiovascular risk factors have not been investigated fully. We studied these factors by investigating senescence-associated β-galactosidase (SA-β-gal) and human telomerase activity in human umbilical venous endothelial cells (HUVECs). Treatment with NO donor (Z)-1-[2-(2-aminoethyl)-N-(2-aminoethyl)amino]diazen-1-ium-1,2-diolate (DETA-NO) and transfection with endothelial NO synthase (eNOS) into HUVECs each decreased the number of SA-β-gal positive cells and increased telomerase activity. The NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME) abolished the effect of eNOS transfection. The physiological concentration of 17β-estradiol activated hTERT, decreased SA-β-gal-positive cells, and caused cell proliferation. However, ICI 182780, an estrogen receptor-specific antagonist, and l-NAME each inhibited these effects. Finally, we investigated the effect of NO bioavailability on high glucose-promoted cellular senescence of HUVECs. Inhibition by eNOS transfection of this cellular senescence under high glucose conditions was less pronounced. Treatment with l-arginine or l-citrulline of eNOS-transfected cells partially inhibited, and combination of l-arginine and l-citrulline with antioxidants strongly prevented, high glucose-induced cellular senescence. These data demonstrate that NO can prevent endothelial senescence, thereby contributing to the anti-senile action of estrogen. The ingestion of NO-boosting substances, including l-arginine, l-citrulline, and antioxidants, can delay endothelial senescence under high glucose. We suggest that the delay in endothelial senescence through NO and/or eNOS activation may have clinical utility in the treatment of atherosclerosis in the elderly.
Keywords: diabetes mellitus, endothelial nitric oxide synthase, estrogen, aging
Aging is known to be a major cardiovascular risk factor (1). Cellular senescence is the limited ability of human cells to divide when cultured in vitro and is usually accompanied by phenotypic changes in morphology, gene expression, and function (2). These changes have been implicated in human aging. Until recently, little attention has been paid to the potential impact of vascular cellular senescence on age-related vascular disorders. Senescent cells from aged animals express increased levels of proinflammatory molecules, suggesting that cellular senescence in vivo contributes to the pathogenesis of human atherosclerosis (3).
The telomere hypothesis is a widely accepted explanation of the occurrence of senescence (4). Telomeres, repetitive DNA sequences at the ends of eukaryotic chromosomes, shorten as a linear function of increasing cellular division, and according to the hypothesis, short telomere length triggers the onset of senescence (5, 6). Telomerase, a ribonucleoprotein, can synthesize new telomeric repeats and restore telomere length. Cellular senescence usually accompanies telomere shortening and increases in senescence-associated β-galactosidase (SA-β-gal) (assayed at pH 6), which is distinguishable from endogenous lysosomal β-gal activity, is considered to be a marker of cellular senescence (7).
According to a free-radical theory, reactive oxygen species (ROS) may be potential candidates responsible for senescence, and oxidative stress may promote senescence by shortening telomere through inactivation of the Src kinase family (8–10). Therefore, not only atherosclerosis but also senescence has been shown to progress via ROS (11). NO is a widespread signaling molecule in the cardiovascular system, which functions in multiple ways to protect against the initiation and progression of atherosclerosis (12, 13). NO prevents the adhesion and aggregation of blood cells and inhibits vascular smooth muscle cell proliferation (14). However, neither the role nor the effect of endothelial NO on senescence is fully known. As NO can abrogate the state of oxidative stress, NO may thus have the potential to affect cellular senescence by scavenging senescent stimuli such as ROS.
Accordingly, the present study was performed to examine whether or not NO and the activation of eNOS can delay endothelial senescence. We also considered estrogen depletion and diabetes mellitus among various cardiovascular risk factors as applied models of the combined effects of NO on both atherosclerosis and cell longevity.
The morbidity of cardiovascular disease dramatically increases after menopause (15). In such cases, estrogen depletion has been speculated as a cause of the disease, and estrogen plays an antiatherogenic role both in vivo and in vitro (16, 17). Although hormone replacement therapy was reported not to prevent cardiovascular disease in a clinical trial, this ineffectiveness was due to the increased frequency of thrombosis produced by estrogen in advanced atherosclerosis and to the adverse effect of coprescribed progesterone (18). The fact that females are known to live several years longer than males world-wide strongly supports the antiatherogenic effect of estrogen.
Diabetes mellitus is also a major cardiovascular risk factor, and the etiology of diabetic atherosclerosis is suggested to include the increase of ROS and the decrease of NO bioavailability as a result of high glucose levels (19). The incidence of cardiovascular diseases is increased in elderly diabetic patients, but the relationship between senescence and diabetes on endothelial function has yet to be elucidated (20). NO is synthesized by NOS, which utilizes l-arginine as a substrate and produces l-citrulline as the second reaction product. l-arginine can be synthesized from l-citrulline in endothelial cells through a recycling pathway (21). This pathway may be the principal mechanism for sustaining localized l-arginine availability for endothelial nitric oxide synthase (eNOS)-catalyzed NO production (21, 22). In the present study, we examined the effect of NO boosting on high glucose and/or senescence by the regulation of eNOS.
Results
NO Delays Cellular Senescence.
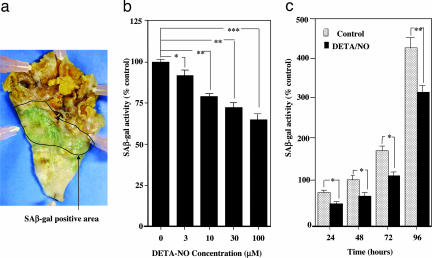
The effect of NO on endothelial cellular senescence was investigated by evaluating SA-β-gal used as a cellular senescence marker and human telomerase activity used as an indicator of elongation of telomere length in human umbilical vein endothelial cells (HUVECs). We also examined SA-β-gal activity in the thoracic aorta and coronary arteries obtained from 3 autopsied elderly individuals. Fig. 1a shows that SA-β-gal activity was observed in the mild atherosclerotic area in human thoracic aorta. Treatment with the NO donor, (Z)-1-[2-(2-aminoethyl)-N-(2-aminoethyl) amino] diazen-1-ium-1,2-diolate (DETA-NO), for 24 h significantly decreased the SA-β-gal activity in HUVECs (Fig. 1 b and c). The effect of DETA-NO was found to be both concentration-dependent (3–100 μM, Fig. 1b) and time-dependent (24–96 h treatment, Fig. 1c). Coincubation with NG-nitro-l-arginine methyl ester (l-NAME) (300 μM) did not affect the action of DETA-NO (data not shown). DETA-NO also increased telomerase activity in HUVECs (data not shown).
Fig. 1.
SA-β-gal activity as cellular senescence. (a) SA-β-gal-positive staining was observed in atherosclerotic lesions of the intimal side of human thoracic aorta, which was obtained by autopsy. No staining was detected in the nonatherosclerotic area and advanced atherosclerotic area, including the necrotic core and ulcer complicated lesion. (b) Concentration-dependent decrease in SA-β-gal activity in HUVECs by DETA-NO. HUVECs were treated with DETA-NO for 24 h. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.0001 vs. DETA-NO-untreated control. (c) Time-dependent decrease in SA-β-gal activity in HUVECs by DETA-NO. HUVECs were treated with 10 μM DETA-NO for 24–96 h. ∗, P < 0.05; ∗∗, P < 0.01 vs. the corresponding control. Control sample, which is treated for 48 h, is expressed as 100%.
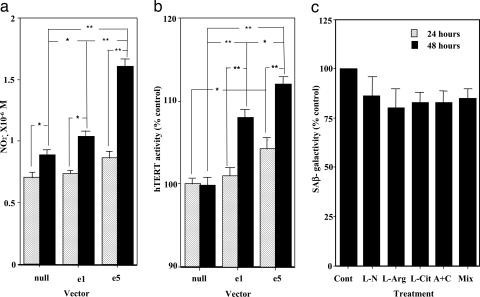
Transfection with eNOS into HEK 293 cells or HUVECs for 48 h increased the NO metabolite, NO2− (Fig. 2a), and also significantly increased telomerase activity (Fig. 2b). On the other hand, the number of SA-β-gal-stained cells was reduced by eNOS transfection (data not shown). Fig. 2c shows the effects of eNOS-related substrate and products on SA-β-gal staining in HUVECs. Coincubation with the NOS inhibitor l-NAME (300 μM) tended to decrease the number of SA-β-gal-stained cells by inhibiting NO release from HUVECs. SA-β-gal-positive staining also tended to decrease in the presence of l-arginine and/or l-citrulline. However, their effects on SA-β-gal-stained cells are not statistically significant even though they were given together.
Fig. 2.
Influence of eNOS modulation on cellular senescence. (a) The effect of transfection with eNOS on nitrite production by HEK 293 cells. Transfection with eNOS into cells was performed; e5 included five times the amount of eNOS vector compared with e1. The nitrite concentrations in the medium 24 and 48 h after transfection are shown. ∗, P < 0.05; ∗∗, P < 0.01. (b) The effect of transfection with eNOS on telomerase activity in HEK 293 cells. The activity of hTERT in cells 24 and 48 h after transfection are shown. ∗, P < 0.05; ∗∗, P < 0.01. (c) The effects of treatment with l-NAME (L-N, 300 μM), l-arginine (L-arg, 1 mM), and l-citrulline (L-cit, 300 μM) alone or in combination (A+C) on SA-β-gal activity in HUVECs. The treatment time was 24 h. Mix = l-arginine, l-citrulline and vitamin E plus vitamin C (each, 100 μM).
Estrogen Delays Cellular Senescence via an NO-Dependent Mechanism.
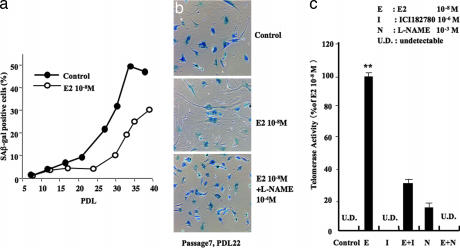
We next investigated the effect of E2 on cellular senescence in HUVECs. At physiological concentrations (10 nM), E2 treatment reduced the number of SA-β-gal-positive cells, especially in large population-doubling level (PDL) cells (Fig. 3a). Fig. 3b shows representative photographs of SA-β-gal-stained cells in HUVECs of PDL 22. E2 decreased the number of SA-β-gal-stained cells, whereas this effect was prevented by coincubation with l-NAME (Fig. 3b). E2 markedly activated telomerase, and this activation was inhibited by ICI 182780, an estrogen receptor-specific antagonist, and by l-NAME (Fig. 3c). These results suggest that the counteracting effect of E2 on senescence involves an eNOS-dependent mechanism by means of activation of estrogen receptors.
Fig. 3.
Effect of estrogen on cellular senescence. (a) The relative levels of SA-β-gal-positive staining cells in different PDL when HUVECs were untreated and treated with 10−8 M E2 for 24 h. Positive staining cells were evaluated by FACscan. (b) Representative photographs of SA-β-gal staining in control, 10−8 M E2-treated, and 10−8 M E2- and 10−4 M l-NAME-treated cells. Note that treatment with E2 decreased the number of SA-β-gal-positive cells, which was prevented by further treatment with l-NAME. Cells were used in PDL 22 at passage 7. (c) The effects of E2 (E, 10−8 M), ICI 182780 (I, 1 μM), and l-NAME (N, 1 mM) on telomerase activity in HUVECs. UD, undetectable. ∗∗, P < 0.01 vs. control.
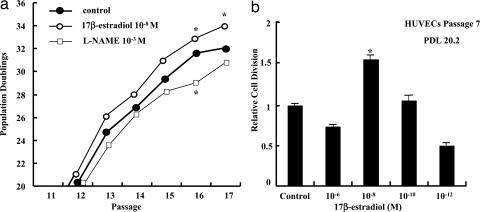
The physiological concentration of E2 also enhanced proliferation of HUVECs (Fig. 4). As the HUVEC proliferating activity tended to slow down in senescent cells, this basal mechanism seems to be different from that underlying the effect of E2 on telomerase and SA-β-gal. On the other hand, l-NAME treatment decreased proliferation of HUVECs in all PDL (Fig. 4a). The peak effect on cell proliferation was achieved with physiological concentrations of E2, whereas higher E2 concentration produced a lesser effect (Fig. 4b).
Fig. 4.
Effects of E2 on endothelial cell proliferation. (a) The effects of E2 (10−8 M) and l-NAME (1 mM) on population doublings in each passage of HUVECs. The treatment time with E2 or l-NAME was 24 h. ∗, P < 0.05 vs. control. (b) The effects of different concentrations of E2 on relative cell division of HUVECs. Cells were used in PDL 20.2 at passage 7. ∗, P < 0.05: cell division vs. control.
The Effect of NO Bioavailability on High-Glucose-Induced Cellular Senescence.
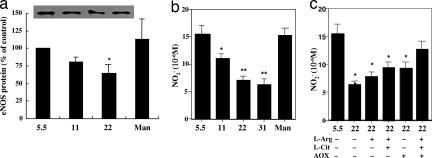
Finally, the effects of NO bioavailability on cellular senescence under high glucose conditions were investigated. Exposure to high glucose for 24 h decreased the expression level of eNOS protein in a manner dependent on the concentration of glucose, resulting in decreases of 19% at 11 mM and 33% at 22 mM glucose compared with the control (5.5 mM glucose) level (Fig. 5a). Mannitol, used as an osmolarity control, had no influence on eNOS protein level. In HUVECs cultured under high glucose conditions (11, 22, and 31 mM) for 3 days, nitrite (NO2−) production was decreased (Fig. 5b) and intracellular ROS production was increased (data not shown) in a manner dependent on the concentration of glucose. Treatment with l-arginine, l-citrulline, and antioxidants (vitamin C and E) alone or in combination showed a significant recovery of the decreased nitrite level under high glucose conditions (Fig. 5c). When l-arginine, l-citrulline and antioxidants were given together, the recovery of nitrite production was more marked.
Fig. 5.
Influence of high glucose on eNOS expression and nitrite production. (a) The effect of exposure to different concentrations of glucose on the level of eNOS protein expression in HUVECs. Mannitol (Man) was given as an osmolarity control. Cells were kept under different glucose conditions for 72 h. ∗, P < 0.05 vs. normal (5.5 mM) glucose. (b) The effect of exposure to different concentrations of glucose on nitrite levels in culture medium of HUVECs. ∗, P < 0.05; ∗∗, P < 0.01 vs. normal glucose. (c) The effects of l-arginine (L-arg, 1 mM), l-citrulline (L-cit, 300 μM), and antioxidants (AOX, 100 μM vitamin E plus 100 μM vitamin C) alone or in combination on nitrite levels in culture medium in HUVECs, which were reduced by 22 mM glucose. ∗, P < 0.05; ∗∗, P < 0.01 vs. normal glucose.
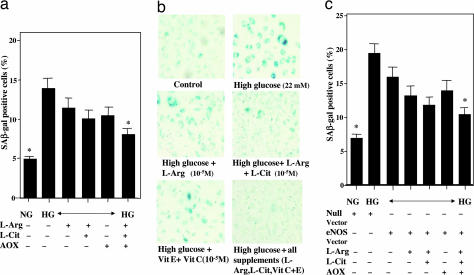
High glucose exposure for 72 h promoted cellular senescence as indicated by increases in SA-β-gal-positive staining (Fig. 6) and decrease in telomerase activity (data not shown). The number of SA-β-gal-positive staining cells under high glucose conditions tended to decrease slightly after incubation with l-arginine, l-citrulline, and antioxidants alone, and was significantly decreased when they were given together (Fig. 6 a and b). Moreover, transfection with eNOS tended to prevent cellular senescence slightly, and the combined presence of l-arginine, l-citrulline, and antioxidants very effectively prevented it under high glucose conditions (Fig. 6c).
Fig. 6.
Influence of high glucose for 72 h on cellular senescence of HUVECs. (a) The effects of l-arginine (L-arg, 1 mM), l-citrulline (L-cit, 300 μM), and antioxidants (AOX, 100 μM vitamin E plus 100 μM vitamin C) on the increase in β-gal-positive stained cells when exposed to high (22 mM) glucose. ∗, P < 0.05 vs. high glucose without any treatment. (b) Representative photographs showing cellular senescence by staining cells with SA-β- gal. (c) Modulation by transfection with eNOS of the effects of l-arginine, l-citrulline, and antioxidants on the increase in SA-β-gal-positive-stained cells when exposed to high glucose. Null vector is control vector of eNOS Vector. ∗, P < 0.05 vs. high glucose without any treatment. NG, normal glucose; HG, high glucose (22 mM).
Discussion
The free-radical theory of aging proposes that degenerative senescence is largely the result of the cumulative effect of ROS (9, 10). It is possible that some association exists between increased oxidative stress and reduced telomerase activity. Interestingly, individuals with shorter white blood cell telomeres tend to show a >2.8-fold higher coronary risk than the highest quartile for telomere length, after adjusting for age (23).
Telomerase counteracts the shortening of telomeres and contains a catalytic subunit, the hTERT (4, 5). The introduction of hTERT into human cells extends both their lifespan and their telomeres to lengths typical of those of young cells (5, 6). The regulation of hTERT involves both transcriptional and posttranscriptional mechanisms. Transcriptional regulation is believed to be the main regulatory mechanism in cancer cells (24). Telomerase activity can be posttranscriptionally regulated by kinases such as protein kinase C (PKC), extracellular signal-regulated kinase 1/2 (ERK1/2), and Akt [Akt/PKB (protein kinase B)] in endothelial cells (8–10). ROS formation leads to an increase in Src-family kinase activation and a reduction of Akt expression in aging endothelial cells. It is speculated that phosphorylation by Akt keeps hTERT in an active status in the nucleus, whereas increasing the activation of Src-family kinases induces the nuclear export of hTERT, thereby reducing the ability to lengthen telomeres and protect from aging. Along with the enhanced ROS formation, we found that a decrease in telomerase activity preceded the onset of replicative senescence. Thus, ROS such as the superoxide radical and H2O2, which are formed during aerobic metabolism, are generally considered to be important regulators of the aging processes, and their production may be mainly due to the actions of NADPH oxidase and the mitochondria (9, 10, 24–26). In the present study, we showed that DETA-NO, an NO donor, and eNOS transfection activate hTERT and increase scavenging of ROS. l-NAME inhibited the effect of eNOS transfection. These results mean that telomerase activity was likely regulated by NO bioavailability. Our data indicated that eNOS transfection has comparable effects to hTERT transfection on both cellular aging and telomerase activity. In addition, these findings might also indicate that endothelial cell aging is linked to the balance between ROS formation and NO bioavailability, which in turn affects telomerase activity.
eNOS transfection has an antiatherosclerotic effect even in cases of advanced atherosclerosis, and the administration of l-arginine with the gene transfer of eNOS enhances the effect of eNOS transfection (27, 28). We showed that the coadministration of antioxidants with l-arginine and l-citrulline produces an enhanced antiatherosclerotic response in advanced atherosclerosis (29). l-arginine seems to increase the production of NO, whereas antioxidants most likely protect the newly formed NO against destruction by ROS. Recent evidence indicates that the bulk of intracellular endothelial l-arginine may not be available for NO production, because intracellular l-arginine for eNOS may be limited by uptake into plasmalemmal caveolae (30). The pathway by which l-citrulline is recycled to l-arginine is localized to the caveolae and it may be the main source of available l-arginine (21, 22, 29, 31). l-citrulline is converted to l-arginine by mammalian cells, including endothelial cells. This recycling pathway might, therefore, play an important role in sustaining the production of NO in endothelial cells by providing available l-arginine, especially in advanced atherosclerosis or diabetes mellitus, when plasma l-arginine levels are depleted.
Physiological concentrations of E2 activate telomerase activity and decrease the number of SA-β-gal-stained cells through the estrogen receptor and NO-dependent mechanisms. E2 treatment also stimulated the proliferation of HUVECs through the estrogen receptor and NO-dependent mechanisms. We reported the possibility that such effects of estrogen were mediated by the direct effect on eNOS and the scavenging effect on ROS-producing enzymes such as NADPH oxidase, especially the p22phox subunit (32, 33). It is, therefore, proposed that estrogen exerts its effect on endothelial cell senescence by increasing NO bioavailability, which may then reduce ROS generation and subsequently prevent the nuclear export of TERT.
Atherosclerosis is an inflammatory disease characterized by endothelial dysfunction, impairment of NO production (1, 12 13), and oxidative stress (11), which can lead not only to cell membrane injury but also to the destruction of NO. Diabetic macroangiopathy occurs under almost the same conditions, with increased levels of superoxide from NADPH oxidase and impairment of NO production (34, 35). In the present study, high-glucose-induced endothelial dysfunction, oxidative stress, and cellular senescence were reversed with the administration of l-arginine, l-citrulline, and antioxidants. A lack of GTP cyclohydrolase I, which is the rate-limiting enzyme of tetrahydrobiopterin (BH4) synthesis, a cofactor of eNOS, also reduces NO production (36). We speculate that not only BH4 but also l-arginine, l-citrulline, and antioxidants are important in diabetic macroangiopathy. Although NO is known to be involved in reducing both oxidative stress and the progression of atherosclerosis, the present study also assessed the consequence of the NO-mediated delay of cellular senescence on the progression of atherosclerosis. The aforesaid notwithstanding, the local expression (bioavailability) of NO remains an important factor in the maintenance of normal tissue function. We also cannot exclude the possibility that other factors than NO is involved in the progressive cellular senescence in diabetes.
Taken together, the present data provide evidence demonstrating an NO-dependent mechanism in the delay of endothelial cell senescence. Consequently, the antiatherosclerotic action of NO is particularly profound under conditions of aging, estrogen depletion, and diabetes mellitus. NO could, therefore, scavenge the age-associated increase in ROS and thereby reduce the coronary risk factor-induced increase in ROS. Moreover, our data indicate that NO may also prevent endothelial cell senescence, possibly by interfering with the redox balance of endothelial cells.
Methods
Materials.
We used 17β-estradiol (Sigma, St. Louis, MO), d-glucose, d-mannitol (Wako, Osaka, Japan), Takara One Step RNA PCR Kit (Takara, Kyoto, Japan), and eNOS monoclonal antibody (BD Biosciences, San Jose, CA). ICI 182780 was kindly provided by Zeneca Pharmaceuticals. l-NAME and DETA-NO were obtained from Sigma-Aldrich (St. Louis, MO). Monoclonal antibodies to β-galactosidase (Chemicon International, Lexington, NY) were used (7, 37).
Cell Culture.
HUVECs were purchased from Clonetics (San Diego, CA) and cultured in low-glucose EBM-2 supplemented with 10% calf serum, EBM-2 including EGM-2 SingleQuots (Clonetics), 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin in a humidified atmosphere of 5% CO2, 95% air. These cells were positive for the endothelial cell-specific von Willebrand factor and angiotensin-1-converting enzyme activity. The cells were seeded into six-well plates, and subconfluent cell monolayers were studied within six to eight passages. Before starting the experimental procedures, the medium was removed and replaced with phenol red-free low-glucose D-MEM supplemented with 1% calf serum, 0.06% glutamine, and 1% penicillin–streptomycin. In some experiments accompanying eNOS transfection, HEK 293 cells were treated instead of HUVECs because of relative ease of transfection. The rate of PDL was calculated at each passage until growth arrest based on the following formula: PDL = (log10Y − log10X)/log102 (Y indicates the number of cells counted at the end of the passage; X is the number of cells seeded). Cumulative population doubling was calculated as the sum of all of the changes in population doubling.
Measurement of Nitrite.
The methods for measuring nitrite (NO2−) production by HUVECs have been previously described by our laboratory. In brief, samples of the incubation culture medium were recovered after centrifugation to remove any precipitated materials. The nitrite concentrations of the supernatants were determined by high-performance liquid chromatography (ENO10; EICOM, Kyoto, Japan) as described (29, 38). The incubated medium was not completely free of nitrite; therefore, an aliquot of medium was assayed by the same process as the medium obtained from the cultured cells. We used the nitrite value obtained in the medium alone as a blank, and it was subtracted from all of the samples.
Flow Cytometric Analysis of ROS Generation.
The determination of intracellular oxidant production in HUVECs was based on the oxidation of 2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (H2DCFDA) resulting in the formation of the fluorescent compound 2′,7′-dichlorofluorescein (DCF) (Molecular Probes, Eugene, OR) (29, 38). Carboxy-H2DCFDA freely diffuses across the cell membrane, is diacylated, and incorporates into hydrophobic lipid regions of the cell. HUVECs were incubated at 37°C for 30 min in PBS in which 2 μl of 5 mM H2DCFDA was added. After incubation, the dye was aspirated and the cells were trypsinized and washed once by centrifugation at 1,670 × g for 5 min to remove trypsin and extracellular H2DCFDA. HUVECs were resuspended in PBS and transferred into 5 ml of polystyrene round-bottom tubes with cell-strainer caps (Becton Dickinson, Franklin Lakes, NJ). They were protected from light and kept cold until ready for analysis on a FACS caliber flow cytometer (Becton Dickinson) set at ≈515- to 545-nm excitation. The emission filters used a 530/30-nm bandpass.
SA-β-Gal.
HUVECs and tissues were fixed and stained for SA-β-gal activity as described (37). In brief, the cells were fixed for 10 min in 2% formaldehyde, 0.2% glutaraldehyde in PBS, and incubated for 12 h at 37°C without CO2 with fresh β-gal staining solution: 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, and 2 mM MgCl2, pH 6.0. The cells were counterstained with 4′6-diamidino-phenylindole (DAPI; 0.2 mg/ml in 10 mM NaCl) for 10 min to count the total cell number. The percentage of SA-β-gal-positive cells was determined by counting the number of blue cells within a sample of 1,000 cells. We also used the Flow Cytometric Analysis.
Human Telomerase Activity.
The quantitative determination of telomerase activity was performed according to the manufacturer's protocol for the TeloTAGGG telomerase PCR ELISAPLUS Kit (Roche Diagnostics, Mannheim, Germany) based on the telomeric repeat application protocol (TRAP) assay. To measure telomerase activity, 2 μg of protein was used in the PCR.
Western Blot Analysis of eNOS.
Total protein was extracted from the endothelial cells and then analyzed by Western blotting (38, 39). Briefly, the protein concentration was determined with a Dc protein assay kit (Bio-Rad Laboratories, Hercules, CA). Samples of cell homogenate (5 μg) were subjected to electrophoresis on polyacrylamide gels, and proteins were transferred to poly(vinylidine difluoride) filter membranes. To reduce any nonspecific binding, the membrane was preincubated for 30 min at room temperature in TTBS (150 mM NaCl/10 mM Tris, pH 8.0/0.05% Tween 20) containing 5% nonfat milk. The membrane was then incubated overnight with the primary antibody at 3:10,000 dilutions in PBS (0.075 μg/ml). The membrane was incubated with the horseradish peroxidase-conjugated secondary antibody (1:10,000 dilution) for 60 min at room temperature. The blots were washed in TTBS and subsequently visualized with the aid of a SuperSignal West Dura Trial Kit (Pierce Biotechnology, Rockford, IL), exposed to x-ray film, and analyzed by the NIH Image Software program produced by Wayne Rasband (National Institutes of Health, Bethesda, MD). Loading of equal amounts of protein was confirmed by Coomassie brilliant blue and Amido black staining of protein in each lane of the same blot.
Construction of an Adenovirus Vector Carrying eNOS and Transfer into Cultured ECs.
Recombinant adenoviruses containing eNOS cDNA were constructed by using the ADENO-QUEST Kit (Quantum, Quebec City, Canada) (27). Briefly, bovine eNOS cDNA (provided by T. Michel, Harvard University, Cambridge, MA) was cloned into the AdBM5pAG vector. The resulting plasmid was then cotransfected with viral DNA into HEK 293 cells. We incubated 5 × 105 HUVECs in a six-well plate for 24 h, then incubated cells with adenoviruses at a multiplicity of infection of 20 for 24 h. For all of the studies, the viral titers were adjusted to 2 × 109 pfu/ml. Adenoviruses carrying an Escherichia coli lacZ gene encoding a nucleus-localized variant of β-gal (Ad. β-gal) or no cDNA (Ad.-null) were also used. We also used eNOS/pcDNA3.1(+) and Qiagen Effectane Transferase Reagent.
Statistics.
All data are given as means ± SEM from at least three independent experiments. Comparisons between the two groups were made based on the nonparametric Mann–Whitney U test. Statistical significance was evaluated with repeated-measures ANOVA by using a least-significant difference (LSD) post hoc test or ANOVA for multiple comparisons (SPSS Software 11.0). Differences were considered to be significant at a value of P < 0.05.
Abbreviations
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- SA-β-gal
senescence-associated β-galactosidase
- hTERT
human telomerase reverse transcriptase
- HUVECs
human umbilical vein endothelial cells
- l-NAME
NG-nitro-l-arginine methyl ester
- DETA-NO
(Z)-1-[2-(2-aminoethyl)-N-(2-aminoethyl) amino] diazen-1-ium-1,2-diolate
- PDL
population-doubling level.
Footnotes
The authors declare no conflict of interest.
References
- 1.Zeiher AM, Drexler H, Saurbier B, Just H. J Clin Invest. 1993;92:652–662. doi: 10.1172/JCI116634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein S. Science. 1990;249:1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann J, Haendeler J, Aicher A, Rossig L, Vasa M, Zeiher AM, Dimmeler S. Circ Res. 2001;89:709–715. doi: 10.1161/hh2001.097796. [DOI] [PubMed] [Google Scholar]
- 4.Liu JP. FASEB J. 1999;13:2091–2104. doi: 10.1096/fasebj.13.15.2091. [DOI] [PubMed] [Google Scholar]
- 5.Hsiao R, Sharma HW, Ramakrishnan S, Keith E, Narayanan R. Anticancer Res. 1997;117:827–832. [PubMed] [Google Scholar]
- 6.Yang J, Chang E, Cherry AM, Bangs CD, Oei Y, Bodnar A, Bronstein A, Chiu CP, Herron GS. J Biol Chem. 1999;274:26141–26148. doi: 10.1074/jbc.274.37.26141. [DOI] [PubMed] [Google Scholar]
- 7.van der Loo B, Fenton MJ, Erusalimsky JD. Exp Cell Res. 1998;241:309–315. doi: 10.1006/excr.1998.4035. [DOI] [PubMed] [Google Scholar]
- 8.Breitschopf K, Zeiher AM, Dimmeler S. FEBS Lett. 2001;493:21–25. doi: 10.1016/s0014-5793(01)02272-4. [DOI] [PubMed] [Google Scholar]
- 9.Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. J Cell Sci. 2004;117:2417–2426. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- 10.Haendeler J, Hoffmann J, Brandes RP, Zeiher AM, Dimmeler S. Mol Cell Biol. 2003;23:4598–4610. doi: 10.1128/MCB.23.13.4598-4610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 12.Ignarro LJ, Napoli C. Curr Atherosclerosis Rep. 2004;6:281–287. doi: 10.1007/s11883-004-0059-9. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi T, Ishikawa T, Naito M, Kuzuya M, Funaki C, Asai KH, Kuzuya F. Atherosclerosis. 1991;87:23–38. doi: 10.1016/0021-9150(91)90229-v. [DOI] [PubMed] [Google Scholar]
- 14.Ignarro LJ, Buga GM, Wei L-H, Bauer PM, Wu G, del Soldato P. Proc Natl Acad Sci USA. 2001;98:4202–4208. doi: 10.1073/pnas.071054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannel WB. J Gend Specif Med. 2002;5:27–37. [PubMed] [Google Scholar]
- 16.Nathan L, Pervin S, Singh R, Rosenfeld M, Chaudhuri G. Circ Res. 1999;85:377–385. doi: 10.1161/01.res.85.4.377. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi T, Jayachandran M, Sumi D, Thakur NK, Esaki T, Muto E, Kano H, Asai Y, Iguchi A. Arterioscler Thromb Vasc Biol. 2000;20:1613–1621. doi: 10.1161/01.atv.20.6.1613. [DOI] [PubMed] [Google Scholar]
- 18.Harman SM, Naftolin F, Brinton EA, Judelson DR. Ann NY Acad Sci. 2005;1052:43–56. doi: 10.1196/annals.1347.004. [DOI] [PubMed] [Google Scholar]
- 19.Cai H, Harrison DG. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 20.Resnick HE, Shorr RI, Kuller L, Franse L, Harris TB. J Clin Epidemiol. 2001;54:869–876. doi: 10.1016/s0895-4356(01)00359-6. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin BL, Solomonson LP, Eichler DC. J Biol Chem. 2004;279:18353–18360. doi: 10.1074/jbc.M308160200. [DOI] [PubMed] [Google Scholar]
- 22.Solomonson LP, Flam BR, Pendleton LC, Goodwin BL, Eichler DC. J Exp Biol. 2003;206:2083–2087. doi: 10.1242/jeb.00361. [DOI] [PubMed] [Google Scholar]
- 23.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. Arterioscler Thromb Vasc Biol. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 24.Buys CH. N Engl J Med. 2000;342:1282–1283. doi: 10.1056/NEJM200004273421710. [DOI] [PubMed] [Google Scholar]
- 25.Montagna W, Carlisle K. Br J Dermatol. 1990;35(Suppl 122):61–70. doi: 10.1111/j.1365-2133.1990.tb16127.x. [DOI] [PubMed] [Google Scholar]
- 26.Channon KM, Guzik TJ. J Physiol Pharmacol. 2002;53:515–524. [PubMed] [Google Scholar]
- 27.Hayashi T, Sumi D, Packiasamy AJ, Matsui-Hirai H, Asai-Tanaka Y, Kano H, Fukatsu A, Tsunekawa T, Miyazaki A, Iguchi A, Ignarro LJ. Cardiovasc Res. 2004;61:339–351. doi: 10.1016/j.cardiores.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 28.Frey A, Schneider-Rasp S, Marienfeld U, Yu JC, Paul M, Poller W, Schmidt HH. Biochem Pharmacol. 1999;58:1155–1166. doi: 10.1016/s0006-2952(99)00196-3. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi T, Packiasamy AJ, Matsui-Hirai H, Miyazaki A, Fukatsu A, Funami J, Iguchi A, Ignarro LJ. Proc Natl Acad Sci USA. 2005;102:13681–13686. doi: 10.1073/pnas.0506595102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardy TA, May JM. Free Radical Biol Med. 2002;32:122–131. doi: 10.1016/s0891-5849(01)00781-x. [DOI] [PubMed] [Google Scholar]
- 31.Flam BR, Hartmann PJ, Harrell-Booth M, Solomonson LP, Eichler DC. Nitric Oxide. 2001;5:187–197. doi: 10.1006/niox.2001.0340. [DOI] [PubMed] [Google Scholar]
- 32.Krotz F, Sohn HY, Pohl U. Arterioscler Thromb Vasc Biol. 2004;24:1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- 33.Jiang F, Drummond GR, Dusting GJ. Endothelium. 2004;11:79–88. doi: 10.1080/10623320490482600. [DOI] [PubMed] [Google Scholar]
- 34.Basta G, Schmidt AM, De Caterina R. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 36.Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, Goh N, Rockett KA, Channon KM. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fenton M, Barker S, Kurz DJ, Erusalimsky JD. Arterioscler Thromb Vasc Biol. 2001;21:220–226. doi: 10.1161/01.atv.21.2.220. [DOI] [PubMed] [Google Scholar]
- 38.Ding QF, Hayashi T, Packiasamy AJ, Miyazaki A, Fukatsu A, Shiraishi H, Nomura T, Iguchi A. Life Sci. 2004;75:3185–3194. doi: 10.1016/j.lfs.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Jayachandran M, Hayashi T, Sumi D, Iguchi A, Miller VM. Am J Physiol Heart Circ Physiol. 2001;281:H1327–H1333. doi: 10.1152/ajpheart.2001.281.3.H1327. [DOI] [PubMed] [Google Scholar]