Abstract
Glioblastoma multiforme (GBM) is the most common subtype of primary malignant brain tumor. Although serotype 5 adenoviral vectors (Ads) have been used successfully in clinical trials for GBM, the capacity of Ads to infect human glioma cells and the expression of adenoviral receptors in GBM cells have been challenged. In this report, we studied the expression of three molecules that have been shown to mediate adenoviral entry into cells, i.e., coxsackie and adenovirus receptor (CAR), integrin αvβ3 (INT), and major histocompatibility complex class I (MHCI), in rodent glioma cell lines and low-passage primary cultures and cell lines from human GBM. We correlated levels of expression of CAR, INT, and MHCI with transduction efficiency elicited by several high-capacity helper-dependent adenoviral vectors (HC-Ads). Expression levels of adenoviral receptors were variable among the different GBM cells studied. HC-Ad-mediated therapeutic gene expression was efficient, ranging between 20 and 80% of the total target cells expressing the encoded transgenes. Our results show no correlation between the levels of CAR, INT, or MHCI molecules and the levels of transgene expression or the number of GBM cells transduced. We conclude that expression levels of adenoviral receptors do not predict their transduction efficiency or biological function.
Keywords: gutless adenovirus vectors, glioblastoma multiforme, HSV1-TK, Flt3L, β-galactosidase, CAR, integrins, MHCI, cell death, TetON
INTRODUCTION
Glioblastoma multiforme (GBM) is incurable despite surgery, radiotherapy, and chemotherapy. Its infiltrative nature and cytological heterogeneity constitute a therapeutic challenge [1,2]. Thus, new therapeutic approaches have been attempted. Adenoviral vectors (Ads) of serotype 5 have been extensively used for gene transfer to human glioblastoma cells in vitro and in vivo. Ads encoding herpes simplex virus type 1 thymidine kinase (HSV1-TK), carboxypeptidase G2, or FasL can infect primary cultures of human glioma cells obtained from surgical biopsies [3] in vitro and exert cytotoxicity [4–6]. Adenoviral vectors also showed higher transduction efficiency than retroviral vectors in human clinical trials [7]. Further, the intratumoral administration of an Ad encoding HSV1-TK to GBM patients increased survival in two different trials [8,9]. However, the efficiency of adenoviral vectors at infecting human glioma cells remains controversial. During the past years, the presence of adenoviral receptors in human glioma cells as well as their correlation with adenoviral transduction efficiency has been highly debated [10–18].
Adenoviral vectors’ entry into cells is thought to be initiated by high-affinity binding of the adenoviral fiber protein knob to the cell surface receptor, coxsackie and adenovirus receptor (CAR) [19]. The interaction of the adenoviral penton protein with cell surface integrins (INT), such as INT αvβ3 and αvβ5, leads to the internalization of the virus by endocytosis [19]. However, this mechanism on its own fails to explain the infection process of Ads into all cell types. In the brain, ablation of CAR binding changes Ad tropism. Mutation of the fiber protein to ablate CAR binding results in loss of oligoden-drocyte transduction and redirection of transduction to neurons and glial cells [20]. Mutated adenoviruses unable to bind CAR and INT still transduce primary human GBM cells and tumor explants [17]. Variable expression of CAR has been detected in human glioblastoma explants [13,15,17,18]. The expression of CAR in several human glioma cell lines has also shown to be very variable [10–13,15,18,21]. Nevertheless, these cells were successfully infected with Ad vectors [10,12,15,18,22].
Another molecule that has been proposed to be involved in the initial binding of adenoviruses to cell membranes is the major histocompatibility complex class I (MHCI) α2 domain [23]. Since this molecule has been detected in human glioma cells [24], it could also be involved in adenoviral entry.
Clinical treatment of GBM with Ad vectors can be compromised by the fact that the majority of adults have been exposed to adenovirus. Thus, to ensure stable, long-term transgene expression, avoiding side effects elicited by antiadenoviral immune responses, high-capacity helper-dependent adenoviral vectors (HC-Ad) have been developed [25–28]. In this paper, we generated two HC-Ad vectors encoding HSV1-TK or Fms-like tyrosine kinase 3 ligand (Flt3L), two therapeutic targets that we have previously shown to be highly efficacious in a preclinical GBM model [29]. We tested their ability to transduce human and murine glioma cells and correlated their transduction efficiency with the levels of CAR, INT, and MHCI. Interestingly, we found no statistically significant correlation between the expression of CAR, INT, or MHCI and the levels of transgene expression, the number of transduced cells, or measures of the transgenes’ biological activity. Our results support HC-Ads as excellent candidates for therapeutic approaches that require gene delivery into human GBM. However, measures of Ad entry receptors remain poor predictors of Ad-mediated transduction efficiency in GBM cells.
RESULTS
Human and Murine Glioma Cells Express Variable Amounts of Adenoviral Receptors
To determine the feasibility of transducing human glioma cells with high-capacity adenoviral vectors, we first studied the expression of several proteins involved in adenovirus binding and internalization. We determined CAR and INT expression by Western blotting using total cellular protein extracts (Fig. 1). We used CHO cells as negative controls due to their lack of expression of CAR and INT [16]. We used HeLa cells as positive controls, since the expression of CAR and INT is well documented in these cells [10]. We found different levels of CAR and INT in human and murine glioma cells (Fig. 1A). U251 cells expressed higher levels of CAR compared to all cells tested. U87 cells expressed comparable levels of CAR compared to HeLa cells. In IN2045 and IN859 human glioma short-term cultures and CNS-1 and GL26 murine glioma cells CAR could not be detected. Expression of INT was detectable in all the human glioma cells analyzed and in mouse GL26 cells; in rat CNS-1 cells, INT levels were undetectable (Fig. 1B).
FIG. 1.

Expression of coxsackie and adenovirus receptor (CAR) and integrin αvβ3 (INT) in human and murine glioma cells. (A) CAR and (B) integrin αvβ3 expression was determined by Western blot analysis in human glioma cell lines (U251, U87), cultures from human glioma biopsies (IN859, IN 2045), rat (CNS-1), and mouse (GL26) glioma cells. CHO and HeLa cells were used as negative and positive controls, respectively. β-Actin was used as loading control. The tops show Western blots for CAR (A), INT (B), and β-actin from glioma, CHO, and HeLa cell lysates. The bottom panels show the integrated density values of CAR or INT in relation to β-actin expression for all cells analyzed.
MHCI was reported to be involved in adenovirus binding and entry into cells [23]; we therefore determined the expression of this protein in glioma cells by flow cytometry (Fig. 2). We detected the expression of MHCI in all human and murine glioma cells. IN2045 human glioma cultures exhibited the highest expression of MHCI, followed by U251. We found the lowest expression of MHCI in U87 human glioma cells and in the murine glioma cells, CNS-1 and GL26 (Fig. 2).
FIG. 2.
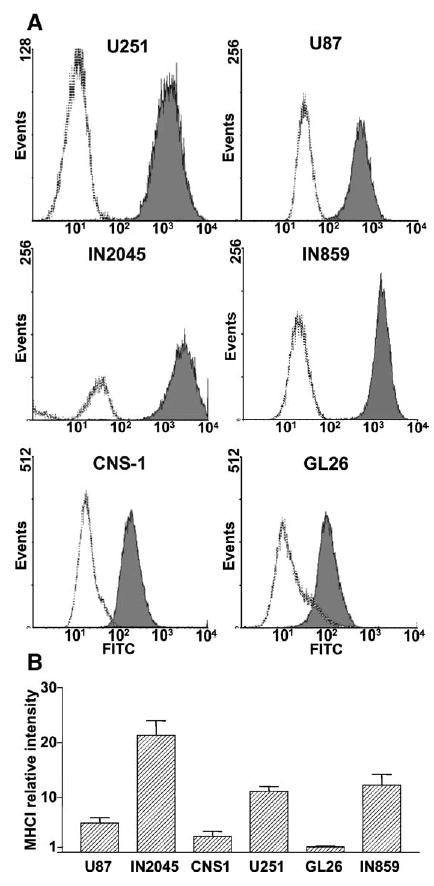
Flow-cytometric analysis of major histocompatibility complex class I (MHCI) expression in human and murine glioma cells. MHCI expression was detected in established human glioma cell lines (U251, U87), short-term cultures from human glioma biopsies (IN859, IN 2045), and rat (CNS-1) and mouse (GL26) glioma cells by flow cytometry. (A) Representative histograms show the intensity of fluorescence for each cell line incubated with FITC-conjugated isotype control (dotted line) or anti-MHCI antibody (solid line, gray area). (B) Columns represent the means ± SEM (n = 3–5) of MHCI fluorescence intensity after subtraction of control isotype intensity. Results were standardized to the intensity of GL26 cells, which exhibited the lowest fluorescence levels of MHCI.
The Murine CMV Promoter Exerts High Levels of Transgene Expression in GBM Cells
For clinical applications, it is important to achieve maximum levels of transgene expression per input viral vector particle to diminish the dose of vectors needed to achieve therapeutic efficacy and eliminate adverse side effects due to high vector doses. To determine conclusively whether the murine CMV promoter would yield higher levels of transgene expression in human glioma cells, we determined transgene expression levels after infection with high-capacity adenoviral vectors encoding β-galactosidase (β-Gal). We infected human and murine glioma cells with regulated HC-Ad vectors encoding the reporter gene, β-Gal, which can be detected by immunocytochemistry and enzymatic activity. These vectors encode the transgene under the control of the TetON system expressed under the control of the murine (HC-mTetON-βGal) or human CMV promoter (HC-hTetON-βGal). Immunocytochemical analysis showed that human and murine glioma cells were efficiently transduced by both vectors with stringent control by doxycycline (Dox) (Fig. 3B). To determine which promoter, mCMV or hCMV, exerts higher expression in the ON state, we compared the levels of β-Gal activity exerted by each of these vectors in all the cell lines (Fig. 3A). In all the human glioma cells, as well as CNS-1 cells, HC-Ad-mTetON-βGal yielded higher transgene expression levels than HC-Ad-hTetON-βGal in the presence of Dox. However, in mouse GL26 cells both vectors yielded similar levels of β-Gal activity. The levels of β-Gal activity per transduced cell were higher when they were infected with HC-Ad-mTetON-βGal in all the human cell lines (Fig. 3C). Among the murine glioma cells, β-Gal activity/cell was higher in CNS-1 cells infected with HC-Ad-mTetON-βGal, while GL26 cells yielded similar β-Gal levels/cell when infected with either HC-Ad vector.
FIG. 3.
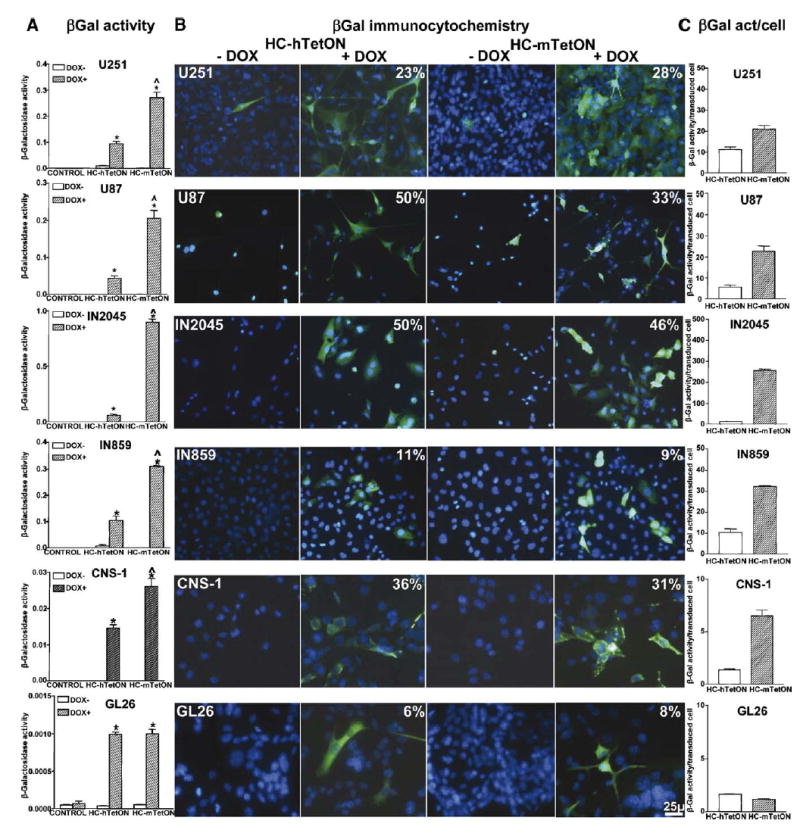
Expression and bioactivity of β-galactosidase from regulatable high-capacity adenoviral vectors encoding the TetON system under the control of the human (HC-Ad-hTetON) or the murine (HC-Ad-mTetON) CMV promoter in human and murine glioma cells. Established human glioma cell lines (U251, U87), cultures from human glioma biopsies (IN859, IN 2045), and rat (CNS-1) and mouse (GL26) glioma cells were infected with HC-Ad vectors encoding β-galactosidase under the control of a TetON system driven by the human CMV (HC-Ad-hTetON) or the murine CMV promoter (HC-Ad-mTetON). Cells were incubated in the presence or absence of the inducer, doxycycline (DOX; 1 μg/ml), and, after 72 h, transgene expression was determined by (A) β-galactosidase activity assay and (B) immunocytochemistry. The percentage of β-Gal-positive cells in the presence of DOX is depicted in the top right corners of the photos. *P < 0.05 vs DOX−; ^P < 0.05 vs HC-Ad-hTetON, two-way ANOVA followed by Duncan’s test. (C) Activity of β-galactosidase per cell transduced with HC-Ad-hTetON or HC-Ad-mTetON. The columns represent the means ± SEM of β-Gal activity per cell expressing β-Gal, as determined by unbiased cell counts of β-Gal-immunoreactive cells.
Construction of HC-Ad Vectors Encoding Therapeutic Transgenes: HSV1-TK and Flt3L
We developed two high-capacity adenoviral vectors encoding the therapeutic transgenes, i.e., HSV1-TK and Flt3L (Fig. 4). Expression of HSV1-TK in combination with Flt3L from Ads is efficacious at inducing GBM regression and long-term survival in a large intracranial model in Lewis rats [29]. Since the murine CMV promoter exerted stronger transgene expression, not only in rat CNS-1 cells, but also in all human glioma cells, the transgenes in these HC-Ad vectors were encoded under the control of the mCMV promoter. HC-Ad-TK expresses HSV1-TK constitutively, under the control of the mCMV promoter. We also constructed and purified HC-Ad-mTetON-Flt3L, which encodes human soluble Flt3L under the control of the regulatable TetON system driven by the mCMV promoter (Fig. 4). Following cloning of all transcriptional cassettes to develop the HC-Ad vector plasmids, we performed restriction digests to map the plasmid DNA constructs (Figs. 4A, 4B, and 4C). After confirming the presence and the required orientation of the TetON sequence and the transgene expression cassettes, we proceeded to rescue, scale up, and purify both HC-Ads as previously described [25,27]. The titer of HC-Ad-TK was 4.6 × 1012 vector particles (vp)/ml containing 106 plaque-forming units (pfu)/ml of helper virus (HV); the titer for HC-Ad-mTetON-Flt3L was 6.97 × 1012 vp/ml containing 105 pfu/ml of HV. Both vector preparations were RCA and LPS negative.
FIG. 4.

Schematic representations of pSTK120-mCMV-TK-polyA ≈ 32 kb (HC-Ad-mCMV-TK, left) and pSTK120.2-[TRE-hsFlt3L-polyA]-[mCMV-rtTA2SM2-pIRES-tTSKid-pA]-Kana ≈36 kb (HC-Ad-mCMV-TetON-Flt3L, right). (A) HC-Ad plasmid maps indicate the orientation of the expression cassettes as well as the constituents and orientation of the mCMV-driven regulatable TetON switch cassette within the pSTK gutless plasmids. (B) Gel electrophoresis and restriction map analysis of HC-Ad plasmid DNA to assess expected band sizes. For both gels, the lanes are as follows: lane 1, hyperladder I (corresponding sizes are labeled to the right of each gel); lane 2, HindIII digest; lane 3, NheI digest; lane 4, PmeI digest; lane 5, hyperladder I. (C) Linear depiction of the HC-Ad vector encoding the TK transgene (top) or hsFlt3L regulated by the mCMV-driven TetON switch (bottom). The constructs indicate the individual components and the orientation of the cassettes and their promoters. Some restriction enzyme sites are shown with the appropriate size fragments, which correspond to the sizes indicated in panel B.
Cytotoxic Effects of HC-Ad-TK on Glioma Cells in the Presence and Absence of Ganciclovir (GCV)
We determined the expression of HSV1-TK by immunocytochemistry. As shown in Fig. 5B, we detected strong expression of TK in all the glioma cells infected with HC-Ad-TK. The transduction efficiency of this vector ranged between 25 and 80%; the highest transduction efficiency was detected in U251 cells. GL26 and IN859 cells exhibited a significantly higher percentage of HC-TK-transduced cells compared to the percentage of β-Gal-expressing cells. This could be due to the fact that expression of TK was assessed by immunocytochemistry using biotinylated secondary antibodies followed by avidin–DAB amplification, a more sensitive technique than immunofluorescence used to detect β-Gal. We detected the cytotoxic effects of HSV1-TK/GCV by flow-cytometric analysis of the cell cycle of cells labeled with propidium iodide [5]. Incubation of cells with 10 μM GCV had no cytotoxic effect per se. However, incubation with GCV after HC-Ad-TK infection exerted potent cytotoxicity in all the cell lines tested (Fig. 5A). We determined levels of 30–50% cell death in all the cell lines, except for IN2045 human GBM cells, which exhibited levels of almost 100% cell death. In U251 human GBM cells, TK expression was cytotoxic in the absence of GCV, causing low levels of cytotoxicity (~20%); in the presence of GCV, cytotoxicity increased to 35%.
FIG. 5.
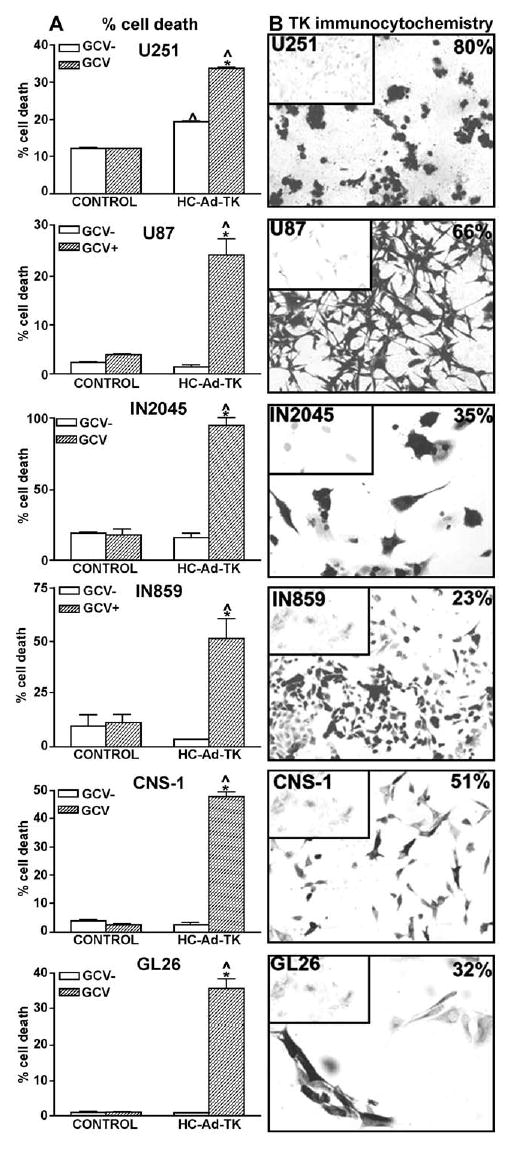
Expression and bioactivity of HSV1-TK from a high-capacity adenoviral vector in human and murine glioma cells. Established human glioma cell lines (U251, U87), cultures from human glioma biopsies (IN859, IN 2045), and rat (CNS-1) and mouse (GL26) glioma cells were infected with 1 transgene-expressing particle/cell of a HC-Ad vector encoding HSV1-TK under the control of the murine CMV promoter (HC-Ad-TK) for 48 h. Cells were incubated in the presence or absence of the prodrug ganciclovir (GCV; 10 μM) for 48 h. (A) Cell death was determined by flow-cytometric analysis of cell cycle. Columns represent the means ± SEM of the percentage of hypodiploid cells (sub-G1). *P < 0.05 vs control without GCV (GCV−); ^P < 0.05 vs mock-infected cells (control), two-way ANOVA followed by Duncan’s test. (B) Transgene expression was determined by immunocytochemistry in the absence of GCV. The percentage of TK-positive cells is depicted in the top right corners of the pictures. Inset: Mock-infected cells.
To determine whether the cytotoxic effects of HC-Ad-TK/GCV were transgene specific, and not due to HC-Ad infection, we infected cells with a HC-Ad encoding β-Gal under the control of the mCMV promoter (HC-Ad-βGal), at the same dose as HC-Ad-TK in the presence or absence of GCV (supplementary data). HC-Ad-βGal did not modify the percentage of cell death of any cell line tested, even in the presence of GCV.
Transgene Expression from HC-Ad-mTetON-Flt3L Is Tightly Regulated in GBM Cells
We determined transgene expression from HC-Ad-mTe-tON-Flt3L by immunocytochemistry and by ELISA in the supernatant of infected cells. HC-Ad-mTetON-Flt3L transduced all glioma cells efficiently (Fig. 6). The expression of Flt3L was tightly regulated by Dox. The transduction efficiency of this vector was high, ranging between 50 and 80% for the human glioma cells and CNS-1 cells, while 27% of infected GL26 expressed Flt3L. Flt3L expressed from HC-mTetON-Flt3L was efficiently released by all the GBM cells (Fig. 6A). The levels of Flt3L in the presence of Dox were around 5–60 nmol/ml, while in the absence of Dox they were undetectable ( P < 0.001). The levels of Flt3L released per transduced cell ranged between 180 and 800 fmol/cell (Fig. 6C).
FIG. 6.

Secretion of Flt3L from the regulatable high-capacity adenoviral vector (HC-Ad-mTetON-Flt3L) in human and murine glioma cells. Established human glioma cell lines (U251, U87), cultures from human glioma biopsies (IN859, IN 2045), and rat (CNS-1) and mouse (GL26) glioma cells were infected with HC-Ad vector encoding Flt3L under the control of a TetON system driven by the murine CMV promoter (HC-Ad-mTetON-Flt3L) for 72 h in the presence or absence of the inducer doxycycline (DOX; 1 μg/ml). (A) Transgene expression was determined in the supernatant of the cultures using an ELISA kit that detects human Flt3L. *P < 0.05 vs DOX−; ^P < 0.05 vs mock-infected cells, two-way ANOVA followed by Duncan’s test. (B) Transgene expression was determined by immunocytochemistry. The percentage of Flt3L-positive cells in the presence of DOX is depicted in the top right corners of the pictures. Inset: Cells infected with HC-Ad-mTetON-Flt3L in the absence of DOX. (C) Numbers indicate the means ± SEM of Flt3L released from each cell expressing Flt3L, as determined by unbiased cell count of Flt3L-immunoreactive cells.
Transgene Expression Levels From HC-Ads Do Not Correlate with Adenoviral Receptor Expression in Glioma Cells
All the GBM cell lines and GBM cells from intraoperative biopsies in short-term culture were efficiently transduced by HC-Ad vectors. To determine if the levels of expression of CAR, INT, or MHCI played a role in adenoviral-mediated transgene expression within GBM cells, we performed a Pierce correlation analysis between their expression levels and the transgene expression levels in all GBM cells tested. The only positive significant correlation was established between MHCI expression and the percentage of HC-Ad-TK/GCV-induced cell death (Table 1).
TABLE 1.
Correlation analysis between transgene expression from different HC-Ad vectors and the levels of expression of CAR, INT, and MHCI in human and murine glioma cells
| Adenoviral receptor | ||||
|---|---|---|---|---|
| Vector | Transgene expression | CAR | INT | MHCI |
| HC-Ad-mTetON-βGal | % cells expressing transgene | 0.04 | 0.01 | 0.63 |
| β-Gal activity | 0.06 | 0.19 | 0.22 | |
| β-Gal activity/cell | 0.01 | 0.01 | 0.12 | |
| HC-Ad-hTetON-βGal | % cells expressing transgene | 0.01 | 0.39 | 0.56 |
| β-Gal activity | 0.06 | 0.21 | 0.01 | |
| β-Gal activity/cell | 0.24 | 0.52 | 0.01 | |
| HC-Ad-TK | % cells expressing transgene | 0.62 | 0.13 | 0.02 |
| % cell death | 0.12 | 0.01 | 0.73* | |
| HC-Ad-TetON-Flt3L | % cells expressing transgene | 0.14 | 0.17 | 0.03 |
| Flt3L release | 0.16 | 0.14 | 0.01 | |
Transgene expression levels (as assessed by β-Gal activity, HSV1-TK/GCV-induced cell death, and Flt3L release and the percentage of cells transduced by HC-Ad-mTetON-βGal, HC-Ad-hTetON-βGal, HC-Ad-TK, and HC-Ad-mTetON-Flt3L) were paired with CAR and INT expression levels, as assessed by Western blot, and MHCI expression levels, as determined by flow cytometry in U251, U87, IN859, IN 2045, CNS-1, and GL26 glioma cells. Pierce correlation analysis was used to determine the correlation coefficient (R2) between the expression of the receptors and transduction efficiency.
P < 0.05.
DISCUSSION
Adenovirus entry into cells follows the binding of Ad to the CAR and the interaction with cell surface INTs αvβ3 and αvβ5, which triggers Ad endocytosis [19]. Additionally, the fiber shaft contains protein epitopes that bind heparin sulfate proteoglycans (HSPG), which can act as an additional receptor for adenovirus [30]. Interestingly, expression of CAR and integrins is tissue- and cell-type specific [13], while expression of HSPG is almost universal [31]. The presence of CAR and its relationship to the capacity of Ad to transduce human glioma cells remain controversial. For example, poor transduction has been attributed to low levels of Ad type 5 receptors in these cells [13,18,32,33]. However, many reports have demonstrated that cells with low to negligible expression of CAR remain susceptible to Ad transduction [12,15,18,22]. In fact, gene therapy clinical trials for GBM showed that Ads efficiently transduce such tumors and are clinically more effective compared to retroviral vectors or standard treatment [7,9,34–36]. In a landmark human GBM in vivo experiment, 10% of glioma cells were transduced 2 days following intratumoral administration of Ad, a very high transduction efficiency compared to the almost negligible levels seen for retroviral transduction of the same tumor type [7]. A recently reported phase I open-label, dose-escalation, multi-institutional GBM trail using an E1B-attenuated adenovirus type 5, ONYX-015, showed that the treatment was well tolerated and the efficacy results were very encouraging, thus warranting further testing in patients with malignant GBM [34].
The capacity of Ad5 vectors to transduce human glioma cells is considered to be a limiting factor for therapeutic success. The importance of this issue concerns all those developing adenoviral-mediated gene therapeutic approaches for GBM. Our own and our colleagues’ successes in treating experimental syngeneic glioma tumors using conditionally cytotoxic, directly cytotoxic, immune-stimulatory, and combined approaches encoded within Ad5-type vectors, and the clinical success and safety of conditional cytotoxic and oncolytic viral approaches for the treatment of GBM, give the elucidation of this issue some urgency (reviewed in [1,37–40]).
We thus examined the correlation between the primary receptors for Ad5 and various indices of transduction and vectors’ therapeutic efficacy, in established glioma cell lines from rats and mice, established cell lines from human GBM, and low-passage cells obtained from GBM intra-operative biopsies. We detected heterogeneous levels of CAR and INT throughout the GBM cells tested. Detailed statistical correlation analysis between the indices of adenoviral receptors and indices of numbers of transduced cells and transgene expression levels failed in the vast majority of cases to reach statistical significance. The lack of correlation between levels of Ad receptors and indices of transduction indicates that levels of primary Ad receptors are not predictive of the efficiency of Ads in transducing GBM cells. The high multiplicity of infection used for infecting target cells in vitro and for gene delivery in vivo in preclinical models and in human trials is likely to play a role in mediating Ad attachment and internalization.
In this article we report that human and murine glioma cells consistently express MHCI, which has been shown to participate in Ad infection [23]. The levels of MHCI expression surprisingly provided the only statistically significant positive correlation with transgene bioactivity across all glioma cells tested. However, the percentage of dead cells after TK/GCV treatment does not reflect solely the efficiency of transduction, since the bystander effect elicited by HSV1-TK plus GCV in actively dividing cells is likely to potentiate tumor cell killing [5,41].
Regulatable systems that can turn “on” and “off” therapeutic gene expression are likely to be preferred in future clinical gene therapeutic approaches, to reduce putative adverse side effects of the therapy [42,43]. Our data show that regulatable HC-Ad vectors successfully express transgenes in a regulated manner in human glioma cells. In agreement with our previous results in the rat brain in vivo [27], HC-Ad-mTetON-βGal proved to yield higher transgene expression levels compared to HC-Ad-hTetON-βGal in all human glioma cells tested. The immunocytochemical analysis of the cells showed that in each cell line, the percentage of cells transduced by both vectors is similar, as expected, since the infection efficiency is dependent on capsid proteins that are identical in both HC-Ad vectors. However, the levels of β-Gal activity per transduced cell were at least twofold higher when the human glioma cells were transduced with HC-Ad-mTetON-βGal than with HC-Ad-hTetON-βGal. Our results demonstrate for the first time, using short-term cultures of human GBM cells and human GBM cell lines, that the murine CMV promoter is stronger than the human CMV promoter when encoded within Ad vectors independent of the species origin of the target cells [27,44,45].
First-generation Ads encoding HSV1-TK and Flt3L were shown to constitute an effective anti-glioma treatment in preclinical models of intracranial, syngeneic GBM in Lewis rats [29]. This therapy leads to cytotoxicity and release of tumor antigens combined with the recruitment and activation of immune cells, especially plasmacytoid dendritic cells within the brain [46], causing the regression of large intracranial GBM masses and increasing the life span of animals [29]. Thus, we constructed two HC-Ad encoding the therapeutic transgenes (i.e., HSV1-TK or Flt3L) that will be implemented for GBM clinical trials. Based on the results with vectors expressing a marker transgene, the expression of both therapeutic transgenes was driven by the murine CMV promoter. HC-Ad-TK and HC-Ad-mTe-tON-Flt3L effectively transduced all glioma cells tested. HSV1-TK expression mediated a strong cytotoxic effect when glioma cells were incubated with GCV. In U251 human glioma cells cytotoxicity was also detected in HC-Ad-TK-infected cells in the absence of GCV, indicating that HSV1-TK is cytotoxic to these cells, a phenomenon observed in other cell lines [5,47] and transgenic mice with cell-targeted expression of HSV1-TK [48]. Although GL26 cells yielded lower levels of transgene expression than other glioma cells, as assessed by quantitative assays, such as β-Gal activity or Flt3L ELISA, GL26 cells were equally as sensitive to apoptosis as other glioma cell lines. However, this is not a direct assessment of the expression levels of HSV1-TK; rather, it depends on the sensitivity of glioma cells to apoptosis induced by adenoviral vectors expressing HSV1-TK, which is also dependent on the bystander effect [4,5,41]. HC-Ad-mTetON-Flt3L also proved to transduce human glioma cells efficiently, with stringent regulation of Flt3L expression by the inducer. An advantage of this approach will be that due to the bystander effect of TK/ GCV [5,41] added to the immune stimulatory effect of Flt3L [29,46], limited numbers of glioma cells will need to be transduced for the therapy to succeed.
Taken together, our results indicate that HC-Ad vectors efficiently transduce human glioma cells and are very attractive gene transfer vectors for GBM gene therapy both in preclinical models and in human trials. Unexpectedly, expression levels of adenoviral entry receptors cannot be used to predict Ad transduction efficiency. Our data strongly support the use of regulatable high-capacity adenoviral vectors for the development of novel glioma therapeutics in humans.
MATERIALS AND METHODS
Cell cultures
Established human glioma cell lines U251 (ATCC, Rockville, MD, USA) and U87; short-term glioma cell cultures derived from surgical biopsies from patients with WHO grade IV astrocytoma, IN859 and IN2045 [5]; Lewis rat CNS-1 glioma cells; C57/BL6 mouse GL26 glioma cells; and HeLa and CHO cells were cultured in DMEM with 10% FBS, 100 U/ml penicillin/streptomycin, 2 mM L-glutamine, 2 mM nonessential amino acids.
High-capacity adenoviral vectors: development of murine CMV-driven regulatable TetON Switch and TK- and Flt3L-encoding cassettes
The [mCMV-rtTA2SM2-pIRES-tTSKid-pA] regulatable TetON switch was generated as described elsewhere [27]. The plasmid pBlueScript II SK(+) (Stratagene, La Jolla, CA, USA) was modified with a polylinker to generate an intermediate cloning plasmid, pBS-MCS1, introducing the following cloning sites: NotI, AscI, NheI, and HindIII. The kanamycin resistance gene was excised using NheI from plasmid pcDNA3.1/Zeo(+)Kanamycin (Clontech) and cloned into the NheI site of pBS-MCS1 to generate pBS-MCS1-Kana. The plasmid pSP72 (Promega, Madison, WI, USA) was modified with a polylinker to add the following restriction sites: BglII, HindIII, BamHI, and ClaI. The mCMV-TK-polyA expression cassette was excised from pΔE1-mCMV-TK-polyA [45] with BamHI and ClaI and inserted into the modified pSP72 plasmid to make pSP72-mCMV-TK-polyA with HindIII sites now flanking the expression cassette. The mCMV-TK-polyA expression cassette was excised with HindIII and cloned into the HindIII site of pBS-MCS1-Kana to make the high-capacity shuttle plasmid pBS-MCS1-mCMV-TK-polyA-Kana.
The plasmid pBlueScript II KS (+)[TRE-βgal-polyA]-Kana [27] was cut with BamHI to remove the β-galactosidase cDNA, and the hsFlt3L cDNA flanked by BglII sites obtained from pSP72Bgl2.hsFlt3L [29] was inserted, to generate pBlueScript II KS (+)[TRE-hsFlt3L-polyA]-Kana. The [mCMV-rtTA2SM2-pIRES-tTSKid-pA] regulatable TetON switch was then excised from pSP72[mCMV-rtTA2SM2-pIRES-tTSKid-pA] with BglII and cloned into the BglII site of pBlueScript II KS (+)[TRE-hsFlt3L-polyA]-Kana to generate the high-capacity shuttle plasmid pBlueScript II KS (+)[TRE-hsFlt3L-polyA]-[mCMV-rtTA2SM2-pIRES-tTSKid-pA]-Kana.
Engineering of HC-Ad plasmids
The cassette mCMV-TK-polyA-Kana was excised from the shuttle plasmid using NotI and cloned into the compatible EagI site of the HC-Ad plasmid pSTK120 [27], generating pSTK120-mCMV-TK-polyA (HC-Ad-mCMV-TK). The insert [TRE-hsFlt3L-polyA]-[mCMV-rtTA2SM2-pIRES-tTSKid-pA]-Kana was excised from the shuttle plasmid using NotI and cloned into the compatible EagI site of HC-Ad plasmid pSTK120.2, generating pSTK120.2-[TRE-hsFlt3L-polyA]-[mCMV-rtTA2SM2-pIRES-tTSKid-pA]-Kana (HC-Ad-mCMV-TetON-Flt3L). The fragments were ligated overnight, transformed into DH5α cells, and plated on LB plates with kanamycin. Plasmids were isolated from overnight cultures (32°C, 165 rpm) and screened by HindIII digestion. Maxiprep purifications were performed on the correct clones (Qiagen, Valencia, CA, USA). Restriction enzymes were used to confirm the integrity of the plasmids.
Production, scale up, and purification of HC-Ad vectors
HC-Ad vectors stocks were generated, scaled up, and purified using 5 μg of HC-Ad plasmid DNA as previously described [25]. Briefly, 1 × 109 116 cells growing in suspension were resuspended in 150 ml of conditioned MEM with 10% FBS and co-infected with 100 vp/cell of vector seed stocks and 1 pfu/cell of helper virus AdNG163R-2 [25]. Virus adsorption was performed for 2 h at 37°C in a 3-L spinner flask using a Bellennium D2005 magnetic stir plate (Bellco, Vineland, NJ, USA). Fresh medium mixture containing 25% MEM with 10% FBS and 75% Joklik’s MEM (Sigma, St. Louis, MO, USA) supplemented with 5% FBS was added to a final volume of 2 L. Co-infected cells were harvested 48 h later and HC-Ad vectors purified by three-step CsCl ultracentrifugations as described before [25,26].
Vector characterization
Viral vector particles per milliliter were measured at OD260 with a spectrophotometer (Beckman Coulter, Fullerton, CA, USA), and transgene-expressing particles per milliliter by TK or Flt3L immunocytochemistry [29]. Helper virus was titered using pfu/ml [25,26]. LPS contamination (Cambrex, East Rutherford, NJ, USA) and replication-competent adenovirus were detected using a biological assay [49].
Western blotting
Nonreducing 10% SDS–PAGE was performed with whole cell lysates. Primary anti-CAR (Upstate Cell Signaling Solutions), anti-integrin αvβ3 (Chemicon International), or anti-actin antibodies (Sigma) were detected with HRP-conjugated antibodies and developed with an ECL kit (Amersham Biosciences).
Immunocytochemistry
Anti-TK and anti-Flt3L antibody [29] binding in paraformaldehyde-fixed cells was detected with biotinylated secondary antibodies, revealed by peroxidase-conjugated avidin–diaminobenzidine. Anti-β-Gal antibody [50] binding was revealed with a fluorescent secondary antibody (Molecular Probes), followed by nuclear staining with DAPI and mounting with ProLong Antifade (Molecular Probes).
Unbiased cell counts
The number of cells transduced with the HC-Ad vectors was estimated at high magnification (40×) by unbiased, stereological techniques, using a Zeiss Axioplan 2 microscope controlled by a Ludl electronic MAC 5000 XY stage control (Ludl Electronics Products Ltd., Hawthorne, NY, USA) and Axioplan Z-axis control (Carl Zeiss Inc., Thornwood, NY, USA) utilizing the Stereo Investigator software version 5.00 (Microbrightfield, Inc., Colchester, VT, USA). The area to be sampled was traced with a ×1.25 objective. The thickness and the size of the counting frames were 1 and 200 × 200 μm, respectively. Twenty sampling sites were counted in each well.
Flow cytometry
Glioma cells (5 × 104/well) were infected with HC-Ad-TK (1 expressing particle/cell) or HC-Ad-βGal (1 blue-forming unit/cell). After 48 h cells were incubated in the presence or absence of the prodrug ganciclovir (10 μM) for an additional 48 h. After trypsinization, cells were fixed in ethanol 75% at −20°C. Cells were incubated in PBS containing propidium iodide (50 μg/ml) and RNase (10 μg/ml). Hypodiploid cells (sub-G1) were counted using a FACScan (Becton–Dickinson).
MHCI detection
Trypsinized glioma cells were incubated with fluorescein-conjugated anti-mouse MHCI (Serotec), mouse IgG2b isotype control, anti-human β2-microglobulin (BD Pharmingen), or IgM isotype control antibodies (37°C for 30 min). MHCI labeling is expressed as MHCI mean intensity after subtraction of isotype control mean intensity.
Flt3L ELISA
Glioma cells (5 × 104/well) were infected with HC-Ad-TetON-Flt3L and incubated with or without 1 μg/ml doxycycline for 72 h. Human Flt3L concentration was determined in 50 μl of supernatant using a commercial ELISA kit (21-377-296; R&D Systems, MN, USA).
β-Galactosidase activity
Cells (5 × 104/well) were infected with 30 blue-forming units of HC-Ad-mTetON-βGal or HC-Ad-hTetON-βGal and incubated for 72 h either with or without 1 μg/ml doxycycline. β-Galactosidase was measured as described before [27].
Statistical analysis
Data were analyzed by two-way ANOVA followed by Duncan’s test. Correlations were determined using Pearce correlation test. P < 0.05 was considered the cut-off point for significance.
Supplementary Material
Acknowledgments
This work was funded by National Institute of Neurological Disorders & Stroke (NINDS) 1RO1 NS44556.01, 1RO3 TW006273-01 to M.G.C., NINDS 1RO1 NS42893, U54 4 NS04-5309 and R21 NS47298 to P.R.L., The Linda Tallen & David Paul Kane Annual Fellowship, the Bram and Elaine Goldsmith Endowed Chair to P.R.L., the Medallions Group Endowed Chair to M.G.C., and the Board of Governors (CSMC). This work was also supported in part by NIH (CA102126), the Susan G. Komen Breast Cancer Foundation (BCTR02-01194), and the Donna and Jesse Garber Award to L.K.M.K.
APPENDIX A. SUPPLEMENTARY DATA
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ymthe. 2006.05.006.
References
- 1.Castro MG, et al. Current and future strategies for the treatment of malignant brain tumors. Pharmacol Ther. 2003;98:71–108. doi: 10.1016/s0163-7258(03)00014-7. [DOI] [PubMed] [Google Scholar]
- 2.Prados MD, et al. Treatment of progressive or recurrent glioblastoma multiforme in adults with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration: a phase I/II multi-institutional trial. J Neurooncol. 2003;65:269–278. doi: 10.1023/b:neon.0000003588.18644.9c. [DOI] [PubMed] [Google Scholar]
- 3.Darling JL, Thomas DG. Response of short-term cultures derived from human malignant glioma to aziridinylbenzoquinone, etoposide and doxorubicin: an in vitro phase II trial. Anticancer Drugs. 2001;12:753–760. doi: 10.1097/00001813-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Glaser T, Castro MG, Lowenstein PR, Weller M. Death receptor-independent cytochrome c release and caspase activation mediate thymidine kinase plus ganciclovir-mediated cytotoxicity in LN-18 and LN-229 human malignant glioma cells. Gene Ther. 2001;8:469–476. doi: 10.1038/sj.gt.3301415. [DOI] [PubMed] [Google Scholar]
- 5.Maleniak TC, Darling JL, Lowenstein PR, Castro MG. Adenovirus-mediated expression of HSV1-TK or Fas ligand induces cell death in primary human glioma-derived cell cultures that are resistant to the chemotherapeutic agent CCNU. Cancer Gene Ther. 2001;8:589–598. doi: 10.1038/sj.cgt.7700348. [DOI] [PubMed] [Google Scholar]
- 6.Cowen RL, et al. Adenovirus vector-mediated delivery of the prodrug-converting enzyme carboxypeptidase G2 in a secreted or GPI-anchored form: high-level expression of this active conditional cytotoxic enzyme at the plasma membrane. Cancer Gene Ther. 2002;9:897–907. doi: 10.1038/sj.cgt.7700514. [DOI] [PubMed] [Google Scholar]
- 7.Puumalainen AM, et al. Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther. 1998;9:1769–1774. doi: 10.1089/hum.1998.9.12-1769. [DOI] [PubMed] [Google Scholar]
- 8.Germano IM, Fable J, Gultekin SH, Silvers A. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas. J Neurooncol. 2003;65:279–289. doi: 10.1023/b:neon.0000003657.95085.56. [DOI] [PubMed] [Google Scholar]
- 9.Immonen A, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004;10:967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Bruning A, Runnebaum IB. CAR is a cell–cell adhesion protein in human cancer cells and is expressionally modulated by dexamethasone, TNFalpha, and TGFbeta. Gene Ther. 2003;10:198–205. doi: 10.1038/sj.gt.3301887. [DOI] [PubMed] [Google Scholar]
- 11.Carson SD. Coxsackievirus and adenovirus receptor (CAR) is modified and shed in membrane vesicles. Biochemistry. 2004;43:8136–8142. doi: 10.1021/bi049778d. [DOI] [PubMed] [Google Scholar]
- 12.Fueyo J, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 13.Fuxe J, et al. Expression of the coxsackie and adenovirus receptor in human astrocytic tumors and xenografts. Int J Cancer. 2003;103:723–729. doi: 10.1002/ijc.10891. [DOI] [PubMed] [Google Scholar]
- 14.Grill J, et al. Combined targeting of adenoviruses to integrins and epidermal growth factor receptors increases gene transfer into primary glioma cells and spheroids. Clin Cancer Res. 2001;7:641–650. [PubMed] [Google Scholar]
- 15.Mori T, Arakawa H, Tokino T, Mineura K, Nakamura Y. Significant increase of adenovirus infectivity in glioma cell lines by extracellular domain of hCAR. Oncol Res. 1999;11:513–521. [PubMed] [Google Scholar]
- 16.Skog J, et al. Efficient internalization into low-passage glioma cell lines using adenoviruses other than type 5: an approach for improvement of gene delivery to brain tumours. J Gen Virol. 2004;85:2627–2638. doi: 10.1099/vir.0.80084-0. [DOI] [PubMed] [Google Scholar]
- 17.van Beusechem VW, et al. Efficient and selective gene transfer into primary human brain tumors by using single-chain antibody-targeted adenoviral vectors with native tropism abolished. J Virol. 2002;76:2753–2762. doi: 10.1128/JVI.76.6.2753-2762.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, et al. Retargeting of adenoviral vector using basic fibroblast growth factor ligand for malignant glioma gene therapy. J Neurosurg. 2005;103:1058–1066. doi: 10.3171/jns.2005.103.6.1058. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Bergelson JM. Adenovirus receptors. J Virol. 2005;79:12125–12131. doi: 10.1128/JVI.79.19.12125-12131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas CE, Edwards P, Wickham TJ, Castro MG, Lowenstein PR. Adenovirus binding to the coxsackievirus and adenovirus receptor or integrins is not required to elicit brain inflammation but is necessary to transduce specific neural cell types. J Virol. 2002;76:3452–3460. doi: 10.1128/JVI.76.7.3452-3460.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Einfeld DA, et al. Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J Virol. 2001;75:11284–11291. doi: 10.1128/JVI.75.23.11284-11291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang KC, et al. Impact of the coxsackie and adenovirus receptor (CAR) on glioma cell growth and invasion: requirement for the C-terminal domain. Int J Cancer. 2005;113:738–745. doi: 10.1002/ijc.20623. [DOI] [PubMed] [Google Scholar]
- 23.Hong SS, Karayan L, Tournier J, Curiel DT, Boulanger PA. Adenovirus type 5 fiber knob binds to MHC class I alpha2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zagzag D, et al. Downregulation of major histocompatibility complex antigens in invading glioma cells: stealth invasion of the brain. Lab Invest. 2005;85:328–341. doi: 10.1038/labinvest.3700233. [DOI] [PubMed] [Google Scholar]
- 25.Palmer D, Ng P. Improved system for helper-dependent adenoviral vector production. Mol Ther. 2003;8:846–852. doi: 10.1016/j.ymthe.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Umana P, et al. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination. Nat Biotechnol. 2001;19:582–585. doi: 10.1038/89349. [DOI] [PubMed] [Google Scholar]
- 27.Xiong W, et al. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehrhardt A, Kay M. Gutted adenovirus: a rising star on the horizon? Gene Ther. 2005;12:1540–1541. doi: 10.1038/sj.gt.3302597. [DOI] [PubMed] [Google Scholar]
- 29.Ali S, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dechecchi MC, Tamanini A, Bonizzato A, Cabrini G. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology. 2000;268:382–390. doi: 10.1006/viro.1999.0171. [DOI] [PubMed] [Google Scholar]
- 31.Parish CR. Heparan sulfate and inflammation. Nat Immunol. 2005;6:861–862. doi: 10.1038/ni0905-861. [DOI] [PubMed] [Google Scholar]
- 32.Asaoka K, Tada M, Sawamura Y, Ikeda J, Abe H. Dependence of efficient adenoviral gene delivery in malignant glioma cells on the expression levels of the coxsackievirus and adenovirus receptor. J Neurosurg. 2000;92:1002–1008. doi: 10.3171/jns.2000.92.6.1002. [DOI] [PubMed] [Google Scholar]
- 33.Kim JS, et al. Enhancement of the adenoviral sensitivity of human ovarian cancer cells by transient expression of coxsackievirus and adenovirus receptor (CAR) Gynecol Oncol. 2002;85:260–265. doi: 10.1006/gyno.2002.6607. [DOI] [PubMed] [Google Scholar]
- 34.Chiocca EA, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 35.Eck SL, et al. Treatment of recurrent or progressive malignant glioma with a recombinant adenovirus expressing human interferon-beta (H5.010CMVhIFN-beta): a phase I trial. Hum Gene Ther. 2001;12:97–113. doi: 10.1089/104303401451013. [DOI] [PubMed] [Google Scholar]
- 36.Lang FF, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 37.Curtin JF, et al. Combining cytotoxic and immune-mediated gene therapy to treat brain tumors. Curr Top Med Chem. 2005;5:1151–1170. doi: 10.2174/156802605774370856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King GD, et al. Gene therapy and targeted toxins for glioma. Curr Gene Ther. 2005;5:535–557. doi: 10.2174/156652305774964631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiocca EA. Oncolytic viruses. Nat Rev Cancer. 2002;2:938–950. doi: 10.1038/nrc948. [DOI] [PubMed] [Google Scholar]
- 40.Gomez-Manzano C, Yung WK, Alemany R, Fueyo J. Genetically modified adenoviruses against gliomas: from bench to bedside. Neurology. 2004;63:418–426. doi: 10.1212/01.wnl.0000133302.15022.7f. [DOI] [PubMed] [Google Scholar]
- 41.Freeman SM, et al. The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53:5274–5283. [PubMed] [Google Scholar]
- 42.Goverdhana S, et al. Regulatable gene expression systems for gene therapy applications: progress and future challenges. Mol Ther. 2005;12:189–211. doi: 10.1016/j.ymthe.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woods NB, Bottero V, Schmidt M, von Kalle C, Verma IM. Gene therapy: therapeutic gene causing lymphoma. Nature. 2006;440:1123. doi: 10.1038/4401123a. [DOI] [PubMed] [Google Scholar]
- 44.Addison CL, Hitt M, Kunsken D, Graham FL. Comparison of the human versus murine cytomegalovirus immediate early gene promoters for transgene expression by adenoviral vectors. J Gen Virol. 1997;78(Pt 7 ):1653–1661. doi: 10.1099/0022-1317-78-7-1653. [DOI] [PubMed] [Google Scholar]
- 45.Gerdes CA, Castro MG, Lowenstein PR. Strong promoters are the key to highly efficient, noninflammatory and noncytotoxic adenoviral-mediated transgene delivery into the brain in vivo. Mol Ther. 2000;2:330–338. doi: 10.1006/mthe.2000.0140. [DOI] [PubMed] [Google Scholar]
- 46.Curtin JF, et al. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol. 2006;176:3566–3577. doi: 10.4049/jimmunol.176.6.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Windeatt S, et al. Adenovirus-mediated herpes simplex virus type-1 thymidine kinase gene therapy suppresses oestrogen-induced pituitary prolactinomas. J Clin Endocrinol Metab. 2000;85:1296–1305. doi: 10.1210/jcem.85.3.6482. [DOI] [PubMed] [Google Scholar]
- 48.Salomon B, et al. A truncated herpes simplex virus thymidine kinase phosphorylates thymidine and nucleoside analogs and does not cause sterility in transgenic mice. Mol Cell Biol. 2006;15:5322–5328. doi: 10.1128/mcb.15.10.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puntel M, et al. Quantification of high-capacity helper-dependent adenoviral vector genomes in vitro and in vivo, using quantitative TaqMan real-time polymerase chain reaction. Hum Gene Ther. 2006;17:531–544. doi: 10.1089/hum.2006.17.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci USA. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


